 Found 60 hits of Enzyme Inhibition Constant Data
Found 60 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218438
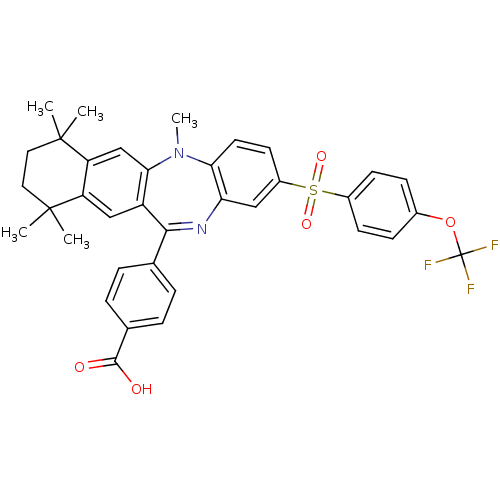
(4-[5,7,7,10,10-pentamethyl-2-(4-trifluoromethoxy-b...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)S(=O)(=O)c1ccc(OC(F)(F)F)cc1 |t:9| Show InChI InChI=1S/C36H33F3N2O5S/c1-34(2)16-17-35(3,4)28-20-31-26(19-27(28)34)32(21-6-8-22(9-7-21)33(42)43)40-29-18-25(14-15-30(29)41(31)5)47(44,45)24-12-10-23(11-13-24)46-36(37,38)39/h6-15,18-20H,16-17H2,1-5H3,(H,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218429
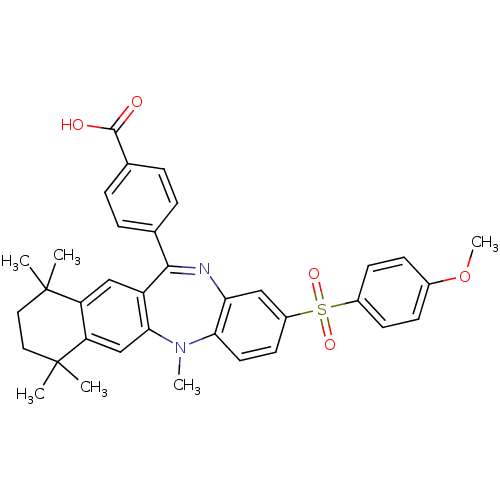
(4-[2-(4-methoxy-benzenesulfonyl)-5,7,7,10,10-penta...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc2N(C)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:25| Show InChI InChI=1S/C36H36N2O5S/c1-35(2)17-18-36(3,4)29-21-32-27(20-28(29)35)33(22-7-9-23(10-8-22)34(39)40)37-30-19-26(15-16-31(30)38(32)5)44(41,42)25-13-11-24(43-6)12-14-25/h7-16,19-21H,17-18H2,1-6H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218448
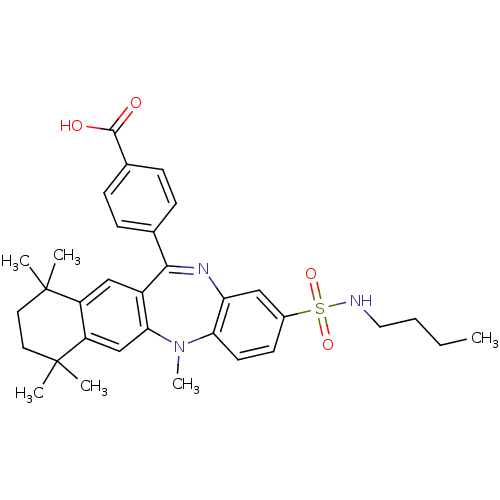
(4-(2-butylsulfamoyl-5,7,7,10,10-pentamethyl-7,8,9,...)Show SMILES CCCCNS(=O)(=O)c1ccc2N(C)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:21| Show InChI InChI=1S/C33H39N3O4S/c1-7-8-17-34-41(39,40)23-13-14-28-27(18-23)35-30(21-9-11-22(12-10-21)31(37)38)24-19-25-26(20-29(24)36(28)6)33(4,5)16-15-32(25,2)3/h9-14,18-20,34H,7-8,15-17H2,1-6H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218438

(4-[5,7,7,10,10-pentamethyl-2-(4-trifluoromethoxy-b...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)S(=O)(=O)c1ccc(OC(F)(F)F)cc1 |t:9| Show InChI InChI=1S/C36H33F3N2O5S/c1-34(2)16-17-35(3,4)28-20-31-26(19-27(28)34)32(21-6-8-22(9-7-21)33(42)43)40-29-18-25(14-15-30(29)41(31)5)47(44,45)24-12-10-23(11-13-24)46-36(37,38)39/h6-15,18-20H,16-17H2,1-5H3,(H,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218433
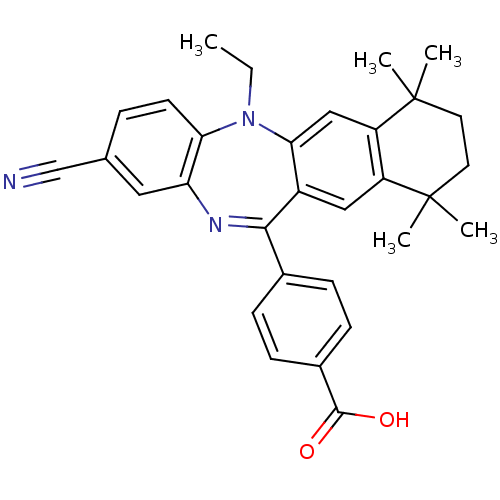
(4-(2-cyano-5-ethyl-7,7,10,10-tetramethyl-7,8,9,10-...)Show SMILES CCN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)C#N |t:10| Show InChI InChI=1S/C31H31N3O2/c1-6-34-26-12-7-19(18-32)15-25(26)33-28(20-8-10-21(11-9-20)29(35)36)22-16-23-24(17-27(22)34)31(4,5)14-13-30(23,2)3/h7-12,15-17H,6,13-14H2,1-5H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218429

