 Found 45 hits of Enzyme Inhibition Constant Data
Found 45 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220573
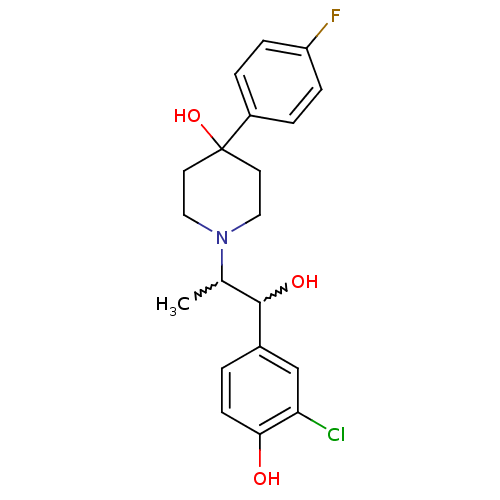
(1-(1-(3-chloro-4-hydroxyphenyl)-1-hydroxypropan-2-...)Show SMILES CC(C(O)c1ccc(O)c(Cl)c1)N1CCC(O)(CC1)c1ccc(F)cc1 |w:1.0,2.2| Show InChI InChI=1S/C20H23ClFNO3/c1-13(19(25)14-2-7-18(24)17(21)12-14)23-10-8-20(26,9-11-23)15-3-5-16(22)6-4-15/h2-7,12-13,19,24-26H,8-11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220589
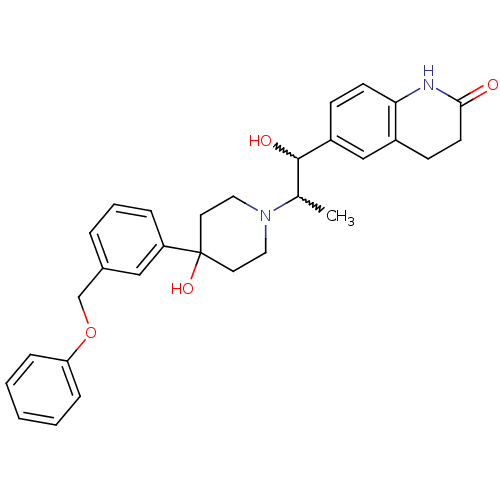
(6-(1-hydroxy-2-(4-hydroxy-4-(3-(phenoxymethyl)phen...)Show SMILES CC(C(O)c1ccc2NC(=O)CCc2c1)N1CCC(O)(CC1)c1cccc(COc2ccccc2)c1 |w:2.2,1.0| Show InChI InChI=1S/C30H34N2O4/c1-21(29(34)24-10-12-27-23(19-24)11-13-28(33)31-27)32-16-14-30(35,15-17-32)25-7-5-6-22(18-25)20-36-26-8-3-2-4-9-26/h2-10,12,18-19,21,29,34-35H,11,13-17,20H2,1H3,(H,31,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220583
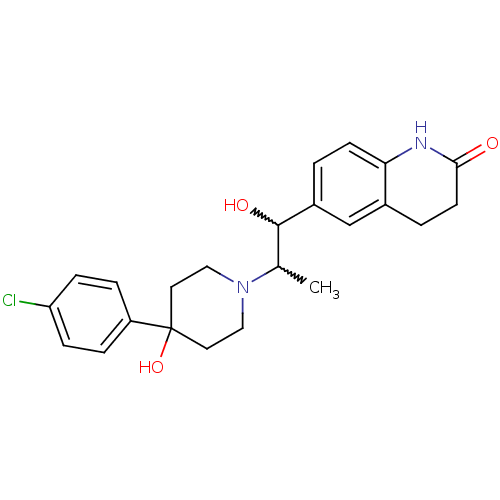
(6-(2-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-...)Show SMILES CC(C(O)c1ccc2NC(=O)CCc2c1)N1CCC(O)(CC1)c1ccc(Cl)cc1 |w:2.2,1.0| Show InChI InChI=1S/C23H27ClN2O3/c1-15(22(28)17-2-8-20-16(14-17)3-9-21(27)25-20)26-12-10-23(29,11-13-26)18-4-6-19(24)7-5-18/h2,4-8,14-15,22,28-29H,3,9-13H2,1H3,(H,25,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220586
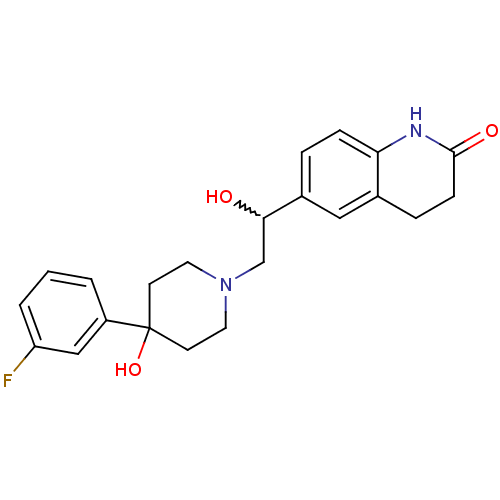
(1-[2-hydroxy-2-(6-hydroxy-biphenyl-3-yl)-1-methyl-...)Show SMILES OC(CN1CCC(O)(CC1)c1cccc(F)c1)c1ccc2NC(=O)CCc2c1 |w:1.0| Show InChI InChI=1S/C22H25FN2O3/c23-18-3-1-2-17(13-18)22(28)8-10-25(11-9-22)14-20(26)16-4-6-19-15(12-16)5-7-21(27)24-19/h1-4,6,12-13,20,26,28H,5,7-11,14H2,(H,24,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220588

