 Found 70 hits of Enzyme Inhibition Constant Data
Found 70 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220703

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-cyclohexyl-4-((...)Show SMILES Cn1nnnc1CC1(CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1)C1CCCCC1 Show InChI InChI=1S/C33H42ClN7O2/c1-40-30(37-38-39-40)21-33(26-9-3-2-4-10-26)15-17-41(18-16-33)32(43)29(19-23-11-13-27(34)14-12-23)36-31(42)28-20-24-7-5-6-8-25(24)22-35-28/h5-8,11-14,26,28-29,35H,2-4,9-10,15-22H2,1H3,(H,36,42)/t28-,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220696
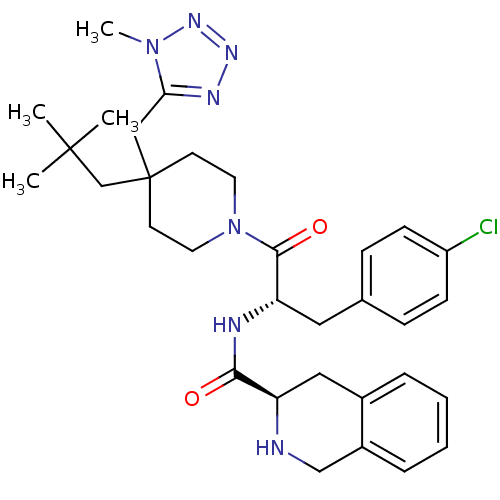
((R)-N-((S)-3-(4-chlorophenyl)-1-(4-((1-methyl-1H-t...)Show SMILES Cn1nnnc1CC1(CC(C)(C)C)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C32H42ClN7O2/c1-31(2,3)21-32(19-28-36-37-38-39(28)4)13-15-40(16-14-32)30(42)27(17-22-9-11-25(33)12-10-22)35-29(41)26-18-23-7-5-6-8-24(23)20-34-26/h5-12,26-27,34H,13-21H2,1-4H3,(H,35,41)/t26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220689

((R)-N-((S)-1-(4-sec-butyl-4-((1-methyl-1H-tetrazol...)Show SMILES CCC(C)C1(Cc2nnnn2C)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 |w:2.2| Show InChI InChI=1S/C31H40ClN7O2/c1-4-21(2)31(19-28-35-36-37-38(28)3)13-15-39(16-14-31)30(41)27(17-22-9-11-25(32)12-10-22)34-29(40)26-18-23-7-5-6-8-24(23)20-33-26/h5-12,21,26-27,33H,4,13-20H2,1-3H3,(H,34,40)/t21?,26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220700