(4-[2-(4-methoxy-benzenesulfonyl)-5,7,7,10,10-penta...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc2N(C)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:25| Show InChI InChI=1S/C36H36N2O5S/c1-35(2)17-18-36(3,4)29-21-32-27(20-28(29)35)33(22-7-9-23(10-8-22)34(39)40)37-30-19-26(15-16-31(30)38(32)5)44(41,42)25-13-11-24(43-6)12-14-25/h7-16,19-21H,17-18H2,1-6H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218434
(4-[5,7,7,10,10-pentamethyl-2-(4-trifluoromethoxy-p...)Show SMILES CN1c2ccc(Sc3ccc(OC(F)(F)F)cc3)cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C |t:22| Show InChI InChI=1S/C36H33F3N2O3S/c1-34(2)16-17-35(3,4)28-20-31-26(19-27(28)34)32(21-6-8-22(9-7-21)33(42)43)40-29-18-25(14-15-30(29)41(31)5)45-24-12-10-23(11-13-24)44-36(37,38)39/h6-15,18-20H,16-17H2,1-5H3,(H,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218448

(4-(2-butylsulfamoyl-5,7,7,10,10-pentamethyl-7,8,9,...)Show SMILES CCCCNS(=O)(=O)c1ccc2N(C)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:21| Show InChI InChI=1S/C33H39N3O4S/c1-7-8-17-34-41(39,40)23-13-14-28-27(18-23)35-30(21-9-11-22(12-10-21)31(37)38)24-19-25-26(20-29(24)36(28)6)33(4,5)16-15-32(25,2)3/h9-14,18-20,34H,7-8,15-17H2,1-6H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218446
(4-[2-(4-chloro-phenylmethanesulfonyl)-5,7,7,10,10-...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)S(=O)(=O)Cc1ccc(Cl)cc1 |t:9| Show InChI InChI=1S/C36H35ClN2O4S/c1-35(2)16-17-36(3,4)29-20-32-27(19-28(29)35)33(23-8-10-24(11-9-23)34(40)41)38-30-18-26(14-15-31(30)39(32)5)44(42,43)21-22-6-12-25(37)13-7-22/h6-15,18-20H,16-17,21H2,1-5H3,(H,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218436
(4-(2-cyano-5,7,7,10,10-pentamethyl-7,8,9,10-tetrah...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)C#N |t:9| Show InChI InChI=1S/C30H29N3O2/c1-29(2)12-13-30(3,4)23-16-26-21(15-22(23)29)27(19-7-9-20(10-8-19)28(34)35)32-24-14-18(17-31)6-11-25(24)33(26)5/h6-11,14-16H,12-13H2,1-5H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218444
(4-[5,7,7,10,10-pentamethyl-2-(pyrrolidine-1-sulfon...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)S(=O)(=O)N1CCCC1 |t:9| Show InChI InChI=1S/C33H37N3O4S/c1-32(2)14-15-33(3,4)26-20-29-24(19-25(26)32)30(21-8-10-22(11-9-21)31(37)38)34-27-18-23(12-13-28(27)35(29)5)41(39,40)36-16-6-7-17-36/h8-13,18-20H,6-7,14-17H2,1-5H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218453
(4-[5,7,7,10,10-pentamethyl-2-(propane-1-sulfonyl)-...)Show SMILES CCCS(=O)(=O)c1ccc2N(C)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:19| Show InChI InChI=1S/C32H36N2O4S/c1-7-16-39(37,38)22-12-13-27-26(17-22)33-29(20-8-10-21(11-9-20)30(35)36)23-18-24-25(19-28(23)34(27)6)32(4,5)15-14-31(24,2)3/h8-13,17-19H,7,14-16H2,1-6H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218428
(4-[5,7,7,10,10-pentamethyl-2-(morpholine-4-sulfony...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)S(=O)(=O)N1CCOCC1 |t:9| Show InChI InChI=1S/C33H37N3O5S/c1-32(2)12-13-33(3,4)26-20-29-24(19-25(26)32)30(21-6-8-22(9-7-21)31(37)38)34-27-18-23(10-11-28(27)35(29)5)42(39,40)36-14-16-41-17-15-36/h6-11,18-20H,12-17H2,1-5H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218427
(4-[5,7,7,10,10-pentamethyl-2-(4-trifluoromethoxy-p...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)S(=O)(=O)Nc1ccc(OC(F)(F)F)cc1 |t:9| Show InChI InChI=1S/C36H34F3N3O5S/c1-34(2)16-17-35(3,4)28-20-31-26(19-27(28)34)32(21-6-8-22(9-7-21)33(43)44)40-29-18-25(14-15-30(29)42(31)5)48(45,46)41-23-10-12-24(13-11-23)47-36(37,38)39/h6-15,18-20,41H,16-17H2,1-5H3,(H,43,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218433