(6-(1-hydroxy-2-(4-hydroxy-4-(3-methoxyphenyl)piper...)Show SMILES COc1cccc(c1)C1(O)CCN(CC1)C(C)C(O)c1ccc2NC(=O)CCc2c1 |w:17.19,15.17| Show InChI InChI=1S/C24H30N2O4/c1-16(23(28)18-6-8-21-17(14-18)7-9-22(27)25-21)26-12-10-24(29,11-13-26)19-4-3-5-20(15-19)30-2/h3-6,8,14-16,23,28-29H,7,9-13H2,1-2H3,(H,25,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220581
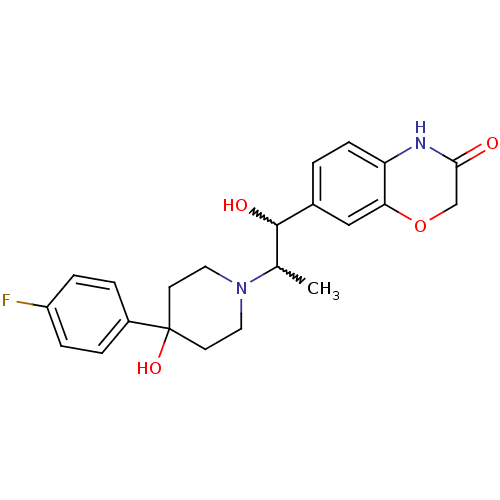
(7-(2-(4-(4-fluorophenyl)-4-hydroxypiperidin-1-yl)-...)Show SMILES CC(C(O)c1ccc2NC(=O)COc2c1)N1CCC(O)(CC1)c1ccc(F)cc1 |w:2.2,1.0| Show InChI InChI=1S/C22H25FN2O4/c1-14(21(27)15-2-7-18-19(12-15)29-13-20(26)24-18)25-10-8-22(28,9-11-25)16-3-5-17(23)6-4-16/h2-7,12,14,21,27-28H,8-11,13H2,1H3,(H,24,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220584