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-hexyl-4-((1-met...)Show SMILES CCCCCCC1(Cc2nnnn2C)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C33H44ClN7O2/c1-3-4-5-8-15-33(22-30-37-38-39-40(30)2)16-18-41(19-17-33)32(43)29(20-24-11-13-27(34)14-12-24)36-31(42)28-21-25-9-6-7-10-26(25)23-35-28/h6-7,9-14,28-29,35H,3-5,8,15-23H2,1-2H3,(H,36,42)/t28-,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220691
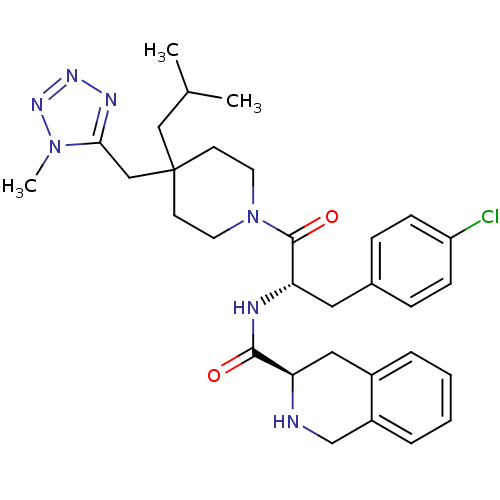
((R)-N-((S)-3-(4-chlorophenyl)-1-(4-isobutyl-4-((1-...)Show SMILES CC(C)CC1(Cc2nnnn2C)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C31H40ClN7O2/c1-21(2)18-31(19-28-35-36-37-38(28)3)12-14-39(15-13-31)30(41)27(16-22-8-10-25(32)11-9-22)34-29(40)26-17-23-6-4-5-7-24(23)20-33-26/h4-11,21,26-27,33H,12-20H2,1-3H3,(H,34,40)/t26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220699
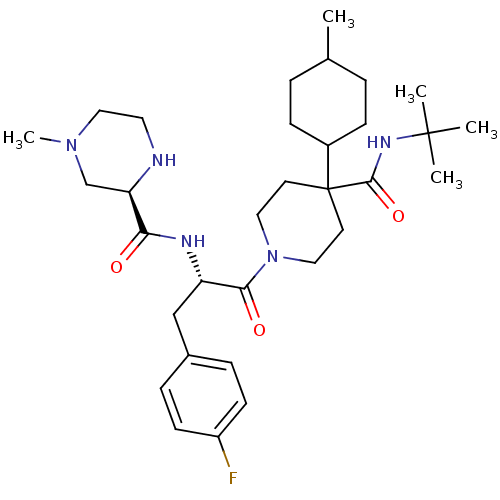
((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-(4-methylc...)Show SMILES CC1CCC(CC1)C1(CCN(CC1)C(=O)[C@H](Cc1ccc(F)cc1)NC(=O)[C@H]1CN(C)CCN1)C(=O)NC(C)(C)C |wU:27.29,wD:15.26,(5.43,-14.96,;4.11,-15.74,;4.11,-17.28,;2.79,-18.06,;1.45,-17.3,;1.43,-15.76,;2.77,-14.98,;.13,-18.08,;1.46,-18.85,;1.46,-20.39,;.12,-21.15,;-1.21,-20.4,;-1.2,-18.85,;.13,-22.69,;1.46,-23.46,;-1.2,-23.47,;-1.2,-25.01,;.14,-25.77,;.13,-27.31,;1.47,-28.08,;2.8,-27.3,;4.14,-28.07,;2.79,-25.75,;1.45,-25,;-2.54,-22.7,;-3.87,-23.47,;-3.87,-25.01,;-5.21,-22.71,;-6.55,-23.49,;-7.89,-22.72,;-9.22,-23.49,;-7.9,-21.16,;-6.56,-20.38,;-5.21,-21.15,;-1.2,-17.31,;-2.54,-18.08,;-1.2,-15.77,;-2.54,-15,;-1.78,-13.66,;-3.32,-16.33,;-3.88,-14.24,)| Show InChI InChI=1S/C32H50FN5O3/c1-22-6-10-24(11-7-22)32(30(41)36-31(2,3)4)14-17-38(18-15-32)29(40)26(20-23-8-12-25(33)13-9-23)35-28(39)27-21-37(5)19-16-34-27/h8-9,12-13,22,24,26-27,34H,6-7,10-11,14-21H2,1-5H3,(H,35,39)(H,36,41)/t22?,24?,26-,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220693