(4-(2-cyano-5-ethyl-7,7,10,10-tetramethyl-7,8,9,10-...)Show SMILES CCN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)C#N |t:10| Show InChI InChI=1S/C31H31N3O2/c1-6-34-26-12-7-19(18-32)15-25(26)33-28(20-8-10-21(11-9-20)29(35)36)22-16-23-24(17-27(22)34)31(4,5)14-13-30(23,2)3/h7-12,15-17H,6,13-14H2,1-5H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218424
(4-(2-cyano-5-ethyl-7,7,10,10-tetramethyl-7,8,9,10-...)Show SMILES CCN1c2ccc(cc2N=C(c2ccc(C(O)=O)c(F)c2)c2cc3c(cc12)C(C)(C)CCC3(C)C)C#N |t:10| Show InChI InChI=1S/C31H30FN3O2/c1-6-35-26-10-7-18(17-33)13-25(26)34-28(19-8-9-20(29(36)37)24(32)14-19)21-15-22-23(16-27(21)35)31(4,5)12-11-30(22,2)3/h7-10,13-16H,6,11-12H2,1-5H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218432
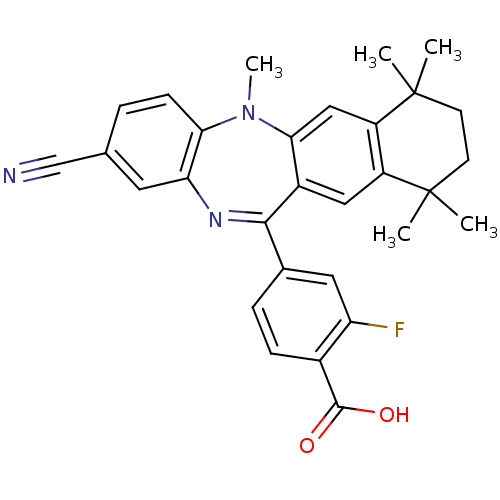
(4-(2-cyano-5,7,7,10,10-pentamethyl-7,8,9,10-tetrah...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(C(O)=O)c(F)c2)c2cc3c(cc12)C(C)(C)CCC3(C)C)C#N |t:9| Show InChI InChI=1S/C30H28FN3O2/c1-29(2)10-11-30(3,4)22-15-26-20(14-21(22)29)27(18-7-8-19(28(35)36)23(31)13-18)33-24-12-17(16-32)6-9-25(24)34(26)5/h6-9,12-15H,10-11H2,1-5H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218441
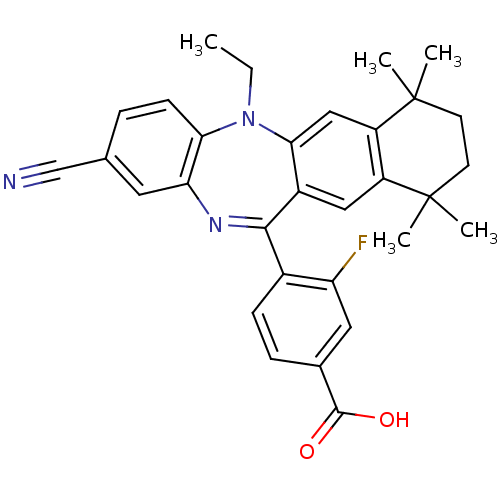
(4-(2-cyano-5-ethyl-7,7,10,10-tetramethyl-7,8,9,10-...)Show SMILES CCN1c2ccc(cc2N=C(c2ccc(cc2F)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)C#N |t:10| Show InChI InChI=1S/C31H30FN3O2/c1-6-35-26-10-7-18(17-33)13-25(26)34-28(20-9-8-19(29(36)37)14-24(20)32)21-15-22-23(16-27(21)35)31(4,5)12-11-30(22,2)3/h7-10,13-16H,6,11-12H2,1-5H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218449
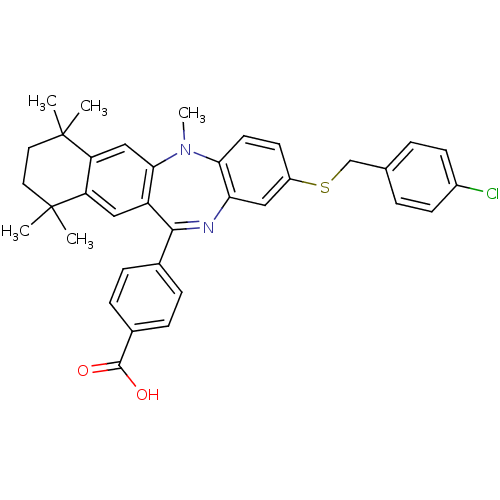
(4-[2-(4-chloro-benzylsulfanyl)-5,7,7,10,10-pentame...)Show SMILES CN1c2ccc(SCc3ccc(Cl)cc3)cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C |t:19| Show InChI InChI=1S/C36H35ClN2O2S/c1-35(2)16-17-36(3,4)29-20-32-27(19-28(29)35)33(23-8-10-24(11-9-23)34(40)41)38-30-18-26(14-15-31(30)39(32)5)42-21-22-6-12-25(37)13-7-22/h6-15,18-20H,16-17,21H2,1-5H3,(H,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218451
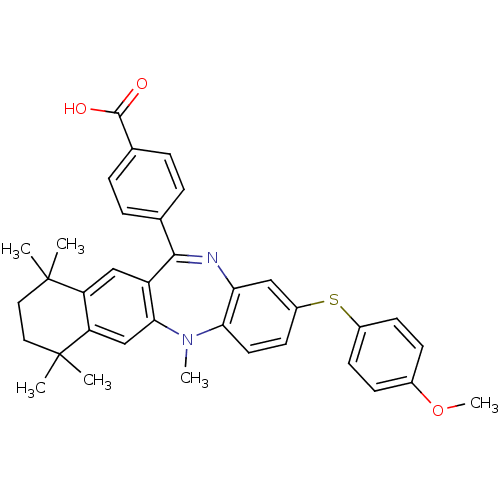
(4-[2-(4-methoxy-phenylsulfanyl)-5,7,7,10,10-pentam...)Show SMILES COc1ccc(Sc2ccc3N(C)c4cc5c(cc4C(=Nc3c2)c2ccc(cc2)C(O)=O)C(C)(C)CCC5(C)C)cc1 |c:20| Show InChI InChI=1S/C36H36N2O3S/c1-35(2)17-18-36(3,4)29-21-32-27(20-28(29)35)33(22-7-9-23(10-8-22)34(39)40)37-30-19-26(15-16-31(30)38(32)5)42-25-13-11-24(41-6)12-14-25/h7-16,19-21H,17-18H2,1-6H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218424

(4-(2-cyano-5-ethyl-7,7,10,10-tetramethyl-7,8,9,10-...)Show SMILES CCN1c2ccc(cc2N=C(c2ccc(C(O)=O)c(F)c2)c2cc3c(cc12)C(C)(C)CCC3(C)C)C#N |t:10| Show InChI InChI=1S/C31H30FN3O2/c1-6-35-26-10-7-18(17-33)13-25(26)34-28(19-8-9-20(29(36)37)24(32)14-19)21-15-22-23(16-27(21)35)31(4,5)12-11-30(22,2)3/h7-10,13-16H,6,11-12H2,1-5H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218425
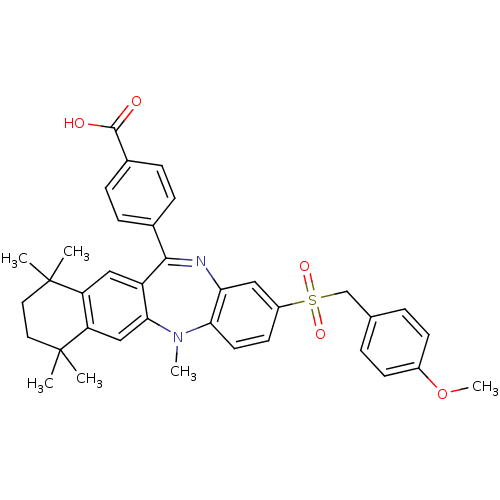
(4-[2-(4-methoxy-phenylmethanesulfonyl)-5,7,7,10,10...)Show SMILES COc1ccc(CS(=O)(=O)c2ccc3N(C)c4cc5c(cc4C(=Nc3c2)c2ccc(cc2)C(O)=O)C(C)(C)CCC5(C)C)cc1 |c:23| Show InChI InChI=1S/C37H38N2O5S/c1-36(2)17-18-37(3,4)30-21-33-28(20-29(30)36)34(24-9-11-25(12-10-24)35(40)41)38-31-19-27(15-16-32(31)39(33)5)45(42,43)22-23-7-13-26(44-6)14-8-23/h7-16,19-21H,17-18,22H2,1-6H3,(H,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218442
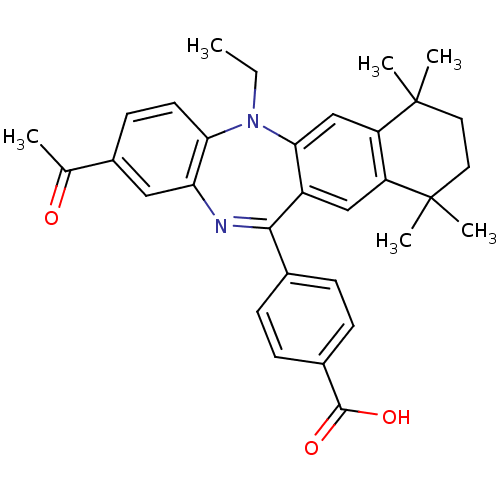
(4-(2-acetyl-5-ethyl-7,7,10,10-tetramethyl-7,8,9,10...)Show SMILES CCN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)C(C)=O |t:10| Show InChI InChI=1S/C32H34N2O3/c1-7-34-27-13-12-22(19(2)35)16-26(27)33-29(20-8-10-21(11-9-20)30(36)37)23-17-24-25(18-28(23)34)32(5,6)15-14-31(24,3)4/h8-13,16-18H,7,14-15H2,1-6H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218436