(6-(2-(4-(4-fluorophenyl)-4-hydroxypiperidin-1-yl)-...)Show SMILES CC(C(O)c1ccc2NC(=O)NCc2c1)N1CCC(O)(CC1)c1ccc(F)cc1 |w:2.2,1.0| Show InChI InChI=1S/C22H26FN3O3/c1-14(20(27)15-2-7-19-16(12-15)13-24-21(28)25-19)26-10-8-22(29,9-11-26)17-3-5-18(23)6-4-17/h2-7,12,14,20,27,29H,8-11,13H2,1H3,(H2,24,25,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220574
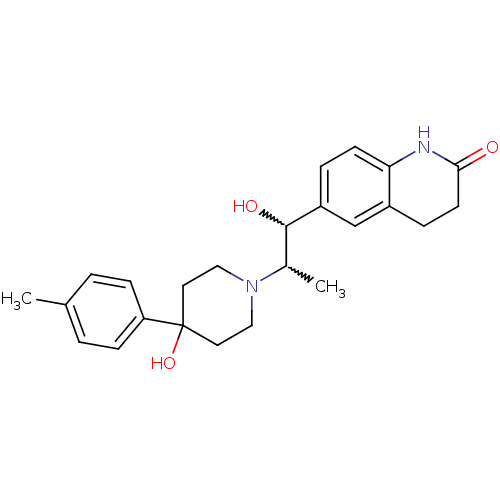
(6-(1-hydroxy-2-(4-hydroxy-4-p-tolylpiperidin-1-yl)...)Show SMILES CC(C(O)c1ccc2NC(=O)CCc2c1)N1CCC(O)(CC1)c1ccc(C)cc1 |w:2.2,1.0| Show InChI InChI=1S/C24H30N2O3/c1-16-3-7-20(8-4-16)24(29)11-13-26(14-12-24)17(2)23(28)19-5-9-21-18(15-19)6-10-22(27)25-21/h3-5,7-9,15,17,23,28-29H,6,10-14H2,1-2H3,(H,25,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220577
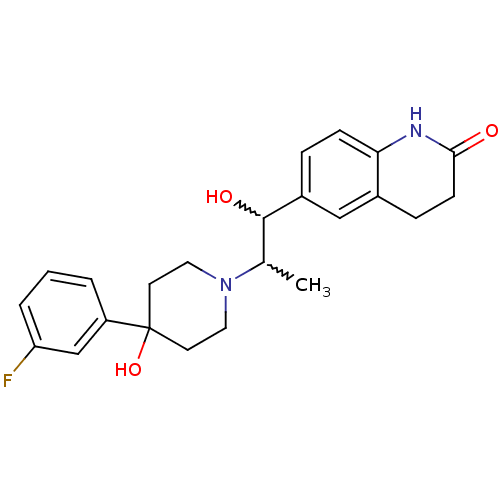
(6-(2-(4-(3-fluorophenyl)-4-hydroxypiperidin-1-yl)-...)Show SMILES CC(C(O)c1ccc2NC(=O)CCc2c1)N1CCC(O)(CC1)c1cccc(F)c1 |w:2.2,1.0| Show InChI InChI=1S/C23H27FN2O3/c1-15(22(28)17-5-7-20-16(13-17)6-8-21(27)25-20)26-11-9-23(29,10-12-26)18-3-2-4-19(24)14-18/h2-5,7,13-15,22,28-29H,6,8-12H2,1H3,(H,25,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220576
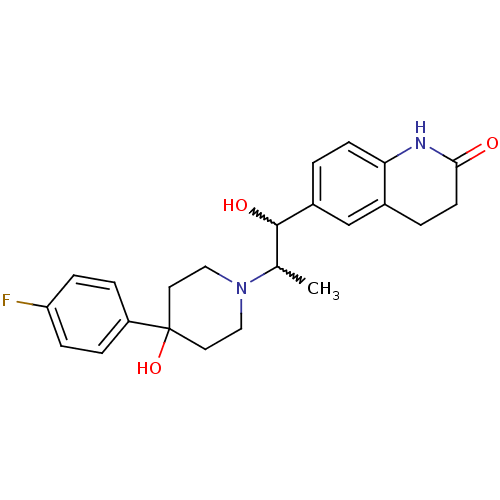
(6-(2-(4-(4-fluorophenyl)-4-hydroxypiperidin-1-yl)-...)Show SMILES CC(C(O)c1ccc2NC(=O)CCc2c1)N1CCC(O)(CC1)c1ccc(F)cc1 |w:2.2,1.0| Show InChI InChI=1S/C23H27FN2O3/c1-15(22(28)17-2-8-20-16(14-17)3-9-21(27)25-20)26-12-10-23(29,11-13-26)18-4-6-19(24)7-5-18/h2,4-8,14-15,22,28-29H,3,9-13H2,1H3,(H,25,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220582