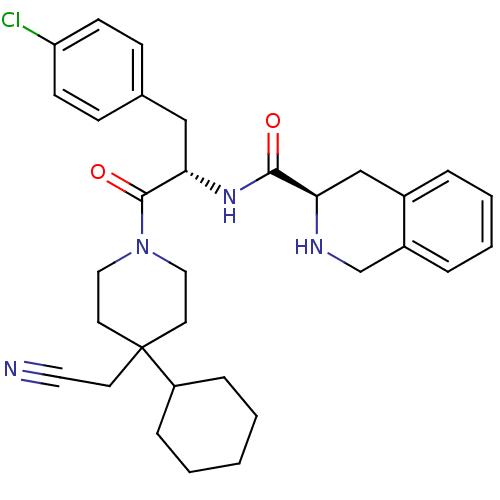
((R)-N-((S)-3-(4-chlorophenyl)-1-(4-(cyanomethyl)-4...)Show SMILES Clc1ccc(C[C@H](NC(=O)[C@H]2Cc3ccccc3CN2)C(=O)N2CCC(CC#N)(CC2)C2CCCCC2)cc1 Show InChI InChI=1S/C32H39ClN4O2/c33-27-12-10-23(11-13-27)20-29(36-30(38)28-21-24-6-4-5-7-25(24)22-35-28)31(39)37-18-15-32(14-17-34,16-19-37)26-8-2-1-3-9-26/h4-7,10-13,26,28-29,35H,1-3,8-9,14-16,18-22H2,(H,36,38)/t28-,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220710
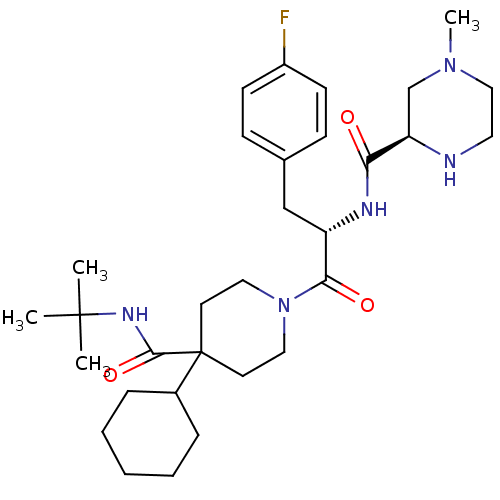
((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-cyclohexyl...)Show SMILES CN1CCN[C@H](C1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C1CCCCC1)C(=O)NC(C)(C)C Show InChI InChI=1S/C31H48FN5O3/c1-30(2,3)35-29(40)31(23-8-6-5-7-9-23)14-17-37(18-15-31)28(39)25(20-22-10-12-24(32)13-11-22)34-27(38)26-21-36(4)19-16-33-26/h10-13,23,25-26,33H,5-9,14-21H2,1-4H3,(H,34,38)(H,35,40)/t25-,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220711
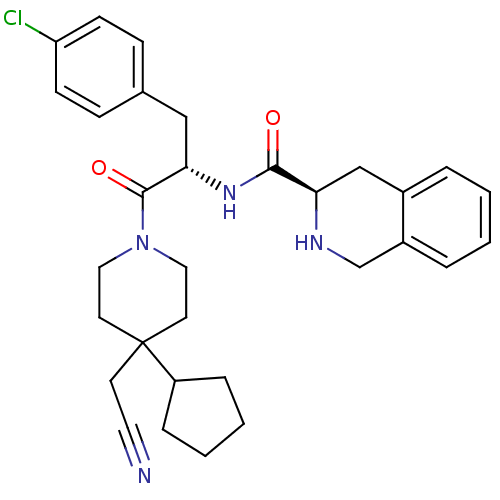
((R)-N-((S)-3-(4-chlorophenyl)-1-(4-(cyanomethyl)-4...)Show SMILES Clc1ccc(C[C@H](NC(=O)[C@H]2Cc3ccccc3CN2)C(=O)N2CCC(CC#N)(CC2)C2CCCC2)cc1 Show InChI InChI=1S/C31H37ClN4O2/c32-26-11-9-22(10-12-26)19-28(35-29(37)27-20-23-5-1-2-6-24(23)21-34-27)30(38)36-17-14-31(13-16-33,15-18-36)25-7-3-4-8-25/h1-2,5-6,9-12,25,27-28,34H,3-4,7-8,13-15,17-21H2,(H,35,37)/t27-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220690
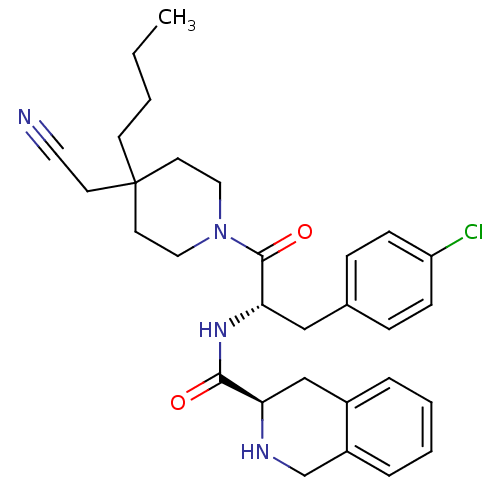
((R)-N-((S)-1-(4-butyl-4-(cyanomethyl)piperidin-1-y...)Show SMILES CCCCC1(CC#N)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C30H37ClN4O2/c1-2-3-12-30(13-16-32)14-17-35(18-15-30)29(37)27(19-22-8-10-25(31)11-9-22)34-28(36)26-20-23-6-4-5-7-24(23)21-33-26/h4-11,26-27,33H,2-3,12-15,17-21H2,1H3,(H,34,36)/t26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220702