(4-(2-cyano-5,7,7,10,10-pentamethyl-7,8,9,10-tetrah...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)C#N |t:9| Show InChI InChI=1S/C30H29N3O2/c1-29(2)12-13-30(3,4)23-16-26-21(15-22(23)29)27(19-7-9-20(10-8-19)28(34)35)32-24-14-18(17-31)6-11-25(24)33(26)5/h6-11,14-16H,12-13H2,1-5H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218452
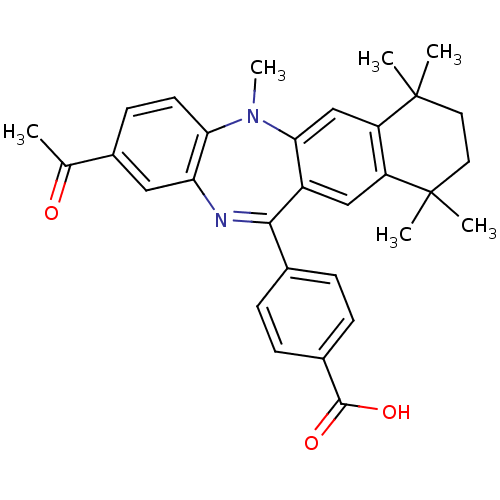
(4-(2-acetyl-5,7,7,10,10-pentamethyl-7,8,9,10-tetra...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)C(C)=O |t:9| Show InChI InChI=1S/C31H32N2O3/c1-18(34)21-11-12-26-25(15-21)32-28(19-7-9-20(10-8-19)29(35)36)22-16-23-24(17-27(22)33(26)6)31(4,5)14-13-30(23,2)3/h7-12,15-17H,13-14H2,1-6H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218453

(4-[5,7,7,10,10-pentamethyl-2-(propane-1-sulfonyl)-...)Show SMILES CCCS(=O)(=O)c1ccc2N(C)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:19| Show InChI InChI=1S/C32H36N2O4S/c1-7-16-39(37,38)22-12-13-27-26(17-22)33-29(20-8-10-21(11-9-20)30(35)36)23-18-24-25(19-28(23)34(27)6)32(4,5)15-14-31(24,2)3/h8-13,17-19H,7,14-16H2,1-6H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218446

(4-[2-(4-chloro-phenylmethanesulfonyl)-5,7,7,10,10-...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)S(=O)(=O)Cc1ccc(Cl)cc1 |t:9| Show InChI InChI=1S/C36H35ClN2O4S/c1-35(2)16-17-36(3,4)29-20-32-27(19-28(29)35)33(23-8-10-24(11-9-23)34(40)41)38-30-18-26(14-15-31(30)39(32)5)44(42,43)21-22-6-12-25(37)13-7-22/h6-15,18-20H,16-17,21H2,1-5H3,(H,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218427

(4-[5,7,7,10,10-pentamethyl-2-(4-trifluoromethoxy-p...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)S(=O)(=O)Nc1ccc(OC(F)(F)F)cc1 |t:9| Show InChI InChI=1S/C36H34F3N3O5S/c1-34(2)16-17-35(3,4)28-20-31-26(19-27(28)34)32(21-6-8-22(9-7-21)33(43)44)40-29-18-25(14-15-30(29)42(31)5)48(45,46)41-23-10-12-24(13-11-23)47-36(37,38)39/h6-15,18-20,41H,16-17H2,1-5H3,(H,43,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218442

(4-(2-acetyl-5-ethyl-7,7,10,10-tetramethyl-7,8,9,10...)Show SMILES CCN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)C(C)=O |t:10| Show InChI InChI=1S/C32H34N2O3/c1-7-34-27-13-12-22(19(2)35)16-26(27)33-29(20-8-10-21(11-9-20)30(36)37)23-17-24-25(18-28(23)34)32(5,6)15-14-31(24,3)4/h8-13,16-18H,7,14-15H2,1-6H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218428