(6-(2-(4-(4-fluorophenyl)-4-hydroxypiperidin-1-yl)-...)Show SMILES CC(C(O)c1ccc2NC(=O)N(C)Cc2c1)N1CCC(O)(CC1)c1ccc(F)cc1 |w:2.2,1.0| Show InChI InChI=1S/C23H28FN3O3/c1-15(21(28)16-3-8-20-17(13-16)14-26(2)22(29)25-20)27-11-9-23(30,10-12-27)18-4-6-19(24)7-5-18/h3-8,13,15,21,28,30H,9-12,14H2,1-2H3,(H,25,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50032651
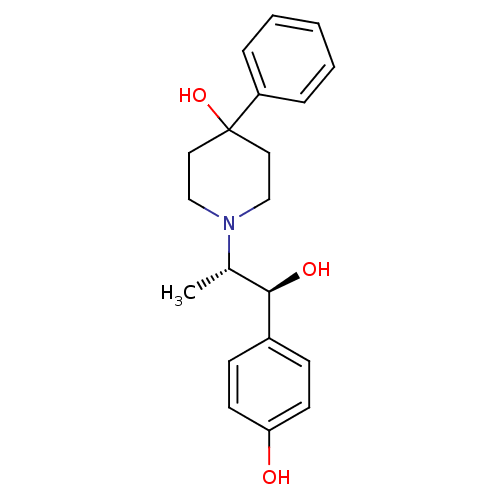
(1-((1S,2S)-1-hydroxy-1-(4-hydroxyphenyl)propan-2-y...)Show SMILES C[C@@H]([C@@H](O)c1ccc(O)cc1)N1CCC(O)(CC1)c1ccccc1 Show InChI InChI=1S/C20H25NO3/c1-15(19(23)16-7-9-18(22)10-8-16)21-13-11-20(24,12-14-21)17-5-3-2-4-6-17/h2-10,15,19,22-24H,11-14H2,1H3/t15-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220578
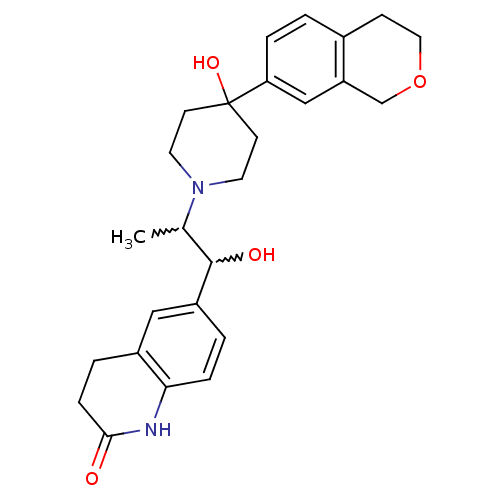
(6-(2-(4-(3,4-dihydro-1H-isochromen-7-yl)-4-hydroxy...)Show SMILES CC(C(O)c1ccc2NC(=O)CCc2c1)N1CCC(O)(CC1)c1ccc2CCOCc2c1 |w:2.2,1.0| Show InChI InChI=1S/C26H32N2O4/c1-17(25(30)20-3-6-23-19(14-20)4-7-24(29)27-23)28-11-9-26(31,10-12-28)22-5-2-18-8-13-32-16-21(18)15-22/h2-3,5-6,14-15,17,25,30-31H,4,7-13,16H2,1H3,(H,27,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220580

(4-(4-fluorophenyl)-1-(1-hydroxy-1-(4-hydroxy-2-met...)Show SMILES CC(C(O)c1ccc(O)cc1C)N1CCC(O)(CC1)c1ccc(F)cc1 |w:1.0,2.2| Show InChI InChI=1S/C21H26FNO3/c1-14-13-18(24)7-8-19(14)20(25)15(2)23-11-9-21(26,10-12-23)16-3-5-17(22)6-4-16/h3-8,13,15,20,24-26H,9-12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220585

(6-(2-(4-(4-fluorophenyl)-4-hydroxypiperidin-1-yl)-...)Show SMILES CC(C(O)c1ccc2NC(=O)OCc2c1)N1CCC(O)(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C22H25FN2O4/c1-14(20(26)15-2-7-19-16(12-15)13-29-21(27)24-19)25-10-8-22(28,9-11-25)17-3-5-18(23)6-4-17/h2-7,12,14,20,26,28H,8-11,13H2,1H3,(H,24,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220586

(1-[2-hydroxy-2-(6-hydroxy-biphenyl-3-yl)-1-methyl-...)Show SMILES OC(CN1CCC(O)(CC1)c1cccc(F)c1)c1ccc2NC(=O)CCc2c1 |w:1.0| Show InChI InChI=1S/C22H25FN2O3/c23-18-3-1-2-17(13-18)22(28)8-10-25(11-9-22)14-20(26)16-4-6-19-15(12-16)5-7-21(27)24-19/h1-4,6,12-13,20,26,28H,5,7-11,14H2,(H,24,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220586

