 Found 132 hits of Enzyme Inhibition Constant Data
Found 132 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50216004
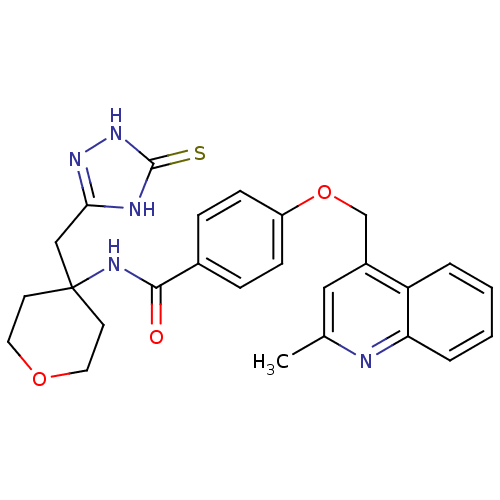
(4-((2-methylquinolin-4-yl)methoxy)-N-(4-((5-thioxo...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(Cc3n[nH]c(=S)[nH]3)CCOCC2)c2ccccc2n1 Show InChI InChI=1S/C26H27N5O3S/c1-17-14-19(21-4-2-3-5-22(21)27-17)16-34-20-8-6-18(7-9-20)24(32)29-26(10-12-33-13-11-26)15-23-28-25(35)31-30-23/h2-9,14H,10-13,15-16H2,1H3,(H,29,32)(H2,28,30,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 223 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50216004

(4-((2-methylquinolin-4-yl)methoxy)-N-(4-((5-thioxo...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(Cc3n[nH]c(=S)[nH]3)CCOCC2)c2ccccc2n1 Show InChI InChI=1S/C26H27N5O3S/c1-17-14-19(21-4-2-3-5-22(21)27-17)16-34-20-8-6-18(7-9-20)24(32)29-26(10-12-33-13-11-26)15-23-28-25(35)31-30-23/h2-9,14H,10-13,15-16H2,1H3,(H,29,32)(H2,28,30,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 635 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50216004

(4-((2-methylquinolin-4-yl)methoxy)-N-(4-((5-thioxo...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(Cc3n[nH]c(=S)[nH]3)CCOCC2)c2ccccc2n1 Show InChI InChI=1S/C26H27N5O3S/c1-17-14-19(21-4-2-3-5-22(21)27-17)16-34-20-8-6-18(7-9-20)24(32)29-26(10-12-33-13-11-26)15-23-28-25(35)31-30-23/h2-9,14H,10-13,15-16H2,1H3,(H,29,32)(H2,28,30,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 753 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215989

(CHEMBL392232 | cis-N-(1-methyl-4-(5-thioxo-4,5-dih...)Show SMILES CN1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C26H28N6O2S/c1-16-13-18(20-5-3-4-6-22(20)27-16)15-34-19-9-7-17(8-10-19)25(33)28-23-14-32(2)12-11-21(23)24-29-26(35)31-30-24/h3-10,13,21,23H,11-12,14-15H2,1-2H3,(H,28,33)(H2,29,30,31,35)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 958 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50216004

(4-((2-methylquinolin-4-yl)methoxy)-N-(4-((5-thioxo...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(Cc3n[nH]c(=S)[nH]3)CCOCC2)c2ccccc2n1 Show InChI InChI=1S/C26H27N5O3S/c1-17-14-19(21-4-2-3-5-22(21)27-17)16-34-20-8-6-18(7-9-20)24(32)29-26(10-12-33-13-11-26)15-23-28-25(35)31-30-23/h2-9,14H,10-13,15-16H2,1H3,(H,29,32)(H2,28,30,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50216004

(4-((2-methylquinolin-4-yl)methoxy)-N-(4-((5-thioxo...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(Cc3n[nH]c(=S)[nH]3)CCOCC2)c2ccccc2n1 Show InChI InChI=1S/C26H27N5O3S/c1-17-14-19(21-4-2-3-5-22(21)27-17)16-34-20-8-6-18(7-9-20)24(32)29-26(10-12-33-13-11-26)15-23-28-25(35)31-30-23/h2-9,14H,10-13,15-16H2,1H3,(H,29,32)(H2,28,30,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50216005
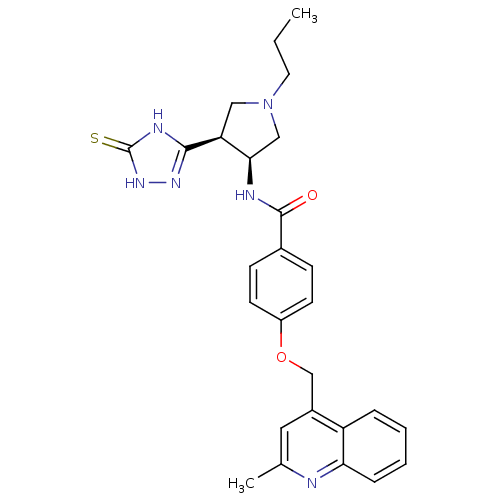
(CHEMBL394294 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES CCCN1C[C@@H](NC(=O)c2ccc(OCc3cc(C)nc4ccccc34)cc2)[C@H](C1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C27H30N6O2S/c1-3-12-33-14-22(25-30-27(36)32-31-25)24(15-33)29-26(34)18-8-10-20(11-9-18)35-16-19-13-17(2)28-23-7-5-4-6-21(19)23/h4-11,13,22,24H,3,12,14-16H2,1-2H3,(H,29,34)(H2,30,31,32,36)/t22-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50216000

(CHEMBL247546 | cis-N-(1-acetyl-4-(5-thioxo-4,5-dih...)Show SMILES CC(=O)N1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C27H28N6O3S/c1-16-13-19(21-5-3-4-6-23(21)28-16)15-36-20-9-7-18(8-10-20)26(35)29-24-14-33(17(2)34)12-11-22(24)25-30-27(37)32-31-25/h3-10,13,22,24H,11-12,14-15H2,1-2H3,(H,29,35)(H2,30,31,32,37)/t22-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50216000

(CHEMBL247546 | cis-N-(1-acetyl-4-(5-thioxo-4,5-dih...)Show SMILES CC(=O)N1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C27H28N6O3S/c1-16-13-19(21-5-3-4-6-23(21)28-16)15-36-20-9-7-18(8-10-20)26(35)29-24-14-33(17(2)34)12-11-22(24)25-30-27(37)32-31-25/h3-10,13,22,24H,11-12,14-15H2,1-2H3,(H,29,35)(H2,30,31,32,37)/t22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50216002

(CHEMBL247347 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CCOC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H25N5O3S/c1-15-12-17(19-4-2-3-5-21(19)26-15)13-33-18-8-6-16(7-9-18)24(31)27-22-10-11-32-14-20(22)23-28-25(34)30-29-23/h2-9,12,20,22H,10-11,13-14H2,1H3,(H,27,31)(H2,28,29,30,34)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50216007

(CHEMBL393443 | tert-butyl 4-(4-((2-methylquinolin-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(Cc3n[nH]c(=S)[nH]3)CCN(CC2)C(=O)OC(C)(C)C)c2ccccc2n1 Show InChI InChI=1S/C31H36N6O4S/c1-20-17-22(24-7-5-6-8-25(24)32-20)19-40-23-11-9-21(10-12-23)27(38)34-31(18-26-33-28(42)36-35-26)13-15-37(16-14-31)29(39)41-30(2,3)4/h5-12,17H,13-16,18-19H2,1-4H3,(H,34,38)(H2,33,35,36,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50216005

(CHEMBL394294 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES CCCN1C[C@@H](NC(=O)c2ccc(OCc3cc(C)nc4ccccc34)cc2)[C@H](C1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C27H30N6O2S/c1-3-12-33-14-22(25-30-27(36)32-31-25)24(15-33)29-26(34)18-8-10-20(11-9-18)35-16-19-13-17(2)28-23-7-5-4-6-21(19)23/h4-11,13,22,24H,3,12,14-16H2,1-2H3,(H,29,34)(H2,30,31,32,36)/t22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50215990
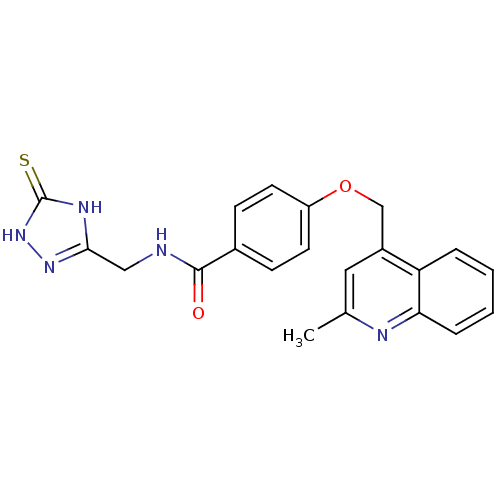
(4-((2-methylquinolin-4-yl)methoxy)-N-((5-thioxo-4,...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCc2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C21H19N5O2S/c1-13-10-15(17-4-2-3-5-18(17)23-13)12-28-16-8-6-14(7-9-16)20(27)22-11-19-24-21(29)26-25-19/h2-10H,11-12H2,1H3,(H,22,27)(H2,24,25,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50215997