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-((1-methyl-1H-t...)Show SMILES Cn1nnnc1CC1(CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1)C1CCOCC1 Show InChI InChI=1S/C32H40ClN7O3/c1-39-29(36-37-38-39)20-32(25-10-16-43-17-11-25)12-14-40(15-13-32)31(42)28(18-22-6-8-26(33)9-7-22)35-30(41)27-19-23-4-2-3-5-24(23)21-34-27/h2-9,25,27-28,34H,10-21H2,1H3,(H,35,41)/t27-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220705

((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-(1-methylc...)Show SMILES CN1CCN[C@H](C1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C(=O)NC(C)(C)C)C1(C)CCCCC1 Show InChI InChI=1S/C32H50FN5O3/c1-30(2,3)36-29(41)32(31(4)13-7-6-8-14-31)15-18-38(19-16-32)28(40)25(21-23-9-11-24(33)12-10-23)35-27(39)26-22-37(5)20-17-34-26/h9-12,25-26,34H,6-8,13-22H2,1-5H3,(H,35,39)(H,36,41)/t25-,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220706
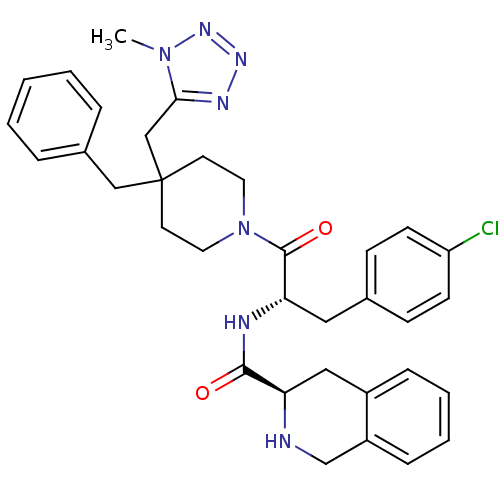
((R)-N-((S)-1-(4-benzyl-4-((1-methyl-1H-tetrazol-5-...)Show SMILES Cn1nnnc1CC1(Cc2ccccc2)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C34H38ClN7O2/c1-41-31(38-39-40-41)22-34(21-25-7-3-2-4-8-25)15-17-42(18-16-34)33(44)30(19-24-11-13-28(35)14-12-24)37-32(43)29-20-26-9-5-6-10-27(26)23-36-29/h2-14,29-30,36H,15-23H2,1H3,(H,37,43)/t29-,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220698

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-(cyanomethyl)-4...)Show SMILES CC(C)C1(CC#N)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C29H35ClN4O2/c1-20(2)29(11-14-31)12-15-34(16-13-29)28(36)26(17-21-7-9-24(30)10-8-21)33-27(35)25-18-22-5-3-4-6-23(22)19-32-25/h3-10,20,25-26,32H,11-13,15-19H2,1-2H3,(H,33,35)/t25-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220697

((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-(cyclohex-...)Show SMILES CN1CCN[C@H](C1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C1CCCC=C1)C(=O)NC(C)(C)C |w:27.29,c:34| Show InChI InChI=1S/C31H46FN5O3/c1-30(2,3)35-29(40)31(23-8-6-5-7-9-23)14-17-37(18-15-31)28(39)25(20-22-10-12-24(32)13-11-22)34-27(38)26-21-36(4)19-16-33-26/h6,8,10-13,23,25-26,33H,5,7,9,14-21H2,1-4H3,(H,34,38)(H,35,40)/t23?,25-,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220701

((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-tert-penty...)Show SMILES CCC(C)(C)C1(CCN(CC1)C(=O)[C@H](Cc1ccc(F)cc1)NC(=O)[C@H]1CN(C)CCN1)C(=O)NC(C)(C)C Show InChI InChI=1S/C30H48FN5O3/c1-8-29(5,6)30(27(39)34-28(2,3)4)13-16-36(17-14-30)26(38)23(19-21-9-11-22(31)12-10-21)33-25(37)24-20-35(7)18-15-32-24/h9-12,23-24,32H,8,13-20H2,1-7H3,(H,33,37)(H,34,39)/t23-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220694