(1-[2-hydroxy-2-(6-hydroxy-biphenyl-3-yl)-1-methyl-...)Show SMILES OC(CN1CCC(O)(CC1)c1cccc(F)c1)c1ccc2NC(=O)CCc2c1 |w:1.0| Show InChI InChI=1S/C22H25FN2O3/c23-18-3-1-2-17(13-18)22(28)8-10-25(11-9-22)14-20(26)16-4-6-19-15(12-16)5-7-21(27)24-19/h1-4,6,12-13,20,26,28H,5,7-11,14H2,(H,24,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220572

(4-(4-fluorophenyl)-1-(1-hydroxy-1-(4-hydroxy-3-met...)Show SMILES CC(C(O)c1ccc(O)c(C)c1)N1CCC(O)(CC1)c1ccc(F)cc1 |w:1.0,2.2| Show InChI InChI=1S/C21H26FNO3/c1-14-13-16(3-8-19(14)24)20(25)15(2)23-11-9-21(26,10-12-23)17-4-6-18(22)7-5-17/h3-8,13,15,20,24-26H,9-12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220587

(6-(2-(4-(4-fluorophenyl)-4-hydroxypiperidin-1-yl)-...)Show SMILES CC(C(O)c1ccc2[nH]c(=O)oc2c1)N1CCC(O)(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C21H23FN2O4/c1-13(19(25)14-2-7-17-18(12-14)28-20(26)23-17)24-10-8-21(27,9-11-24)15-3-5-16(22)6-4-15/h2-7,12-13,19,25,27H,8-11H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220575

(4-(4-fluoro-phenyl)-1-[2-hydroxy-2-(6-hydroxy-biph...)Show SMILES CC(C(O)c1ccc(O)c(c1)-c1ccccc1)N1CCC(O)(CC1)c1ccc(F)cc1 |w:2.2,1.0| Show InChI InChI=1S/C26H28FNO3/c1-18(28-15-13-26(31,14-16-28)21-8-10-22(27)11-9-21)25(30)20-7-12-24(29)23(17-20)19-5-3-2-4-6-19/h2-12,17-18,25,29-31H,13-16H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220581

(7-(2-(4-(4-fluorophenyl)-4-hydroxypiperidin-1-yl)-...)Show SMILES CC(C(O)c1ccc2NC(=O)COc2c1)N1CCC(O)(CC1)c1ccc(F)cc1 |w:2.2,1.0| Show InChI InChI=1S/C22H25FN2O4/c1-14(21(27)15-2-7-18-19(12-15)29-13-20(26)24-18)25-10-8-22(28,9-11-25)16-3-5-17(23)6-4-16/h2-7,12,14,21,27-28H,8-11,13H2,1H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from hERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220585

(6-(2-(4-(4-fluorophenyl)-4-hydroxypiperidin-1-yl)-...)Show SMILES CC(C(O)c1ccc2NC(=O)OCc2c1)N1CCC(O)(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C22H25FN2O4/c1-14(20(26)15-2-7-19-16(12-15)13-29-21(27)24-19)25-10-8-22(28,9-11-25)17-3-5-18(23)6-4-17/h2-7,12,14,20,26,28H,8-11,13H2,1H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from hERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220587

(6-(2-(4-(4-fluorophenyl)-4-hydroxypiperidin-1-yl)-...)Show SMILES CC(C(O)c1ccc2[nH]c(=O)oc2c1)N1CCC(O)(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C21H23FN2O4/c1-13(19(25)14-2-7-17-18(12-14)28-20(26)23-17)24-10-8-21(27,9-11-24)15-3-5-16(22)6-4-15/h2-7,12-13,19,25,27H,8-11H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from hERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220582

(6-(2-(4-(4-fluorophenyl)-4-hydroxypiperidin-1-yl)-...)Show SMILES CC(C(O)c1ccc2NC(=O)N(C)Cc2c1)N1CCC(O)(CC1)c1ccc(F)cc1 |w:2.2,1.0| Show InChI InChI=1S/C23H28FN3O3/c1-15(21(28)16-3-8-20-17(13-16)14-26(2)22(29)25-20)27-11-9-23(30,10-12-27)18-4-6-19(24)7-5-18/h3-8,13,15,21,28,30H,9-12,14H2,1-2H3,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from hERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220574