(4-((2-methylquinolin-4-yl)methoxy)-N-(2-(5-thioxo-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCCc2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C22H21N5O2S/c1-14-12-16(18-4-2-3-5-19(18)24-14)13-29-17-8-6-15(7-9-17)21(28)23-11-10-20-25-22(30)27-26-20/h2-9,12H,10-11,13H2,1H3,(H,23,28)(H2,25,26,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50216003

(CHEMBL247346 | cis-N-(1-acetyl-4-(5-thioxo-4,5-dih...)Show SMILES CC(=O)N1C[C@@H](NC(=O)c2ccc(OCc3cc(C)nc4ccccc34)cc2)[C@H](C1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C26H26N6O3S/c1-15-11-18(20-5-3-4-6-22(20)27-15)14-35-19-9-7-17(8-10-19)25(34)28-23-13-32(16(2)33)12-21(23)24-29-26(36)31-30-24/h3-11,21,23H,12-14H2,1-2H3,(H,28,34)(H2,29,30,31,36)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50215998
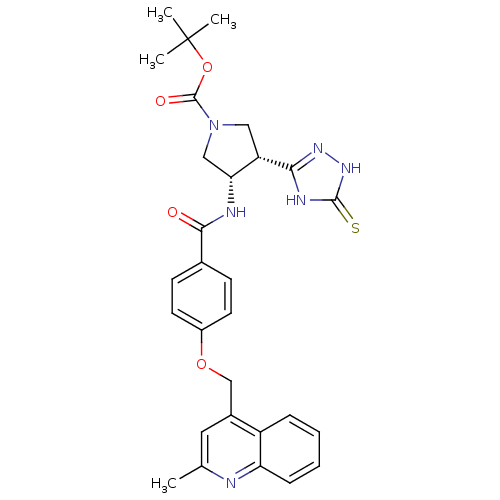
(CHEMBL247141 | cis-tert-butyl 3-(4-((2-methylquino...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(C[C@@H]2c2n[nH]c(=S)[nH]2)C(=O)OC(C)(C)C)c2ccccc2n1 Show InChI InChI=1S/C29H32N6O4S/c1-17-13-19(21-7-5-6-8-23(21)30-17)16-38-20-11-9-18(10-12-20)26(36)31-24-15-35(28(37)39-29(2,3)4)14-22(24)25-32-27(40)34-33-25/h5-13,22,24H,14-16H2,1-4H3,(H,31,36)(H2,32,33,34,40)/t22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50215994

(CHEMBL247743 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(CC[C@@H]2c2n[nH]c(=S)[nH]2)S(C)(=O)=O)c2ccccc2n1 Show InChI InChI=1S/C26H28N6O4S2/c1-16-13-18(20-5-3-4-6-22(20)27-16)15-36-19-9-7-17(8-10-19)25(33)28-23-14-32(38(2,34)35)12-11-21(23)24-29-26(37)31-30-24/h3-10,13,21,23H,11-12,14-15H2,1-2H3,(H,28,33)(H2,29,30,31,37)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50216001

(CHEMBL247947 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(CC#C)CC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C28H28N6O2S/c1-3-13-34-14-12-23(26-31-28(37)33-32-26)25(16-34)30-27(35)19-8-10-21(11-9-19)36-17-20-15-18(2)29-24-7-5-4-6-22(20)24/h1,4-11,15,23,25H,12-14,16-17H2,2H3,(H,30,35)(H2,31,32,33,37)/t23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50215991
(4-(quinolin-4-ylmethoxy)-N-(3-(5-thioxo-4,5-dihydr...)Show SMILES O=C(NCCCc1n[nH]c(=S)[nH]1)c1ccc(OCc2ccnc3ccccc23)cc1 Show InChI InChI=1S/C22H21N5O2S/c28-21(24-12-3-6-20-25-22(30)27-26-20)15-7-9-17(10-8-15)29-14-16-11-13-23-19-5-2-1-4-18(16)19/h1-2,4-5,7-11,13H,3,6,12,14H2,(H,24,28)(H2,25,26,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50215989

(CHEMBL392232 | cis-N-(1-methyl-4-(5-thioxo-4,5-dih...)Show SMILES CN1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C26H28N6O2S/c1-16-13-18(20-5-3-4-6-22(20)27-16)15-34-19-9-7-17(8-10-19)25(33)28-23-14-32(2)12-11-21(23)24-29-26(35)31-30-24/h3-10,13,21,23H,11-12,14-15H2,1-2H3,(H,28,33)(H2,29,30,31,35)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50216008

(CHEMBL392231 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CNCC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H26N6O2S/c1-15-12-17(19-4-2-3-5-21(19)27-15)14-33-18-8-6-16(7-9-18)24(32)28-22-13-26-11-10-20(22)23-29-25(34)31-30-23/h2-9,12,20,22,26H,10-11,13-14H2,1H3,(H,28,32)(H2,29,30,31,34)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50215999

(CHEMBL394084 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CNC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C24H24N6O2S/c1-14-10-16(18-4-2-3-5-20(18)26-14)13-32-17-8-6-15(7-9-17)23(31)27-21-12-25-11-19(21)22-28-24(33)30-29-22/h2-10,19,21,25H,11-13H2,1H3,(H,27,31)(H2,28,29,30,33)/t19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50216006
(CHEMBL247946 | cis-N-(1-isopropyl-4-(5-thioxo-4,5-...)Show SMILES CC(C)N1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C28H32N6O2S/c1-17(2)34-13-12-23(26-31-28(37)33-32-26)25(15-34)30-27(35)19-8-10-21(11-9-19)36-16-20-14-18(3)29-24-7-5-4-6-22(20)24/h4-11,14,17,23,25H,12-13,15-16H2,1-3H3,(H,30,35)(H2,31,32,33,37)/t23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50216009
(CHEMBL247348 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CCNC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H26N6O2S/c1-15-12-17(19-4-2-3-5-21(19)27-15)14-33-18-8-6-16(7-9-18)24(32)28-22-10-11-26-13-20(22)23-29-25(34)31-30-23/h2-9,12,20,22,26H,10-11,13-14H2,1H3,(H,28,32)(H2,29,30,31,34)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50216010

((R)-3-amino-1-((R)-3-methyl-1-(5-thioxo-4,5-dihydr...)Show SMILES CC(C)C[C@@H](N1CC[C@](N)(C1=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C28H32N6O2S/c1-17(2)14-24(25-31-27(37)33-32-25)34-13-12-28(29,26(34)35)20-8-10-21(11-9-20)36-16-19-15-18(3)30-23-7-5-4-6-22(19)23/h4-11,15,17,24H,12-14,16,29H2,1-3H3,(H2,31,32,33,37)/t24-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50215995

(CHEMBL247742 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES CCCN1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C28H32N6O2S/c1-3-13-34-14-12-23(26-31-28(37)33-32-26)25(16-34)30-27(35)19-8-10-21(11-9-19)36-17-20-15-18(2)29-24-7-5-4-6-22(20)24/h4-11,15,23,25H,3,12-14,16-17H2,1-2H3,(H,30,35)(H2,31,32,33,37)/t23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50215992

(CHEMBL247545 | cis-tert-butyl 3-(4-((2-methylquino...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(CC[C@@H]2c2n[nH]c(=S)[nH]2)C(=O)OC(C)(C)C)c2ccccc2n1 Show InChI InChI=1S/C30H34N6O4S/c1-18-15-20(22-7-5-6-8-24(22)31-18)17-39-21-11-9-19(10-12-21)27(37)32-25-16-36(29(38)40-30(2,3)4)14-13-23(25)26-33-28(41)35-34-26/h5-12,15,23,25H,13-14,16-17H2,1-4H3,(H,32,37)(H2,33,34,35,41)/t23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50215996
(CHEMBL392837 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CCC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H25N5O2S/c1-15-13-17(19-5-2-3-7-21(19)26-15)14-32-18-11-9-16(10-12-18)24(31)27-22-8-4-6-20(22)23-28-25(33)30-29-23/h2-3,5,7,9-13,20,22H,4,6,8,14H2,1H3,(H,27,31)(H2,28,29,30,33)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50215993