(4-[5,7,7,10,10-pentamethyl-2-(morpholine-4-sulfony...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)S(=O)(=O)N1CCOCC1 |t:9| Show InChI InChI=1S/C33H37N3O5S/c1-32(2)12-13-33(3,4)26-20-29-24(19-25(26)32)30(21-6-8-22(9-7-21)31(37)38)34-27-18-23(10-11-28(27)35(29)5)42(39,40)36-14-16-41-17-15-36/h6-11,18-20H,12-17H2,1-5H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218431
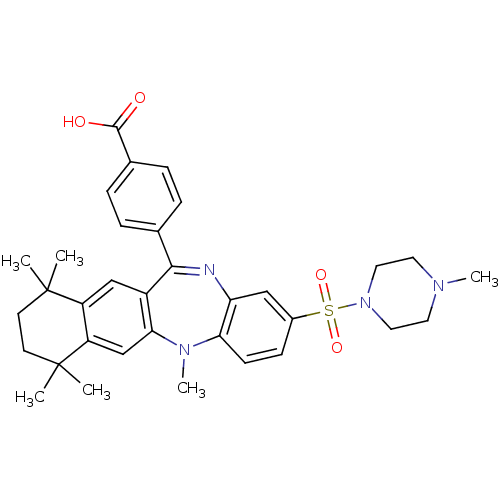
(4-[5,7,7,10,10-pentamethyl-2-(4-methyl-piperazine-...)Show SMILES CN1CCN(CC1)S(=O)(=O)c1ccc2N(C)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:24| Show InChI InChI=1S/C34H40N4O4S/c1-33(2)13-14-34(3,4)27-21-30-25(20-26(27)33)31(22-7-9-23(10-8-22)32(39)40)35-28-19-24(11-12-29(28)37(30)6)43(41,42)38-17-15-36(5)16-18-38/h7-12,19-21H,13-18H2,1-6H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218434

(4-[5,7,7,10,10-pentamethyl-2-(4-trifluoromethoxy-p...)Show SMILES CN1c2ccc(Sc3ccc(OC(F)(F)F)cc3)cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C |t:22| Show InChI InChI=1S/C36H33F3N2O3S/c1-34(2)16-17-35(3,4)28-20-31-26(19-27(28)34)32(21-6-8-22(9-7-21)33(42)43)40-29-18-25(14-15-30(29)41(31)5)45-24-12-10-23(11-13-24)44-36(37,38)39/h6-15,18-20H,16-17H2,1-5H3,(H,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218444

(4-[5,7,7,10,10-pentamethyl-2-(pyrrolidine-1-sulfon...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)S(=O)(=O)N1CCCC1 |t:9| Show InChI InChI=1S/C33H37N3O4S/c1-32(2)14-15-33(3,4)26-20-29-24(19-25(26)32)30(21-8-10-22(11-9-21)31(37)38)34-27-18-23(12-13-28(27)35(29)5)41(39,40)36-16-6-7-17-36/h8-13,18-20H,6-7,14-17H2,1-5H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218441

(4-(2-cyano-5-ethyl-7,7,10,10-tetramethyl-7,8,9,10-...)Show SMILES CCN1c2ccc(cc2N=C(c2ccc(cc2F)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)C#N |t:10| Show InChI InChI=1S/C31H30FN3O2/c1-6-35-26-10-7-18(17-33)13-25(26)34-28(20-9-8-19(29(36)37)14-24(20)32)21-15-22-23(16-27(21)35)31(4,5)12-11-30(22,2)3/h7-10,13-16H,6,11-12H2,1-5H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218440
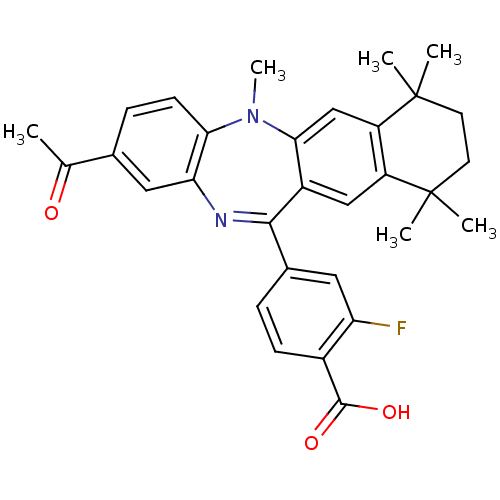
(4-(2-acetyl-5,7,7,10,10-pentamethyl-7,8,9,10-tetra...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(C(O)=O)c(F)c2)c2cc3c(cc12)C(C)(C)CCC3(C)C)C(C)=O |t:9| Show InChI InChI=1S/C31H31FN2O3/c1-17(35)18-8-10-26-25(14-18)33-28(19-7-9-20(29(36)37)24(32)13-19)21-15-22-23(16-27(21)34(26)6)31(4,5)12-11-30(22,2)3/h7-10,13-16H,11-12H2,1-6H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218452

(4-(2-acetyl-5,7,7,10,10-pentamethyl-7,8,9,10-tetra...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)C(C)=O |t:9| Show InChI InChI=1S/C31H32N2O3/c1-18(34)21-11-12-26-25(15-21)32-28(19-7-9-20(10-8-19)29(35)36)22-16-23-24(17-27(22)33(26)6)31(4,5)14-13-30(23,2)3/h7-12,15-17H,13-14H2,1-6H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218432

(4-(2-cyano-5,7,7,10,10-pentamethyl-7,8,9,10-tetrah...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(C(O)=O)c(F)c2)c2cc3c(cc12)C(C)(C)CCC3(C)C)C#N |t:9| Show InChI InChI=1S/C30H28FN3O2/c1-29(2)10-11-30(3,4)22-15-26-20(14-21(22)29)27(18-7-8-19(28(35)36)23(31)13-18)33-24-12-17(16-32)6-9-25(24)34(26)5/h6-9,12-15H,10-11H2,1-5H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218439
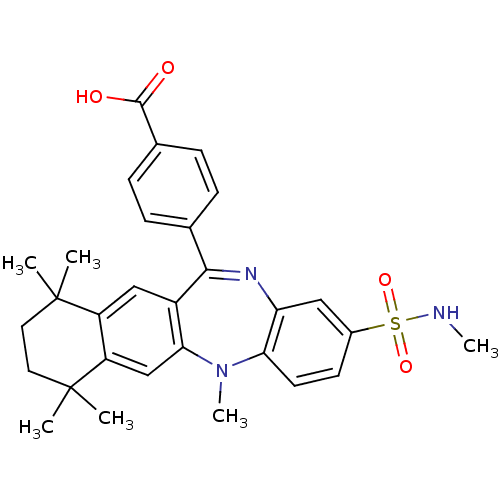
(4-(5,7,7,10,10-pentamethyl-2-methylsulfamoyl-7,8,9...)Show SMILES CNS(=O)(=O)c1ccc2N(C)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:18| Show InChI InChI=1S/C30H33N3O4S/c1-29(2)13-14-30(3,4)23-17-26-21(16-22(23)29)27(18-7-9-19(10-8-18)28(34)35)32-24-15-20(38(36,37)31-5)11-12-25(24)33(26)6/h7-12,15-17,31H,13-14H2,1-6H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218439