(6-(1-hydroxy-2-(4-hydroxy-4-p-tolylpiperidin-1-yl)...)Show SMILES CC(C(O)c1ccc2NC(=O)CCc2c1)N1CCC(O)(CC1)c1ccc(C)cc1 |w:2.2,1.0| Show InChI InChI=1S/C24H30N2O3/c1-16-3-7-20(8-4-16)24(29)11-13-26(14-12-24)17(2)23(28)19-5-9-21-18(15-19)6-10-22(27)25-21/h3-5,7-9,15,17,23,28-29H,6,10-14H2,1-2H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from hERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50220573

(1-(1-(3-chloro-4-hydroxyphenyl)-1-hydroxypropan-2-...)Show SMILES CC(C(O)c1ccc(O)c(Cl)c1)N1CCC(O)(CC1)c1ccc(F)cc1 |w:1.0,2.2| Show InChI InChI=1S/C20H23ClFNO3/c1-13(19(25)14-2-7-18(24)17(21)12-14)23-10-8-20(26,9-11-23)15-3-5-16(22)6-4-15/h2-7,12-13,19,24-26H,8-11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from CYP2D6 |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50220580

(4-(4-fluorophenyl)-1-(1-hydroxy-1-(4-hydroxy-2-met...)Show SMILES CC(C(O)c1ccc(O)cc1C)N1CCC(O)(CC1)c1ccc(F)cc1 |w:1.0,2.2| Show InChI InChI=1S/C21H26FNO3/c1-14-13-18(24)7-8-19(14)20(25)15(2)23-11-9-21(26,10-12-23)16-3-5-17(22)6-4-16/h3-8,13,15,20,24-26H,9-12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from CYP2D6 |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220579
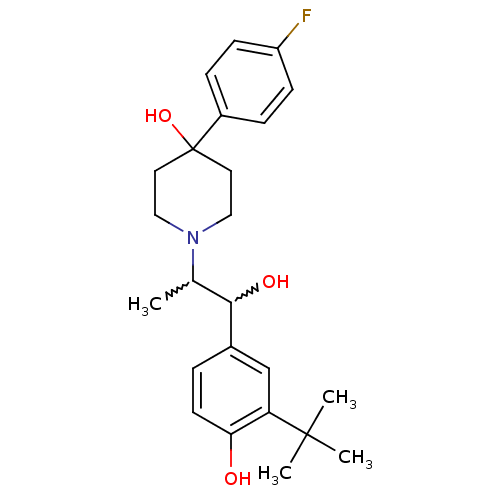
(1-(1-(3-tert-butyl-4-hydroxyphenyl)-1-hydroxypropa...)Show SMILES CC(C(O)c1ccc(O)c(c1)C(C)(C)C)N1CCC(O)(CC1)c1ccc(F)cc1 |w:2.2,1.0| Show InChI InChI=1S/C24H32FNO3/c1-16(22(28)17-5-10-21(27)20(15-17)23(2,3)4)26-13-11-24(29,12-14-26)18-6-8-19(25)9-7-18/h5-10,15-16,22,27-29H,11-14H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220584

(6-(2-(4-(4-fluorophenyl)-4-hydroxypiperidin-1-yl)-...)Show SMILES CC(C(O)c1ccc2NC(=O)NCc2c1)N1CCC(O)(CC1)c1ccc(F)cc1 |w:2.2,1.0| Show InChI InChI=1S/C22H26FN3O3/c1-14(20(27)15-2-7-19-16(12-15)13-24-21(28)25-19)26-10-8-22(29,9-11-26)17-3-5-18(23)6-4-17/h2-7,12,14,20,27,29H,8-11,13H2,1H3,(H2,24,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from hERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50220575

(4-(4-fluoro-phenyl)-1-[2-hydroxy-2-(6-hydroxy-biph...)Show SMILES CC(C(O)c1ccc(O)c(c1)-c1ccccc1)N1CCC(O)(CC1)c1ccc(F)cc1 |w:2.2,1.0| Show InChI InChI=1S/C26H28FNO3/c1-18(28-15-13-26(31,14-16-28)21-8-10-22(27)11-9-21)25(30)20-7-12-24(29)23(17-20)19-5-3-2-4-6-19/h2-12,17-18,25,29-31H,13-16H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from CYP2D6 |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220576