(CHEMBL248524 | N-(2-methyl-1-(5-thioxo-4,5-dihydro...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC(C)(C)Cc2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C24H25N5O2S/c1-15-12-17(19-6-4-5-7-20(19)25-15)14-31-18-10-8-16(9-11-18)22(30)27-24(2,3)13-21-26-23(32)29-28-21/h4-12H,13-14H2,1-3H3,(H,27,30)(H2,26,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215998

(CHEMBL247141 | cis-tert-butyl 3-(4-((2-methylquino...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(C[C@@H]2c2n[nH]c(=S)[nH]2)C(=O)OC(C)(C)C)c2ccccc2n1 Show InChI InChI=1S/C29H32N6O4S/c1-17-13-19(21-7-5-6-8-23(21)30-17)16-38-20-11-9-18(10-12-20)26(36)31-24-15-35(28(37)39-29(2,3)4)14-22(24)25-32-27(40)34-33-25/h5-13,22,24H,14-16H2,1-4H3,(H,31,36)(H2,32,33,34,40)/t22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50215990

(4-((2-methylquinolin-4-yl)methoxy)-N-((5-thioxo-4,...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCc2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C21H19N5O2S/c1-13-10-15(17-4-2-3-5-18(17)23-13)12-28-16-8-6-14(7-9-16)20(27)22-11-19-24-21(29)26-25-19/h2-10H,11-12H2,1H3,(H,22,27)(H2,24,25,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50215992

(CHEMBL247545 | cis-tert-butyl 3-(4-((2-methylquino...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(CC[C@@H]2c2n[nH]c(=S)[nH]2)C(=O)OC(C)(C)C)c2ccccc2n1 Show InChI InChI=1S/C30H34N6O4S/c1-18-15-20(22-7-5-6-8-24(22)31-18)17-39-21-11-9-19(10-12-21)27(37)32-25-16-36(29(38)40-30(2,3)4)14-13-23(25)26-33-28(41)35-34-26/h5-12,15,23,25H,13-14,16-17H2,1-4H3,(H,32,37)(H2,33,34,35,41)/t23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50215996

(CHEMBL392837 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CCC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H25N5O2S/c1-15-13-17(19-5-2-3-7-21(19)26-15)14-32-18-11-9-16(10-12-18)24(31)27-22-8-4-6-20(22)23-28-25(33)30-29-23/h2-3,5,7,9-13,20,22H,4,6,8,14H2,1H3,(H,27,31)(H2,28,29,30,33)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50215989

(CHEMBL392232 | cis-N-(1-methyl-4-(5-thioxo-4,5-dih...)Show SMILES CN1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C26H28N6O2S/c1-16-13-18(20-5-3-4-6-22(20)27-16)15-34-19-9-7-17(8-10-19)25(33)28-23-14-32(2)12-11-21(23)24-29-26(35)31-30-24/h3-10,13,21,23H,11-12,14-15H2,1-2H3,(H,28,33)(H2,29,30,31,35)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50215998

(CHEMBL247141 | cis-tert-butyl 3-(4-((2-methylquino...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(C[C@@H]2c2n[nH]c(=S)[nH]2)C(=O)OC(C)(C)C)c2ccccc2n1 Show InChI InChI=1S/C29H32N6O4S/c1-17-13-19(21-7-5-6-8-23(21)30-17)16-38-20-11-9-18(10-12-20)26(36)31-24-15-35(28(37)39-29(2,3)4)14-22(24)25-32-27(40)34-33-25/h5-13,22,24H,14-16H2,1-4H3,(H,31,36)(H2,32,33,34,40)/t22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50215995

(CHEMBL247742 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES CCCN1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C28H32N6O2S/c1-3-13-34-14-12-23(26-31-28(37)33-32-26)25(16-34)30-27(35)19-8-10-21(11-9-19)36-17-20-15-18(2)29-24-7-5-4-6-22(20)24/h4-11,15,23,25H,3,12-14,16-17H2,1-2H3,(H,30,35)(H2,31,32,33,37)/t23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50216010

((R)-3-amino-1-((R)-3-methyl-1-(5-thioxo-4,5-dihydr...)Show SMILES CC(C)C[C@@H](N1CC[C@](N)(C1=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C28H32N6O2S/c1-17(2)14-24(25-31-27(37)33-32-25)34-13-12-28(29,26(34)35)20-8-10-21(11-9-20)36-16-19-15-18(3)30-23-7-5-4-6-22(19)23/h4-11,15,17,24H,12-14,16,29H2,1-3H3,(H2,31,32,33,37)/t24-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50216002

(CHEMBL247347 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CCOC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H25N5O3S/c1-15-12-17(19-4-2-3-5-21(19)26-15)13-33-18-8-6-16(7-9-18)24(31)27-22-10-11-32-14-20(22)23-28-25(34)30-29-23/h2-9,12,20,22H,10-11,13-14H2,1H3,(H,27,31)(H2,28,29,30,34)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50216007

(CHEMBL393443 | tert-butyl 4-(4-((2-methylquinolin-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(Cc3n[nH]c(=S)[nH]3)CCN(CC2)C(=O)OC(C)(C)C)c2ccccc2n1 Show InChI InChI=1S/C31H36N6O4S/c1-20-17-22(24-7-5-6-8-25(24)32-20)19-40-23-11-9-21(10-12-23)27(38)34-31(18-26-33-28(42)36-35-26)13-15-37(16-14-31)29(39)41-30(2,3)4/h5-12,17H,13-16,18-19H2,1-4H3,(H,34,38)(H2,33,35,36,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50216008

(CHEMBL392231 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CNCC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H26N6O2S/c1-15-12-17(19-4-2-3-5-21(19)27-15)14-33-18-8-6-16(7-9-18)24(32)28-22-13-26-11-10-20(22)23-29-25(34)31-30-23/h2-9,12,20,22,26H,10-11,13-14H2,1H3,(H,28,32)(H2,29,30,31,34)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50216001

(CHEMBL247947 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(CC#C)CC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C28H28N6O2S/c1-3-13-34-14-12-23(26-31-28(37)33-32-26)25(16-34)30-27(35)19-8-10-21(11-9-19)36-17-20-15-18(2)29-24-7-5-4-6-22(20)24/h1,4-11,15,23,25H,12-14,16-17H2,2H3,(H,30,35)(H2,31,32,33,37)/t23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50216005

(CHEMBL394294 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES CCCN1C[C@@H](NC(=O)c2ccc(OCc3cc(C)nc4ccccc34)cc2)[C@H](C1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C27H30N6O2S/c1-3-12-33-14-22(25-30-27(36)32-31-25)24(15-33)29-26(34)18-8-10-20(11-9-18)35-16-19-13-17(2)28-23-7-5-4-6-21(19)23/h4-11,13,22,24H,3,12,14-16H2,1-2H3,(H,29,34)(H2,30,31,32,36)/t22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50215993

(CHEMBL248524 | N-(2-methyl-1-(5-thioxo-4,5-dihydro...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC(C)(C)Cc2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C24H25N5O2S/c1-15-12-17(19-6-4-5-7-20(19)25-15)14-31-18-10-8-16(9-11-18)22(30)27-24(2,3)13-21-26-23(32)29-28-21/h4-12H,13-14H2,1-3H3,(H,27,30)(H2,26,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50215997

(4-((2-methylquinolin-4-yl)methoxy)-N-(2-(5-thioxo-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCCc2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C22H21N5O2S/c1-14-12-16(18-4-2-3-5-19(18)24-14)13-29-17-8-6-15(7-9-17)21(28)23-11-10-20-25-22(30)27-26-20/h2-9,12H,10-11,13H2,1H3,(H,23,28)(H2,25,26,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50216009

(CHEMBL247348 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CCNC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H26N6O2S/c1-15-12-17(19-4-2-3-5-21(19)27-15)14-33-18-8-6-16(7-9-18)24(32)28-22-10-11-26-13-20(22)23-29-25(34)31-30-23/h2-9,12,20,22,26H,10-11,13-14H2,1H3,(H,28,32)(H2,29,30,31,34)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50216000