(4-(5,7,7,10,10-pentamethyl-2-methylsulfamoyl-7,8,9...)Show SMILES CNS(=O)(=O)c1ccc2N(C)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:18| Show InChI InChI=1S/C30H33N3O4S/c1-29(2)13-14-30(3,4)23-17-26-21(16-22(23)29)27(18-7-9-19(10-8-18)28(34)35)32-24-15-20(38(36,37)31-5)11-12-25(24)33(26)6/h7-12,15-17,31H,13-14H2,1-6H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218425

(4-[2-(4-methoxy-phenylmethanesulfonyl)-5,7,7,10,10...)Show SMILES COc1ccc(CS(=O)(=O)c2ccc3N(C)c4cc5c(cc4C(=Nc3c2)c2ccc(cc2)C(O)=O)C(C)(C)CCC5(C)C)cc1 |c:23| Show InChI InChI=1S/C37H38N2O5S/c1-36(2)17-18-37(3,4)30-21-33-28(20-29(30)36)34(24-9-11-25(12-10-24)35(40)41)38-31-19-27(15-16-32(31)39(33)5)45(42,43)22-23-7-13-26(44-6)14-8-23/h7-16,19-21H,17-18,22H2,1-6H3,(H,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218435
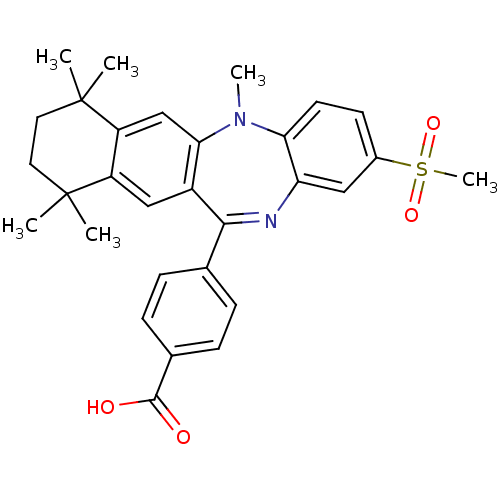
(4-(2-methanesulfonyl-5,7,7,10,10-pentamethyl-7,8,9...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)S(C)(=O)=O |t:9| Show InChI InChI=1S/C30H32N2O4S/c1-29(2)13-14-30(3,4)23-17-26-21(16-22(23)29)27(18-7-9-19(10-8-18)28(33)34)31-24-15-20(37(6,35)36)11-12-25(24)32(26)5/h7-12,15-17H,13-14H2,1-6H3,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218451

(4-[2-(4-methoxy-phenylsulfanyl)-5,7,7,10,10-pentam...)Show SMILES COc1ccc(Sc2ccc3N(C)c4cc5c(cc4C(=Nc3c2)c2ccc(cc2)C(O)=O)C(C)(C)CCC5(C)C)cc1 |c:20| Show InChI InChI=1S/C36H36N2O3S/c1-35(2)17-18-36(3,4)29-21-32-27(20-28(29)35)33(22-7-9-23(10-8-22)34(39)40)37-30-19-26(15-16-31(30)38(32)5)42-25-13-11-24(41-6)12-14-25/h7-16,19-21H,17-18H2,1-6H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218449

(4-[2-(4-chloro-benzylsulfanyl)-5,7,7,10,10-pentame...)Show SMILES CN1c2ccc(SCc3ccc(Cl)cc3)cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C |t:19| Show InChI InChI=1S/C36H35ClN2O2S/c1-35(2)16-17-36(3,4)29-20-32-27(19-28(29)35)33(23-8-10-24(11-9-23)34(40)41)38-30-18-26(14-15-31(30)39(32)5)42-21-22-6-12-25(37)13-7-22/h6-15,18-20H,16-17,21H2,1-5H3,(H,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218435

(4-(2-methanesulfonyl-5,7,7,10,10-pentamethyl-7,8,9...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)S(C)(=O)=O |t:9| Show InChI InChI=1S/C30H32N2O4S/c1-29(2)13-14-30(3,4)23-17-26-21(16-22(23)29)27(18-7-9-19(10-8-18)28(33)34)31-24-15-20(37(6,35)36)11-12-25(24)32(26)5/h7-12,15-17H,13-14H2,1-6H3,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218440

(4-(2-acetyl-5,7,7,10,10-pentamethyl-7,8,9,10-tetra...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(C(O)=O)c(F)c2)c2cc3c(cc12)C(C)(C)CCC3(C)C)C(C)=O |t:9| Show InChI InChI=1S/C31H31FN2O3/c1-17(35)18-8-10-26-25(14-18)33-28(19-7-9-20(29(36)37)24(32)13-19)21-15-22-23(16-27(21)34(26)6)31(4,5)12-11-30(22,2)3/h7-10,13-16H,11-12H2,1-6H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218431