(6-(2-(4-(4-fluorophenyl)-4-hydroxypiperidin-1-yl)-...)Show SMILES CC(C(O)c1ccc2NC(=O)CCc2c1)N1CCC(O)(CC1)c1ccc(F)cc1 |w:2.2,1.0| Show InChI InChI=1S/C23H27FN2O3/c1-15(22(28)17-2-8-20-16(14-17)3-9-21(27)25-20)26-12-10-23(29,11-13-26)18-4-6-19(24)7-5-18/h2,4-8,14-15,22,28-29H,3,9-13H2,1H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from hERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220586

(1-[2-hydroxy-2-(6-hydroxy-biphenyl-3-yl)-1-methyl-...)Show SMILES OC(CN1CCC(O)(CC1)c1cccc(F)c1)c1ccc2NC(=O)CCc2c1 |w:1.0| Show InChI InChI=1S/C22H25FN2O3/c23-18-3-1-2-17(13-18)22(28)8-10-25(11-9-22)14-20(26)16-4-6-19-15(12-16)5-7-21(27)24-19/h1-4,6,12-13,20,26,28H,5,7-11,14H2,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from hERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220577

(6-(2-(4-(3-fluorophenyl)-4-hydroxypiperidin-1-yl)-...)Show SMILES CC(C(O)c1ccc2NC(=O)CCc2c1)N1CCC(O)(CC1)c1cccc(F)c1 |w:2.2,1.0| Show InChI InChI=1S/C23H27FN2O3/c1-15(22(28)17-5-7-20-16(13-17)6-8-21(27)25-20)26-11-9-23(29,10-12-26)18-3-2-4-19(24)14-18/h2-5,7,13-15,22,28-29H,6,8-12H2,1H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from hERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032651

(1-((1S,2S)-1-hydroxy-1-(4-hydroxyphenyl)propan-2-y...)Show SMILES C[C@@H]([C@@H](O)c1ccc(O)cc1)N1CCC(O)(CC1)c1ccccc1 Show InChI InChI=1S/C20H25NO3/c1-15(19(23)16-7-9-18(22)10-8-16)21-13-11-20(24,12-14-21)17-5-3-2-4-6-17/h2-10,15,19,22-24H,11-14H2,1H3/t15-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from hERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50032651

(1-((1S,2S)-1-hydroxy-1-(4-hydroxyphenyl)propan-2-y...)Show SMILES C[C@@H]([C@@H](O)c1ccc(O)cc1)N1CCC(O)(CC1)c1ccccc1 Show InChI InChI=1S/C20H25NO3/c1-15(19(23)16-7-9-18(22)10-8-16)21-13-11-20(24,12-14-21)17-5-3-2-4-6-17/h2-10,15,19,22-24H,11-14H2,1H3/t15-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from CYP2D6 |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50220587

(6-(2-(4-(4-fluorophenyl)-4-hydroxypiperidin-1-yl)-...)Show SMILES CC(C(O)c1ccc2[nH]c(=O)oc2c1)N1CCC(O)(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C21H23FN2O4/c1-13(19(25)14-2-7-17-18(12-14)28-20(26)23-17)24-10-8-21(27,9-11-24)15-3-5-16(22)6-4-15/h2-7,12-13,19,25,27H,8-11H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from CYP2D6 |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220588

(6-(1-hydroxy-2-(4-hydroxy-4-(3-methoxyphenyl)piper...)Show SMILES COc1cccc(c1)C1(O)CCN(CC1)C(C)C(O)c1ccc2NC(=O)CCc2c1 |w:17.19,15.17| Show InChI InChI=1S/C24H30N2O4/c1-16(23(28)18-6-8-21-17(14-18)7-9-22(27)25-21)26-12-10-24(29,11-13-26)19-4-3-5-20(15-19)30-2/h3-6,8,14-16,23,28-29H,7,9-13H2,1-2H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from hERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220578

(6-(2-(4-(3,4-dihydro-1H-isochromen-7-yl)-4-hydroxy...)Show SMILES CC(C(O)c1ccc2NC(=O)CCc2c1)N1CCC(O)(CC1)c1ccc2CCOCc2c1 |w:2.2,1.0| Show InChI InChI=1S/C26H32N2O4/c1-17(25(30)20-3-6-23-19(14-20)4-7-24(29)27-23)28-11-9-26(31,10-12-28)22-5-2-18-8-13-32-16-21(18)15-22/h2-3,5-6,14-15,17,25,30-31H,4,7-13,16H2,1H3,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from hERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220586