(CHEMBL247546 | cis-N-(1-acetyl-4-(5-thioxo-4,5-dih...)Show SMILES CC(=O)N1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C27H28N6O3S/c1-16-13-19(21-5-3-4-6-23(21)28-16)15-36-20-9-7-18(8-10-20)26(35)29-24-14-33(17(2)34)12-11-22(24)25-30-27(37)32-31-25/h3-10,13,22,24H,11-12,14-15H2,1-2H3,(H,29,35)(H2,30,31,32,37)/t22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50215994

(CHEMBL247743 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(CC[C@@H]2c2n[nH]c(=S)[nH]2)S(C)(=O)=O)c2ccccc2n1 Show InChI InChI=1S/C26H28N6O4S2/c1-16-13-18(20-5-3-4-6-22(20)27-16)15-36-19-9-7-17(8-10-19)25(33)28-23-14-32(38(2,34)35)12-11-21(23)24-29-26(37)31-30-24/h3-10,13,21,23H,11-12,14-15H2,1-2H3,(H,28,33)(H2,29,30,31,37)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50216003

(CHEMBL247346 | cis-N-(1-acetyl-4-(5-thioxo-4,5-dih...)Show SMILES CC(=O)N1C[C@@H](NC(=O)c2ccc(OCc3cc(C)nc4ccccc34)cc2)[C@H](C1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C26H26N6O3S/c1-15-11-18(20-5-3-4-6-22(20)27-15)14-35-19-9-7-17(8-10-19)25(34)28-23-13-32(16(2)33)12-21(23)24-29-26(36)31-30-24/h3-11,21,23H,12-14H2,1-2H3,(H,28,34)(H2,29,30,31,36)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50215999

(CHEMBL394084 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CNC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C24H24N6O2S/c1-14-10-16(18-4-2-3-5-20(18)26-14)13-32-17-8-6-15(7-9-17)23(31)27-21-12-25-11-19(21)22-28-24(33)30-29-22/h2-10,19,21,25H,11-13H2,1H3,(H,27,31)(H2,28,29,30,33)/t19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50216006

(CHEMBL247946 | cis-N-(1-isopropyl-4-(5-thioxo-4,5-...)Show SMILES CC(C)N1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C28H32N6O2S/c1-17(2)34-13-12-23(26-31-28(37)33-32-26)25(15-34)30-27(35)19-8-10-21(11-9-19)36-16-20-14-18(3)29-24-7-5-4-6-22(20)24/h4-11,14,17,23,25H,12-13,15-16H2,1-3H3,(H,30,35)(H2,31,32,33,37)/t23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50215991

(4-(quinolin-4-ylmethoxy)-N-(3-(5-thioxo-4,5-dihydr...)Show SMILES O=C(NCCCc1n[nH]c(=S)[nH]1)c1ccc(OCc2ccnc3ccccc23)cc1 Show InChI InChI=1S/C22H21N5O2S/c28-21(24-12-3-6-20-25-22(30)27-26-20)15-7-9-17(10-8-15)29-14-16-11-13-23-19-5-2-1-4-18(16)19/h1-2,4-5,7-11,13H,3,6,12,14H2,(H,24,28)(H2,25,26,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50216001

(CHEMBL247947 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(CC#C)CC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C28H28N6O2S/c1-3-13-34-14-12-23(26-31-28(37)33-32-26)25(16-34)30-27(35)19-8-10-21(11-9-19)36-17-20-15-18(2)29-24-7-5-4-6-22(20)24/h1,4-11,15,23,25H,12-14,16-17H2,2H3,(H,30,35)(H2,31,32,33,37)/t23-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50215995

(CHEMBL247742 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES CCCN1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C28H32N6O2S/c1-3-13-34-14-12-23(26-31-28(37)33-32-26)25(16-34)30-27(35)19-8-10-21(11-9-19)36-17-20-15-18(2)29-24-7-5-4-6-22(20)24/h4-11,15,23,25H,3,12-14,16-17H2,1-2H3,(H,30,35)(H2,31,32,33,37)/t23-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50216003

(CHEMBL247346 | cis-N-(1-acetyl-4-(5-thioxo-4,5-dih...)Show SMILES CC(=O)N1C[C@@H](NC(=O)c2ccc(OCc3cc(C)nc4ccccc34)cc2)[C@H](C1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C26H26N6O3S/c1-15-11-18(20-5-3-4-6-22(20)27-15)14-35-19-9-7-17(8-10-19)25(34)28-23-13-32(16(2)33)12-21(23)24-29-26(36)31-30-24/h3-11,21,23H,12-14H2,1-2H3,(H,28,34)(H2,29,30,31,36)/t21-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50215992

(CHEMBL247545 | cis-tert-butyl 3-(4-((2-methylquino...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(CC[C@@H]2c2n[nH]c(=S)[nH]2)C(=O)OC(C)(C)C)c2ccccc2n1 Show InChI InChI=1S/C30H34N6O4S/c1-18-15-20(22-7-5-6-8-24(22)31-18)17-39-21-11-9-19(10-12-21)27(37)32-25-16-36(29(38)40-30(2,3)4)14-13-23(25)26-33-28(41)35-34-26/h5-12,15,23,25H,13-14,16-17H2,1-4H3,(H,32,37)(H2,33,34,35,41)/t23-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50216007

(CHEMBL393443 | tert-butyl 4-(4-((2-methylquinolin-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(Cc3n[nH]c(=S)[nH]3)CCN(CC2)C(=O)OC(C)(C)C)c2ccccc2n1 Show InChI InChI=1S/C31H36N6O4S/c1-20-17-22(24-7-5-6-8-25(24)32-20)19-40-23-11-9-21(10-12-23)27(38)34-31(18-26-33-28(42)36-35-26)13-15-37(16-14-31)29(39)41-30(2,3)4/h5-12,17H,13-16,18-19H2,1-4H3,(H,34,38)(H2,33,35,36,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50216010

((R)-3-amino-1-((R)-3-methyl-1-(5-thioxo-4,5-dihydr...)Show SMILES CC(C)C[C@@H](N1CC[C@](N)(C1=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C28H32N6O2S/c1-17(2)14-24(25-31-27(37)33-32-25)34-13-12-28(29,26(34)35)20-8-10-21(11-9-20)36-16-19-15-18(3)30-23-7-5-4-6-22(19)23/h4-11,15,17,24H,12-14,16,29H2,1-3H3,(H2,31,32,33,37)/t24-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50215999

(CHEMBL394084 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CNC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C24H24N6O2S/c1-14-10-16(18-4-2-3-5-20(18)26-14)13-32-17-8-6-15(7-9-17)23(31)27-21-12-25-11-19(21)22-28-24(33)30-29-22/h2-10,19,21,25H,11-13H2,1H3,(H,27,31)(H2,28,29,30,33)/t19-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50216002

(CHEMBL247347 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CCOC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H25N5O3S/c1-15-12-17(19-4-2-3-5-21(19)26-15)13-33-18-8-6-16(7-9-18)24(31)27-22-10-11-32-14-20(22)23-28-25(34)30-29-23/h2-9,12,20,22H,10-11,13-14H2,1H3,(H,27,31)(H2,28,29,30,34)/t20-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50216009

(CHEMBL247348 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CCNC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H26N6O2S/c1-15-12-17(19-4-2-3-5-21(19)27-15)14-33-18-8-6-16(7-9-18)24(32)28-22-10-11-26-13-20(22)23-29-25(34)31-30-23/h2-9,12,20,22,26H,10-11,13-14H2,1H3,(H,28,32)(H2,29,30,31,34)/t20-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50215990

(4-((2-methylquinolin-4-yl)methoxy)-N-((5-thioxo-4,...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCc2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C21H19N5O2S/c1-13-10-15(17-4-2-3-5-18(17)23-13)12-28-16-8-6-14(7-9-16)20(27)22-11-19-24-21(29)26-25-19/h2-10H,11-12H2,1H3,(H,22,27)(H2,24,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50215997

(4-((2-methylquinolin-4-yl)methoxy)-N-(2-(5-thioxo-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCCc2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C22H21N5O2S/c1-14-12-16(18-4-2-3-5-19(18)24-14)13-29-17-8-6-15(7-9-17)21(28)23-11-10-20-25-22(30)27-26-20/h2-9,12H,10-11,13H2,1H3,(H,23,28)(H2,25,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50215991

(4-(quinolin-4-ylmethoxy)-N-(3-(5-thioxo-4,5-dihydr...)Show SMILES O=C(NCCCc1n[nH]c(=S)[nH]1)c1ccc(OCc2ccnc3ccccc23)cc1 Show InChI InChI=1S/C22H21N5O2S/c28-21(24-12-3-6-20-25-22(30)27-26-20)15-7-9-17(10-8-15)29-14-16-11-13-23-19-5-2-1-4-18(16)19/h1-2,4-5,7-11,13H,3,6,12,14H2,(H,24,28)(H2,25,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50215994