(4-[5,7,7,10,10-pentamethyl-2-(4-methyl-piperazine-...)Show SMILES CN1CCN(CC1)S(=O)(=O)c1ccc2N(C)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:24| Show InChI InChI=1S/C34H40N4O4S/c1-33(2)13-14-34(3,4)27-21-30-25(20-26(27)33)31(22-7-9-23(10-8-22)32(39)40)35-28-19-24(11-12-29(28)37(30)6)43(41,42)38-17-15-36(5)16-18-38/h7-12,19-21H,13-18H2,1-6H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218450
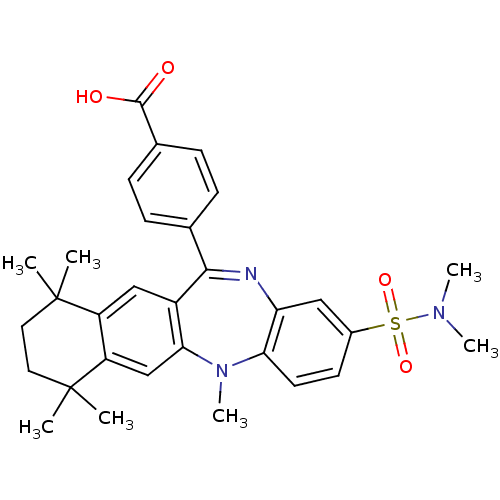
(4-(2-dimethylsulfamoyl-5,7,7,10,10-pentamethyl-7,8...)Show SMILES CN(C)S(=O)(=O)c1ccc2N(C)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:19| Show InChI InChI=1S/C31H35N3O4S/c1-30(2)14-15-31(3,4)24-18-27-22(17-23(24)30)28(19-8-10-20(11-9-19)29(35)36)32-25-16-21(39(37,38)33(5)6)12-13-26(25)34(27)7/h8-13,16-18H,14-15H2,1-7H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218430
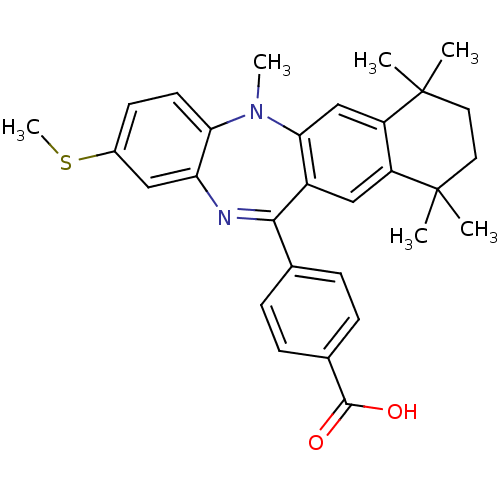
(4-(5,7,7,10,10-pentamethyl-2-methylsulfanyl-7,8,9,...)Show SMILES CSc1ccc2N(C)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:15| Show InChI InChI=1S/C30H32N2O2S/c1-29(2)13-14-30(3,4)23-17-26-21(16-22(23)29)27(18-7-9-19(10-8-18)28(33)34)31-24-15-20(35-6)11-12-25(24)32(26)5/h7-12,15-17H,13-14H2,1-6H3,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218443
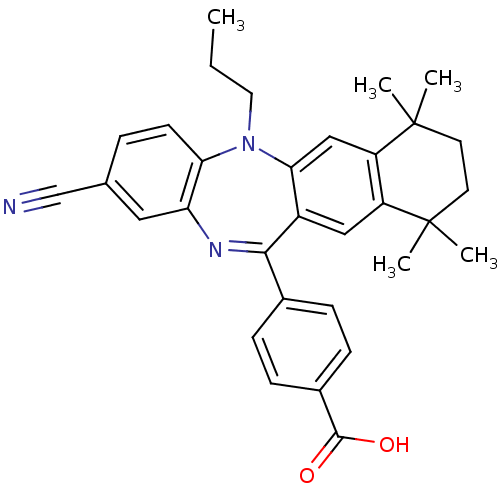
(4-(2-cyano-7,7,10,10-tetramethyl-5-propyl-7,8,9,10...)Show SMILES CCCN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)C#N |t:11| Show InChI InChI=1S/C32H33N3O2/c1-6-15-35-27-12-7-20(19-33)16-26(27)34-29(21-8-10-22(11-9-21)30(36)37)23-17-24-25(18-28(23)35)32(4,5)14-13-31(24,2)3/h7-12,16-18H,6,13-15H2,1-5H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218443

(4-(2-cyano-7,7,10,10-tetramethyl-5-propyl-7,8,9,10...)Show SMILES CCCN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)C#N |t:11| Show InChI InChI=1S/C32H33N3O2/c1-6-15-35-27-12-7-20(19-33)16-26(27)34-29(21-8-10-22(11-9-21)30(36)37)23-17-24-25(18-28(23)35)32(4,5)14-13-31(24,2)3/h7-12,16-18H,6,13-15H2,1-5H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218450

(4-(2-dimethylsulfamoyl-5,7,7,10,10-pentamethyl-7,8...)Show SMILES CN(C)S(=O)(=O)c1ccc2N(C)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:19| Show InChI InChI=1S/C31H35N3O4S/c1-30(2)14-15-31(3,4)24-18-27-22(17-23(24)30)28(19-8-10-20(11-9-19)29(35)36)32-25-16-21(39(37,38)33(5)6)12-13-26(25)34(27)7/h8-13,16-18H,14-15H2,1-7H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218423
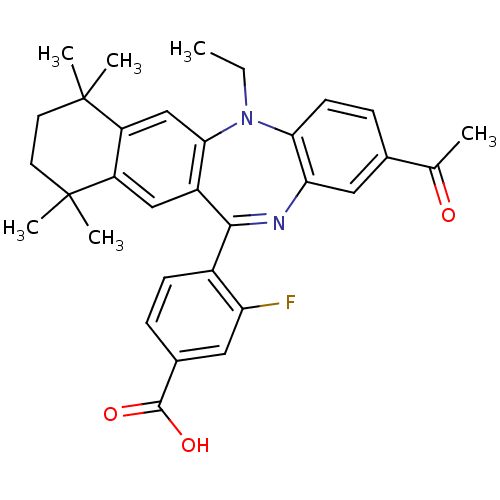
(4-(2-acetyl-5-ethyl-7,7,10,10-tetramethyl-7,8,9,10...)Show SMILES CCN1c2ccc(cc2N=C(c2ccc(cc2F)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)C(C)=O |t:10| Show InChI InChI=1S/C32H33FN2O3/c1-7-35-27-11-9-19(18(2)36)15-26(27)34-29(21-10-8-20(30(37)38)14-25(21)33)22-16-23-24(17-28(22)35)32(5,6)13-12-31(23,3)4/h8-11,14-17H,7,12-13H2,1-6H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218430

(4-(5,7,7,10,10-pentamethyl-2-methylsulfanyl-7,8,9,...)Show SMILES CSc1ccc2N(C)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:15| Show InChI InChI=1S/C30H32N2O2S/c1-29(2)13-14-30(3,4)23-17-26-21(16-22(23)29)27(18-7-9-19(10-8-18)28(33)34)31-24-15-20(35-6)11-12-25(24)32(26)5/h7-12,15-17H,13-14H2,1-6H3,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218423