(1-[2-hydroxy-2-(6-hydroxy-biphenyl-3-yl)-1-methyl-...)Show SMILES OC(CN1CCC(O)(CC1)c1cccc(F)c1)c1ccc2NC(=O)CCc2c1 |w:1.0| Show InChI InChI=1S/C22H25FN2O3/c23-18-3-1-2-17(13-18)22(28)8-10-25(11-9-22)14-20(26)16-4-6-19-15(12-16)5-7-21(27)24-19/h1-4,6,12-13,20,26,28H,5,7-11,14H2,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from hERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220586

(1-[2-hydroxy-2-(6-hydroxy-biphenyl-3-yl)-1-methyl-...)Show SMILES OC(CN1CCC(O)(CC1)c1cccc(F)c1)c1ccc2NC(=O)CCc2c1 |w:1.0| Show InChI InChI=1S/C22H25FN2O3/c23-18-3-1-2-17(13-18)22(28)8-10-25(11-9-22)14-20(26)16-4-6-19-15(12-16)5-7-21(27)24-19/h1-4,6,12-13,20,26,28H,5,7-11,14H2,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from hERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50220581

(7-(2-(4-(4-fluorophenyl)-4-hydroxypiperidin-1-yl)-...)Show SMILES CC(C(O)c1ccc2NC(=O)COc2c1)N1CCC(O)(CC1)c1ccc(F)cc1 |w:2.2,1.0| Show InChI InChI=1S/C22H25FN2O4/c1-14(21(27)15-2-7-18-19(12-15)29-13-20(26)24-18)25-10-8-22(28,9-11-25)16-3-5-17(23)6-4-16/h2-7,12,14,21,27-28H,8-11,13H2,1H3,(H,24,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from CYP2D6 |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50220576

(6-(2-(4-(4-fluorophenyl)-4-hydroxypiperidin-1-yl)-...)Show SMILES CC(C(O)c1ccc2NC(=O)CCc2c1)N1CCC(O)(CC1)c1ccc(F)cc1 |w:2.2,1.0| Show InChI InChI=1S/C23H27FN2O3/c1-15(22(28)17-2-8-20-16(14-17)3-9-21(27)25-20)26-12-10-23(29,11-13-26)18-4-6-19(24)7-5-18/h2,4-8,14-15,22,28-29H,3,9-13H2,1H3,(H,25,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from CYP2D6 |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50220584

(6-(2-(4-(4-fluorophenyl)-4-hydroxypiperidin-1-yl)-...)Show SMILES CC(C(O)c1ccc2NC(=O)NCc2c1)N1CCC(O)(CC1)c1ccc(F)cc1 |w:2.2,1.0| Show InChI InChI=1S/C22H26FN3O3/c1-14(20(27)15-2-7-19-16(12-15)13-24-21(28)25-19)26-10-8-22(29,9-11-26)17-3-5-18(23)6-4-17/h2-7,12,14,20,27,29H,8-11,13H2,1H3,(H2,24,25,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from CYP2D6 |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50220585

(6-(2-(4-(4-fluorophenyl)-4-hydroxypiperidin-1-yl)-...)Show SMILES CC(C(O)c1ccc2NC(=O)OCc2c1)N1CCC(O)(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C22H25FN2O4/c1-14(20(26)15-2-7-19-16(12-15)13-29-21(27)24-19)25-10-8-22(28,9-11-25)17-3-5-18(23)6-4-17/h2-7,12,14,20,26,28H,8-11,13H2,1H3,(H,24,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from CYP2D6 |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50220582

(6-(2-(4-(4-fluorophenyl)-4-hydroxypiperidin-1-yl)-...)Show SMILES CC(C(O)c1ccc2NC(=O)N(C)Cc2c1)N1CCC(O)(CC1)c1ccc(F)cc1 |w:2.2,1.0| Show InChI InChI=1S/C23H28FN3O3/c1-15(21(28)16-3-8-20-17(13-16)14-26(2)22(29)25-20)27-11-9-23(30,10-12-27)18-4-6-19(24)7-5-18/h3-8,13,15,21,28,30H,9-12,14H2,1-2H3,(H,25,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]bufuralol from CYP2D6 |
Bioorg Med Chem Lett 17: 5558-62 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.014
BindingDB Entry DOI: 10.7270/Q2R21138 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data