(CHEMBL247743 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(CC[C@@H]2c2n[nH]c(=S)[nH]2)S(C)(=O)=O)c2ccccc2n1 Show InChI InChI=1S/C26H28N6O4S2/c1-16-13-18(20-5-3-4-6-22(20)27-16)15-36-19-9-7-17(8-10-19)25(33)28-23-14-32(38(2,34)35)12-11-21(23)24-29-26(37)31-30-24/h3-10,13,21,23H,11-12,14-15H2,1-2H3,(H,28,33)(H2,29,30,31,37)/t21-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50215998

(CHEMBL247141 | cis-tert-butyl 3-(4-((2-methylquino...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(C[C@@H]2c2n[nH]c(=S)[nH]2)C(=O)OC(C)(C)C)c2ccccc2n1 Show InChI InChI=1S/C29H32N6O4S/c1-17-13-19(21-7-5-6-8-23(21)30-17)16-38-20-11-9-18(10-12-20)26(36)31-24-15-35(28(37)39-29(2,3)4)14-22(24)25-32-27(40)34-33-25/h5-13,22,24H,14-16H2,1-4H3,(H,31,36)(H2,32,33,34,40)/t22-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50216008

(CHEMBL392231 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CNCC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H26N6O2S/c1-15-12-17(19-4-2-3-5-21(19)27-15)14-33-18-8-6-16(7-9-18)24(32)28-22-13-26-11-10-20(22)23-29-25(34)31-30-23/h2-9,12,20,22,26H,10-11,13-14H2,1H3,(H,28,32)(H2,29,30,31,34)/t20-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50215996

(CHEMBL392837 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CCC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H25N5O2S/c1-15-13-17(19-5-2-3-7-21(19)26-15)14-32-18-11-9-16(10-12-18)24(31)27-22-8-4-6-20(22)23-28-25(33)30-29-23/h2-3,5,7,9-13,20,22H,4,6,8,14H2,1H3,(H,27,31)(H2,28,29,30,33)/t20-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50216006

(CHEMBL247946 | cis-N-(1-isopropyl-4-(5-thioxo-4,5-...)Show SMILES CC(C)N1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C28H32N6O2S/c1-17(2)34-13-12-23(26-31-28(37)33-32-26)25(15-34)30-27(35)19-8-10-21(11-9-19)36-16-20-14-18(3)29-24-7-5-4-6-22(20)24/h4-11,14,17,23,25H,12-13,15-16H2,1-3H3,(H,30,35)(H2,31,32,33,37)/t23-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50215993

(CHEMBL248524 | N-(2-methyl-1-(5-thioxo-4,5-dihydro...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC(C)(C)Cc2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C24H25N5O2S/c1-15-12-17(19-6-4-5-7-20(19)25-15)14-31-18-10-8-16(9-11-18)22(30)27-24(2,3)13-21-26-23(32)29-28-21/h4-12H,13-14H2,1-3H3,(H,27,30)(H2,26,28,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50215989

(CHEMBL392232 | cis-N-(1-methyl-4-(5-thioxo-4,5-dih...)Show SMILES CN1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C26H28N6O2S/c1-16-13-18(20-5-3-4-6-22(20)27-16)15-34-19-9-7-17(8-10-19)25(33)28-23-14-32(2)12-11-21(23)24-29-26(35)31-30-24/h3-10,13,21,23H,11-12,14-15H2,1-2H3,(H,28,33)(H2,29,30,31,35)/t21-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50215989

(CHEMBL392232 | cis-N-(1-methyl-4-(5-thioxo-4,5-dih...)Show SMILES CN1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C26H28N6O2S/c1-16-13-18(20-5-3-4-6-22(20)27-16)15-34-19-9-7-17(8-10-19)25(33)28-23-14-32(2)12-11-21(23)24-29-26(35)31-30-24/h3-10,13,21,23H,11-12,14-15H2,1-2H3,(H,28,33)(H2,29,30,31,35)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215993

(CHEMBL248524 | N-(2-methyl-1-(5-thioxo-4,5-dihydro...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC(C)(C)Cc2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C24H25N5O2S/c1-15-12-17(19-6-4-5-7-20(19)25-15)14-31-18-10-8-16(9-11-18)22(30)27-24(2,3)13-21-26-23(32)29-28-21/h4-12H,13-14H2,1-3H3,(H,27,30)(H2,26,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215995

(CHEMBL247742 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES CCCN1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C28H32N6O2S/c1-3-13-34-14-12-23(26-31-28(37)33-32-26)25(16-34)30-27(35)19-8-10-21(11-9-19)36-17-20-15-18(2)29-24-7-5-4-6-22(20)24/h4-11,15,23,25H,3,12-14,16-17H2,1-2H3,(H,30,35)(H2,31,32,33,37)/t23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215990

(4-((2-methylquinolin-4-yl)methoxy)-N-((5-thioxo-4,...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCc2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C21H19N5O2S/c1-13-10-15(17-4-2-3-5-18(17)23-13)12-28-16-8-6-14(7-9-16)20(27)22-11-19-24-21(29)26-25-19/h2-10H,11-12H2,1H3,(H,22,27)(H2,24,25,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215992

(CHEMBL247545 | cis-tert-butyl 3-(4-((2-methylquino...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(CC[C@@H]2c2n[nH]c(=S)[nH]2)C(=O)OC(C)(C)C)c2ccccc2n1 Show InChI InChI=1S/C30H34N6O4S/c1-18-15-20(22-7-5-6-8-24(22)31-18)17-39-21-11-9-19(10-12-21)27(37)32-25-16-36(29(38)40-30(2,3)4)14-13-23(25)26-33-28(41)35-34-26/h5-12,15,23,25H,13-14,16-17H2,1-4H3,(H,32,37)(H2,33,34,35,41)/t23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215991

(4-(quinolin-4-ylmethoxy)-N-(3-(5-thioxo-4,5-dihydr...)Show SMILES O=C(NCCCc1n[nH]c(=S)[nH]1)c1ccc(OCc2ccnc3ccccc23)cc1 Show InChI InChI=1S/C22H21N5O2S/c28-21(24-12-3-6-20-25-22(30)27-26-20)15-7-9-17(10-8-15)29-14-16-11-13-23-19-5-2-1-4-18(16)19/h1-2,4-5,7-11,13H,3,6,12,14H2,(H,24,28)(H2,25,26,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50216006

(CHEMBL247946 | cis-N-(1-isopropyl-4-(5-thioxo-4,5-...)Show SMILES CC(C)N1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C28H32N6O2S/c1-17(2)34-13-12-23(26-31-28(37)33-32-26)25(15-34)30-27(35)19-8-10-21(11-9-19)36-16-20-14-18(3)29-24-7-5-4-6-22(20)24/h4-11,14,17,23,25H,12-13,15-16H2,1-3H3,(H,30,35)(H2,31,32,33,37)/t23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50216008

(CHEMBL392231 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CNCC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H26N6O2S/c1-15-12-17(19-4-2-3-5-21(19)27-15)14-33-18-8-6-16(7-9-18)24(32)28-22-13-26-11-10-20(22)23-29-25(34)31-30-23/h2-9,12,20,22,26H,10-11,13-14H2,1H3,(H,28,32)(H2,29,30,31,34)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215994

(CHEMBL247743 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(CC[C@@H]2c2n[nH]c(=S)[nH]2)S(C)(=O)=O)c2ccccc2n1 Show InChI InChI=1S/C26H28N6O4S2/c1-16-13-18(20-5-3-4-6-22(20)27-16)15-36-19-9-7-17(8-10-19)25(33)28-23-14-32(38(2,34)35)12-11-21(23)24-29-26(37)31-30-24/h3-10,13,21,23H,11-12,14-15H2,1-2H3,(H,28,33)(H2,29,30,31,37)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215996

(CHEMBL392837 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CCC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H25N5O2S/c1-15-13-17(19-5-2-3-7-21(19)26-15)14-32-18-11-9-16(10-12-18)24(31)27-22-8-4-6-20(22)23-28-25(33)30-29-23/h2-3,5,7,9-13,20,22H,4,6,8,14H2,1H3,(H,27,31)(H2,28,29,30,33)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215997