(4-(2-acetyl-5-ethyl-7,7,10,10-tetramethyl-7,8,9,10...)Show SMILES CCN1c2ccc(cc2N=C(c2ccc(cc2F)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)C(C)=O |t:10| Show InChI InChI=1S/C32H33FN2O3/c1-7-35-27-11-9-19(18(2)36)15-26(27)34-29(21-10-8-20(30(37)38)14-25(21)33)22-16-23-24(17-28(22)35)32(5,6)13-12-31(23,3)4/h8-11,14-17H,7,12-13H2,1-6H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218445
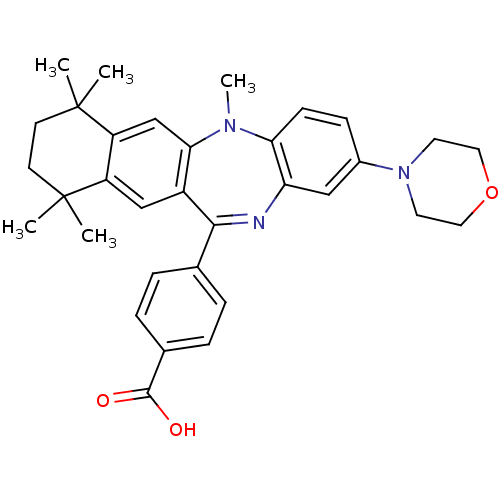
(4-(5,7,7,10,10-pentamethyl-2-morpholin-4-yl-7,8,9,...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)N1CCOCC1 |t:9| Show InChI InChI=1S/C33H37N3O3/c1-32(2)12-13-33(3,4)26-20-29-24(19-25(26)32)30(21-6-8-22(9-7-21)31(37)38)34-27-18-23(10-11-28(27)35(29)5)36-14-16-39-17-15-36/h6-11,18-20H,12-17H2,1-5H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218447
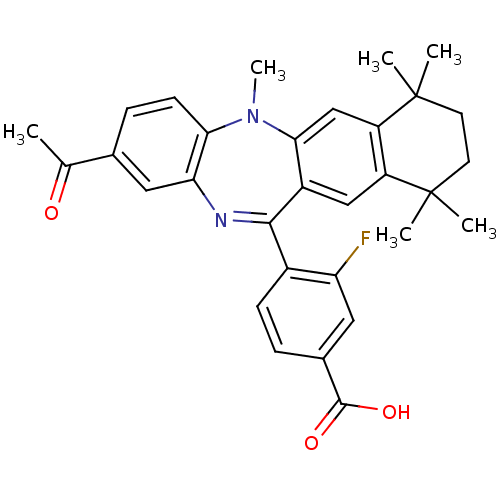
(4-(2-acetyl-5,7,7,10,10-pentamethyl-7,8,9,10-tetra...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2F)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)C(C)=O |t:9| Show InChI InChI=1S/C31H31FN2O3/c1-17(35)18-8-10-26-25(14-18)33-28(20-9-7-19(29(36)37)13-24(20)32)21-15-22-23(16-27(21)34(26)6)31(4,5)12-11-30(22,2)3/h7-10,13-16H,11-12H2,1-6H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218426
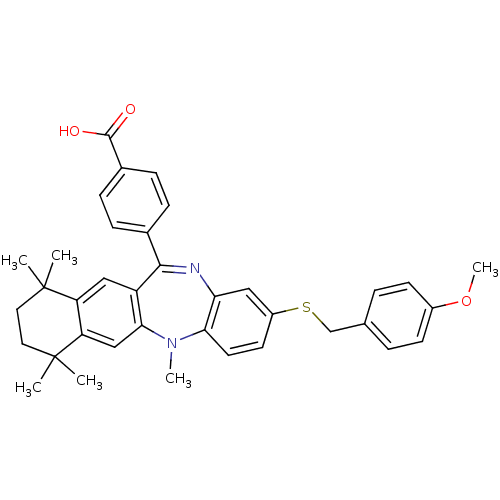
(4-[2-(4-methoxy-benzylsulfanyl)-5,7,7,10,10-pentam...)Show SMILES COc1ccc(CSc2ccc3N(C)c4cc5c(cc4C(=Nc3c2)c2ccc(cc2)C(O)=O)C(C)(C)CCC5(C)C)cc1 |c:21| Show InChI InChI=1S/C37H38N2O3S/c1-36(2)17-18-37(3,4)30-21-33-28(20-29(30)36)34(24-9-11-25(12-10-24)35(40)41)38-31-19-27(15-16-32(31)39(33)5)43-22-23-7-13-26(42-6)14-8-23/h7-16,19-21H,17-18,22H2,1-6H3,(H,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218426

(4-[2-(4-methoxy-benzylsulfanyl)-5,7,7,10,10-pentam...)Show SMILES COc1ccc(CSc2ccc3N(C)c4cc5c(cc4C(=Nc3c2)c2ccc(cc2)C(O)=O)C(C)(C)CCC5(C)C)cc1 |c:21| Show InChI InChI=1S/C37H38N2O3S/c1-36(2)17-18-37(3,4)30-21-33-28(20-29(30)36)34(24-9-11-25(12-10-24)35(40)41)38-31-19-27(15-16-32(31)39(33)5)43-22-23-7-13-26(42-6)14-8-23/h7-16,19-21H,17-18,22H2,1-6H3,(H,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218445

(4-(5,7,7,10,10-pentamethyl-2-morpholin-4-yl-7,8,9,...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)N1CCOCC1 |t:9| Show InChI InChI=1S/C33H37N3O3/c1-32(2)12-13-33(3,4)26-20-29-24(19-25(26)32)30(21-6-8-22(9-7-21)31(37)38)34-27-18-23(10-11-28(27)35(29)5)36-14-16-39-17-15-36/h6-11,18-20H,12-17H2,1-5H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218437
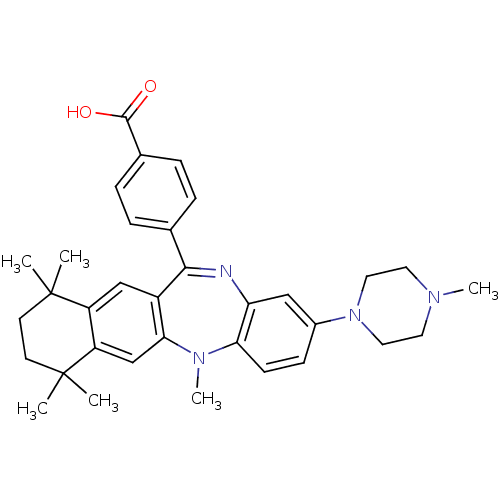
(4-[5,7,7,10,10-pentamethyl-2-(4-methyl-piperazin-1...)Show SMILES CN1CCN(CC1)c1ccc2N(C)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:21| Show InChI InChI=1S/C34H40N4O2/c1-33(2)13-14-34(3,4)27-21-30-25(20-26(27)33)31(22-7-9-23(10-8-22)32(39)40)35-28-19-24(11-12-29(28)37(30)6)38-17-15-36(5)16-18-38/h7-12,19-21H,13-18H2,1-6H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data