(4-((2-methylquinolin-4-yl)methoxy)-N-(2-(5-thioxo-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCCc2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C22H21N5O2S/c1-14-12-16(18-4-2-3-5-19(18)24-14)13-29-17-8-6-15(7-9-17)21(28)23-11-10-20-25-22(30)27-26-20/h2-9,12H,10-11,13H2,1H3,(H,23,28)(H2,25,26,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215999

(CHEMBL394084 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CNC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C24H24N6O2S/c1-14-10-16(18-4-2-3-5-20(18)26-14)13-32-17-8-6-15(7-9-17)23(31)27-21-12-25-11-19(21)22-28-24(33)30-29-22/h2-10,19,21,25H,11-13H2,1H3,(H,27,31)(H2,28,29,30,33)/t19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50216001

(CHEMBL247947 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(CC#C)CC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C28H28N6O2S/c1-3-13-34-14-12-23(26-31-28(37)33-32-26)25(16-34)30-27(35)19-8-10-21(11-9-19)36-17-20-15-18(2)29-24-7-5-4-6-22(20)24/h1,4-11,15,23,25H,12-14,16-17H2,2H3,(H,30,35)(H2,31,32,33,37)/t23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50216009

(CHEMBL247348 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CCNC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H26N6O2S/c1-15-12-17(19-4-2-3-5-21(19)27-15)14-33-18-8-6-16(7-9-18)24(32)28-22-10-11-26-13-20(22)23-29-25(34)31-30-23/h2-9,12,20,22,26H,10-11,13-14H2,1H3,(H,28,32)(H2,29,30,31,34)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50216000

(CHEMBL247546 | cis-N-(1-acetyl-4-(5-thioxo-4,5-dih...)Show SMILES CC(=O)N1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C27H28N6O3S/c1-16-13-19(21-5-3-4-6-23(21)28-16)15-36-20-9-7-18(8-10-20)26(35)29-24-14-33(17(2)34)12-11-22(24)25-30-27(37)32-31-25/h3-10,13,22,24H,11-12,14-15H2,1-2H3,(H,29,35)(H2,30,31,32,37)/t22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50216007

(CHEMBL393443 | tert-butyl 4-(4-((2-methylquinolin-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(Cc3n[nH]c(=S)[nH]3)CCN(CC2)C(=O)OC(C)(C)C)c2ccccc2n1 Show InChI InChI=1S/C31H36N6O4S/c1-20-17-22(24-7-5-6-8-25(24)32-20)19-40-23-11-9-21(10-12-23)27(38)34-31(18-26-33-28(42)36-35-26)13-15-37(16-14-31)29(39)41-30(2,3)4/h5-12,17H,13-16,18-19H2,1-4H3,(H,34,38)(H2,33,35,36,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50216005

(CHEMBL394294 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES CCCN1C[C@@H](NC(=O)c2ccc(OCc3cc(C)nc4ccccc34)cc2)[C@H](C1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C27H30N6O2S/c1-3-12-33-14-22(25-30-27(36)32-31-25)24(15-33)29-26(34)18-8-10-20(11-9-18)35-16-19-13-17(2)28-23-7-5-4-6-21(19)23/h4-11,13,22,24H,3,12,14-16H2,1-2H3,(H,29,34)(H2,30,31,32,36)/t22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50216003

(CHEMBL247346 | cis-N-(1-acetyl-4-(5-thioxo-4,5-dih...)Show SMILES CC(=O)N1C[C@@H](NC(=O)c2ccc(OCc3cc(C)nc4ccccc34)cc2)[C@H](C1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C26H26N6O3S/c1-15-11-18(20-5-3-4-6-22(20)27-15)14-35-19-9-7-17(8-10-19)25(34)28-23-13-32(16(2)33)12-21(23)24-29-26(36)31-30-24/h3-11,21,23H,12-14H2,1-2H3,(H,28,34)(H2,29,30,31,36)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50216002

(CHEMBL247347 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CCOC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H25N5O3S/c1-15-12-17(19-4-2-3-5-21(19)26-15)13-33-18-8-6-16(7-9-18)24(31)27-22-10-11-32-14-20(22)23-28-25(34)30-29-23/h2-9,12,20,22H,10-11,13-14H2,1H3,(H,27,31)(H2,28,29,30,34)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50216010

((R)-3-amino-1-((R)-3-methyl-1-(5-thioxo-4,5-dihydr...)Show SMILES CC(C)C[C@@H](N1CC[C@](N)(C1=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C28H32N6O2S/c1-17(2)14-24(25-31-27(37)33-32-25)34-13-12-28(29,26(34)35)20-8-10-21(11-9-20)36-16-19-15-18(3)30-23-7-5-4-6-22(19)23/h4-11,15,17,24H,12-14,16,29H2,1-3H3,(H2,31,32,33,37)/t24-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50216007

(CHEMBL393443 | tert-butyl 4-(4-((2-methylquinolin-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(Cc3n[nH]c(=S)[nH]3)CCN(CC2)C(=O)OC(C)(C)C)c2ccccc2n1 Show InChI InChI=1S/C31H36N6O4S/c1-20-17-22(24-7-5-6-8-25(24)32-20)19-40-23-11-9-21(10-12-23)27(38)34-31(18-26-33-28(42)36-35-26)13-15-37(16-14-31)29(39)41-30(2,3)4/h5-12,17H,13-16,18-19H2,1-4H3,(H,34,38)(H2,33,35,36,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50216006

(CHEMBL247946 | cis-N-(1-isopropyl-4-(5-thioxo-4,5-...)Show SMILES CC(C)N1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C28H32N6O2S/c1-17(2)34-13-12-23(26-31-28(37)33-32-26)25(15-34)30-27(35)19-8-10-21(11-9-19)36-16-20-14-18(3)29-24-7-5-4-6-22(20)24/h4-11,14,17,23,25H,12-13,15-16H2,1-3H3,(H,30,35)(H2,31,32,33,37)/t23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50215993

(CHEMBL248524 | N-(2-methyl-1-(5-thioxo-4,5-dihydro...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC(C)(C)Cc2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C24H25N5O2S/c1-15-12-17(19-6-4-5-7-20(19)25-15)14-31-18-10-8-16(9-11-18)22(30)27-24(2,3)13-21-26-23(32)29-28-21/h4-12H,13-14H2,1-3H3,(H,27,30)(H2,26,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50216009

(CHEMBL247348 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CCNC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H26N6O2S/c1-15-12-17(19-4-2-3-5-21(19)27-15)14-33-18-8-6-16(7-9-18)24(32)28-22-10-11-26-13-20(22)23-29-25(34)31-30-23/h2-9,12,20,22,26H,10-11,13-14H2,1H3,(H,28,32)(H2,29,30,31,34)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50216003

(CHEMBL247346 | cis-N-(1-acetyl-4-(5-thioxo-4,5-dih...)Show SMILES CC(=O)N1C[C@@H](NC(=O)c2ccc(OCc3cc(C)nc4ccccc34)cc2)[C@H](C1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C26H26N6O3S/c1-15-11-18(20-5-3-4-6-22(20)27-15)14-35-19-9-7-17(8-10-19)25(34)28-23-13-32(16(2)33)12-21(23)24-29-26(36)31-30-24/h3-11,21,23H,12-14H2,1-2H3,(H,28,34)(H2,29,30,31,36)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50215991

(4-(quinolin-4-ylmethoxy)-N-(3-(5-thioxo-4,5-dihydr...)Show SMILES O=C(NCCCc1n[nH]c(=S)[nH]1)c1ccc(OCc2ccnc3ccccc23)cc1 Show InChI InChI=1S/C22H21N5O2S/c28-21(24-12-3-6-20-25-22(30)27-26-20)15-7-9-17(10-8-15)29-14-16-11-13-23-19-5-2-1-4-18(16)19/h1-2,4-5,7-11,13H,3,6,12,14H2,(H,24,28)(H2,25,26,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50215995

(CHEMBL247742 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES CCCN1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C28H32N6O2S/c1-3-13-34-14-12-23(26-31-28(37)33-32-26)25(16-34)30-27(35)19-8-10-21(11-9-19)36-17-20-15-18(2)29-24-7-5-4-6-22(20)24/h4-11,15,23,25H,3,12-14,16-17H2,1-2H3,(H,30,35)(H2,31,32,33,37)/t23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50215997

(4-((2-methylquinolin-4-yl)methoxy)-N-(2-(5-thioxo-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCCc2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C22H21N5O2S/c1-14-12-16(18-4-2-3-5-19(18)24-14)13-29-17-8-6-15(7-9-17)21(28)23-11-10-20-25-22(30)27-26-20/h2-9,12H,10-11,13H2,1H3,(H,23,28)(H2,25,26,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50216010

((R)-3-amino-1-((R)-3-methyl-1-(5-thioxo-4,5-dihydr...)Show SMILES CC(C)C[C@@H](N1CC[C@](N)(C1=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C28H32N6O2S/c1-17(2)14-24(25-31-27(37)33-32-25)34-13-12-28(29,26(34)35)20-8-10-21(11-9-20)36-16-19-15-18(3)30-23-7-5-4-6-22(19)23/h4-11,15,17,24H,12-14,16,29H2,1-3H3,(H2,31,32,33,37)/t24-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50215996

(CHEMBL392837 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CCC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H25N5O2S/c1-15-13-17(19-5-2-3-7-21(19)26-15)14-32-18-11-9-16(10-12-18)24(31)27-22-8-4-6-20(22)23-28-25(33)30-29-23/h2-3,5,7,9-13,20,22H,4,6,8,14H2,1H3,(H,27,31)(H2,28,29,30,33)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50215990

(4-((2-methylquinolin-4-yl)methoxy)-N-((5-thioxo-4,...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCc2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C21H19N5O2S/c1-13-10-15(17-4-2-3-5-18(17)23-13)12-28-16-8-6-14(7-9-16)20(27)22-11-19-24-21(29)26-25-19/h2-10H,11-12H2,1H3,(H,22,27)(H2,24,25,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50216001

(CHEMBL247947 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(CC#C)CC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C28H28N6O2S/c1-3-13-34-14-12-23(26-31-28(37)33-32-26)25(16-34)30-27(35)19-8-10-21(11-9-19)36-17-20-15-18(2)29-24-7-5-4-6-22(20)24/h1,4-11,15,23,25H,12-14,16-17H2,2H3,(H,30,35)(H2,31,32,33,37)/t23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50215998

(CHEMBL247141 | cis-tert-butyl 3-(4-((2-methylquino...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(C[C@@H]2c2n[nH]c(=S)[nH]2)C(=O)OC(C)(C)C)c2ccccc2n1 Show InChI InChI=1S/C29H32N6O4S/c1-17-13-19(21-7-5-6-8-23(21)30-17)16-38-20-11-9-18(10-12-20)26(36)31-24-15-35(28(37)39-29(2,3)4)14-22(24)25-32-27(40)34-33-25/h5-13,22,24H,14-16H2,1-4H3,(H,31,36)(H2,32,33,34,40)/t22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50216000

(CHEMBL247546 | cis-N-(1-acetyl-4-(5-thioxo-4,5-dih...)Show SMILES CC(=O)N1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C27H28N6O3S/c1-16-13-19(21-5-3-4-6-23(21)28-16)15-36-20-9-7-18(8-10-20)26(35)29-24-14-33(17(2)34)12-11-22(24)25-30-27(37)32-31-25/h3-10,13,22,24H,11-12,14-15H2,1-2H3,(H,29,35)(H2,30,31,32,37)/t22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50215999

(CHEMBL394084 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CNC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C24H24N6O2S/c1-14-10-16(18-4-2-3-5-20(18)26-14)13-32-17-8-6-15(7-9-17)23(31)27-21-12-25-11-19(21)22-28-24(33)30-29-22/h2-10,19,21,25H,11-13H2,1H3,(H,27,31)(H2,28,29,30,33)/t19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50215992

(CHEMBL247545 | cis-tert-butyl 3-(4-((2-methylquino...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(CC[C@@H]2c2n[nH]c(=S)[nH]2)C(=O)OC(C)(C)C)c2ccccc2n1 Show InChI InChI=1S/C30H34N6O4S/c1-18-15-20(22-7-5-6-8-24(22)31-18)17-39-21-11-9-19(10-12-21)27(37)32-25-16-36(29(38)40-30(2,3)4)14-13-23(25)26-33-28(41)35-34-26/h5-12,15,23,25H,13-14,16-17H2,1-4H3,(H,32,37)(H2,33,34,35,41)/t23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50215994

(CHEMBL247743 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(CC[C@@H]2c2n[nH]c(=S)[nH]2)S(C)(=O)=O)c2ccccc2n1 Show InChI InChI=1S/C26H28N6O4S2/c1-16-13-18(20-5-3-4-6-22(20)27-16)15-36-19-9-7-17(8-10-19)25(33)28-23-14-32(38(2,34)35)12-11-21(23)24-29-26(37)31-30-24/h3-10,13,21,23H,11-12,14-15H2,1-2H3,(H,28,33)(H2,29,30,31,37)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50216008

(CHEMBL392231 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CNCC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H26N6O2S/c1-15-12-17(19-4-2-3-5-21(19)27-15)14-33-18-8-6-16(7-9-18)24(32)28-22-13-26-11-10-20(22)23-29-25(34)31-30-23/h2-9,12,20,22,26H,10-11,13-14H2,1H3,(H,28,32)(H2,29,30,31,34)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50216002

(CHEMBL247347 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CCOC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H25N5O3S/c1-15-12-17(19-4-2-3-5-21(19)26-15)13-33-18-8-6-16(7-9-18)24(31)27-22-10-11-32-14-20(22)23-28-25(34)30-29-23/h2-9,12,20,22H,10-11,13-14H2,1H3,(H,27,31)(H2,28,29,30,34)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50216005

(CHEMBL394294 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES CCCN1C[C@@H](NC(=O)c2ccc(OCc3cc(C)nc4ccccc34)cc2)[C@H](C1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C27H30N6O2S/c1-3-12-33-14-22(25-30-27(36)32-31-25)24(15-33)29-26(34)18-8-10-20(11-9-18)35-16-19-13-17(2)28-23-7-5-4-6-21(19)23/h4-11,13,22,24H,3,12,14-16H2,1-2H3,(H,29,34)(H2,30,31,32,36)/t22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50216003

(CHEMBL247346 | cis-N-(1-acetyl-4-(5-thioxo-4,5-dih...)Show SMILES CC(=O)N1C[C@@H](NC(=O)c2ccc(OCc3cc(C)nc4ccccc34)cc2)[C@H](C1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C26H26N6O3S/c1-15-11-18(20-5-3-4-6-22(20)27-15)14-35-19-9-7-17(8-10-19)25(34)28-23-13-32(16(2)33)12-21(23)24-29-26(36)31-30-24/h3-11,21,23H,12-14H2,1-2H3,(H,28,34)(H2,29,30,31,36)/t21-,23+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50216000

(CHEMBL247546 | cis-N-(1-acetyl-4-(5-thioxo-4,5-dih...)Show SMILES CC(=O)N1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C27H28N6O3S/c1-16-13-19(21-5-3-4-6-23(21)28-16)15-36-20-9-7-18(8-10-20)26(35)29-24-14-33(17(2)34)12-11-22(24)25-30-27(37)32-31-25/h3-10,13,22,24H,11-12,14-15H2,1-2H3,(H,29,35)(H2,30,31,32,37)/t22-,24+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50216002

(CHEMBL247347 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CCOC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H25N5O3S/c1-15-12-17(19-4-2-3-5-21(19)26-15)13-33-18-8-6-16(7-9-18)24(31)27-22-10-11-32-14-20(22)23-28-25(34)30-29-23/h2-9,12,20,22H,10-11,13-14H2,1H3,(H,27,31)(H2,28,29,30,34)/t20-,22+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50216008

(CHEMBL392231 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CNCC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H26N6O2S/c1-15-12-17(19-4-2-3-5-21(19)27-15)14-33-18-8-6-16(7-9-18)24(32)28-22-13-26-11-10-20(22)23-29-25(34)31-30-23/h2-9,12,20,22,26H,10-11,13-14H2,1H3,(H,28,32)(H2,29,30,31,34)/t20-,22+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50216005

(CHEMBL394294 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES CCCN1C[C@@H](NC(=O)c2ccc(OCc3cc(C)nc4ccccc34)cc2)[C@H](C1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C27H30N6O2S/c1-3-12-33-14-22(25-30-27(36)32-31-25)24(15-33)29-26(34)18-8-10-20(11-9-18)35-16-19-13-17(2)28-23-7-5-4-6-21(19)23/h4-11,13,22,24H,3,12,14-16H2,1-2H3,(H,29,34)(H2,30,31,32,36)/t22-,24+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50215998

(CHEMBL247141 | cis-tert-butyl 3-(4-((2-methylquino...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(C[C@@H]2c2n[nH]c(=S)[nH]2)C(=O)OC(C)(C)C)c2ccccc2n1 Show InChI InChI=1S/C29H32N6O4S/c1-17-13-19(21-7-5-6-8-23(21)30-17)16-38-20-11-9-18(10-12-20)26(36)31-24-15-35(28(37)39-29(2,3)4)14-22(24)25-32-27(40)34-33-25/h5-13,22,24H,14-16H2,1-4H3,(H,31,36)(H2,32,33,34,40)/t22-,24+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50215995

(CHEMBL247742 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES CCCN1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C28H32N6O2S/c1-3-13-34-14-12-23(26-31-28(37)33-32-26)25(16-34)30-27(35)19-8-10-21(11-9-19)36-17-20-15-18(2)29-24-7-5-4-6-22(20)24/h4-11,15,23,25H,3,12-14,16-17H2,1-2H3,(H,30,35)(H2,31,32,33,37)/t23-,25+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50215989

(CHEMBL392232 | cis-N-(1-methyl-4-(5-thioxo-4,5-dih...)Show SMILES CN1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C26H28N6O2S/c1-16-13-18(20-5-3-4-6-22(20)27-16)15-34-19-9-7-17(8-10-19)25(33)28-23-14-32(2)12-11-21(23)24-29-26(35)31-30-24/h3-10,13,21,23H,11-12,14-15H2,1-2H3,(H,28,33)(H2,29,30,31,35)/t21-,23+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50216004

(4-((2-methylquinolin-4-yl)methoxy)-N-(4-((5-thioxo...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(Cc3n[nH]c(=S)[nH]3)CCOCC2)c2ccccc2n1 Show InChI InChI=1S/C26H27N5O3S/c1-17-14-19(21-4-2-3-5-22(21)27-17)16-34-20-8-6-18(7-9-20)24(32)29-26(10-12-33-13-11-26)15-23-28-25(35)31-30-23/h2-9,14H,10-13,15-16H2,1H3,(H,29,32)(H2,28,30,31,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50215994

(CHEMBL247743 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(CC[C@@H]2c2n[nH]c(=S)[nH]2)S(C)(=O)=O)c2ccccc2n1 Show InChI InChI=1S/C26H28N6O4S2/c1-16-13-18(20-5-3-4-6-22(20)27-16)15-36-19-9-7-17(8-10-19)25(33)28-23-14-32(38(2,34)35)12-11-21(23)24-29-26(37)31-30-24/h3-10,13,21,23H,11-12,14-15H2,1-2H3,(H,28,33)(H2,29,30,31,37)/t21-,23+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50216006

(CHEMBL247946 | cis-N-(1-isopropyl-4-(5-thioxo-4,5-...)Show SMILES CC(C)N1CC[C@@H]([C@@H](C1)NC(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C28H32N6O2S/c1-17(2)34-13-12-23(26-31-28(37)33-32-26)25(15-34)30-27(35)19-8-10-21(11-9-19)36-16-20-14-18(3)29-24-7-5-4-6-22(20)24/h4-11,14,17,23,25H,12-13,15-16H2,1-3H3,(H,30,35)(H2,31,32,33,37)/t23-,25+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50216007

(CHEMBL393443 | tert-butyl 4-(4-((2-methylquinolin-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(Cc3n[nH]c(=S)[nH]3)CCN(CC2)C(=O)OC(C)(C)C)c2ccccc2n1 Show InChI InChI=1S/C31H36N6O4S/c1-20-17-22(24-7-5-6-8-25(24)32-20)19-40-23-11-9-21(10-12-23)27(38)34-31(18-26-33-28(42)36-35-26)13-15-37(16-14-31)29(39)41-30(2,3)4/h5-12,17H,13-16,18-19H2,1-4H3,(H,34,38)(H2,33,35,36,42) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50215996

(CHEMBL392837 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CCC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H25N5O2S/c1-15-13-17(19-5-2-3-7-21(19)26-15)14-32-18-11-9-16(10-12-18)24(31)27-22-8-4-6-20(22)23-28-25(33)30-29-23/h2-3,5,7,9-13,20,22H,4,6,8,14H2,1H3,(H,27,31)(H2,28,29,30,33)/t20-,22+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50216009

(CHEMBL247348 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CCNC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H26N6O2S/c1-15-12-17(19-4-2-3-5-21(19)27-15)14-33-18-8-6-16(7-9-18)24(32)28-22-10-11-26-13-20(22)23-29-25(34)31-30-23/h2-9,12,20,22,26H,10-11,13-14H2,1H3,(H,28,32)(H2,29,30,31,34)/t20-,22+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50215993

(CHEMBL248524 | N-(2-methyl-1-(5-thioxo-4,5-dihydro...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC(C)(C)Cc2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C24H25N5O2S/c1-15-12-17(19-6-4-5-7-20(19)25-15)14-31-18-10-8-16(9-11-18)22(30)27-24(2,3)13-21-26-23(32)29-28-21/h4-12H,13-14H2,1-3H3,(H,27,30)(H2,26,28,29,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50216001

(CHEMBL247947 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(CC#C)CC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C28H28N6O2S/c1-3-13-34-14-12-23(26-31-28(37)33-32-26)25(16-34)30-27(35)19-8-10-21(11-9-19)36-17-20-15-18(2)29-24-7-5-4-6-22(20)24/h1,4-11,15,23,25H,12-14,16-17H2,2H3,(H,30,35)(H2,31,32,33,37)/t23-,25+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50215992

(CHEMBL247545 | cis-tert-butyl 3-(4-((2-methylquino...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(CC[C@@H]2c2n[nH]c(=S)[nH]2)C(=O)OC(C)(C)C)c2ccccc2n1 Show InChI InChI=1S/C30H34N6O4S/c1-18-15-20(22-7-5-6-8-24(22)31-18)17-39-21-11-9-19(10-12-21)27(37)32-25-16-36(29(38)40-30(2,3)4)14-13-23(25)26-33-28(41)35-34-26/h5-12,15,23,25H,13-14,16-17H2,1-4H3,(H,32,37)(H2,33,34,35,41)/t23-,25+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50215999

(CHEMBL394084 | cis-4-((2-methylquinolin-4-yl)metho...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CNC[C@@H]2c2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C24H24N6O2S/c1-14-10-16(18-4-2-3-5-20(18)26-14)13-32-17-8-6-15(7-9-17)23(31)27-21-12-25-11-19(21)22-28-24(33)30-29-22/h2-10,19,21,25H,11-13H2,1H3,(H,27,31)(H2,28,29,30,33)/t19-,21+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50215997

(4-((2-methylquinolin-4-yl)methoxy)-N-(2-(5-thioxo-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCCc2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C22H21N5O2S/c1-14-12-16(18-4-2-3-5-19(18)24-14)13-29-17-8-6-15(7-9-17)21(28)23-11-10-20-25-22(30)27-26-20/h2-9,12H,10-11,13H2,1H3,(H,23,28)(H2,25,26,27,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50215991

(4-(quinolin-4-ylmethoxy)-N-(3-(5-thioxo-4,5-dihydr...)Show SMILES O=C(NCCCc1n[nH]c(=S)[nH]1)c1ccc(OCc2ccnc3ccccc23)cc1 Show InChI InChI=1S/C22H21N5O2S/c28-21(24-12-3-6-20-25-22(30)27-26-20)15-7-9-17(10-8-15)29-14-16-11-13-23-19-5-2-1-4-18(16)19/h1-2,4-5,7-11,13H,3,6,12,14H2,(H,24,28)(H2,25,26,27,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 294 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50216010

((R)-3-amino-1-((R)-3-methyl-1-(5-thioxo-4,5-dihydr...)Show SMILES CC(C)C[C@@H](N1CC[C@](N)(C1=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)c1n[nH]c(=S)[nH]1 Show InChI InChI=1S/C28H32N6O2S/c1-17(2)14-24(25-31-27(37)33-32-25)34-13-12-28(29,26(34)35)20-8-10-21(11-9-20)36-16-19-15-18(3)30-23-7-5-4-6-22(19)23/h4-11,15,17,24H,12-14,16,29H2,1-3H3,(H2,31,32,33,37)/t24-,28-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50215990

(4-((2-methylquinolin-4-yl)methoxy)-N-((5-thioxo-4,...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCc2n[nH]c(=S)[nH]2)c2ccccc2n1 Show InChI InChI=1S/C21H19N5O2S/c1-13-10-15(17-4-2-3-5-18(17)23-13)12-28-16-8-6-14(7-9-16)20(27)22-11-19-24-21(29)26-25-19/h2-10H,11-12H2,1H3,(H,22,27)(H2,24,25,26,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data