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-(4-chlorophenyl...)Show SMILES Cn1nnnc1CC1(CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1)c1ccc(Cl)cc1 Show InChI InChI=1S/C33H35Cl2N7O2/c1-41-30(38-39-40-41)20-33(25-8-12-27(35)13-9-25)14-16-42(17-15-33)32(44)29(18-22-6-10-26(34)11-7-22)37-31(43)28-19-23-4-2-3-5-24(23)21-36-28/h2-13,28-29,36H,14-21H2,1H3,(H,37,43)/t28-,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220688
((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-(4,4-dimet...)Show SMILES CN1CCN[C@H](C1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C1CCC(C)(C)CC1)C(=O)NC(C)(C)C Show InChI InChI=1S/C33H52FN5O3/c1-31(2,3)37-30(42)33(24-11-13-32(4,5)14-12-24)15-18-39(19-16-33)29(41)26(21-23-7-9-25(34)10-8-23)36-28(40)27-22-38(6)20-17-35-27/h7-10,24,26-27,35H,11-22H2,1-6H3,(H,36,40)(H,37,42)/t26-,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220708

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-(cyanomethyl)-4...)Show SMILES Clc1ccc(C[C@H](NC(=O)[C@H]2Cc3ccccc3CN2)C(=O)N2CCC(CC#N)(CC2)C2CC2)cc1 Show InChI InChI=1S/C29H33ClN4O2/c30-24-9-5-20(6-10-24)17-26(33-27(35)25-18-21-3-1-2-4-22(21)19-32-25)28(36)34-15-12-29(11-14-31,13-16-34)23-7-8-23/h1-6,9-10,23,25-26,32H,7-8,11-13,15-19H2,(H,33,35)/t25-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220695

((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-(cyclobuty...)Show SMILES CN1CCN[C@H](C1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC2CCC2)(CC1)C(=O)NC(C)(C)C Show InChI InChI=1S/C30H46FN5O3/c1-29(2,3)34-28(39)30(19-22-6-5-7-22)12-15-36(16-13-30)27(38)24(18-21-8-10-23(31)11-9-21)33-26(37)25-20-35(4)17-14-32-25/h8-11,22,24-25,32H,5-7,12-20H2,1-4H3,(H,33,37)(H,34,39)/t24-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 177 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220707

((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-(4-oxocycl...)Show SMILES CN1CCN[C@H](C1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C1CCC(=O)C=C1)C(=O)NC(C)(C)C |c:35| Show InChI InChI=1S/C31H44FN5O4/c1-30(2,3)35-29(41)31(22-7-11-24(38)12-8-22)13-16-37(17-14-31)28(40)25(19-21-5-9-23(32)10-6-21)34-27(39)26-20-36(4)18-15-33-26/h5-7,9-11,22,25-26,33H,8,12-20H2,1-4H3,(H,34,39)(H,35,41)/t22?,25-,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 343 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220704

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-(cyanomethyl)-4...)Show SMILES CCC1(CC#N)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C28H33ClN4O2/c1-2-28(11-14-30)12-15-33(16-13-28)27(35)25(17-20-7-9-23(29)10-8-20)32-26(34)24-18-21-5-3-4-6-22(21)19-31-24/h3-10,24-25,31H,2,11-13,15-19H2,1H3,(H,32,34)/t24-,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 759 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220692

((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-(cycloprop...)Show SMILES CN1CCN[C@H](C1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC2CC2)(CC1)C(=O)NC(C)(C)C Show InChI InChI=1S/C29H44FN5O3/c1-28(2,3)33-27(38)29(18-21-5-6-21)11-14-35(15-12-29)26(37)23(17-20-7-9-22(30)10-8-20)32-25(36)24-19-34(4)16-13-31-24/h7-10,21,23-24,31H,5-6,11-19H2,1-4H3,(H,32,36)(H,33,38)/t23-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220709
((R)-N-((S)-3-(4-chlorophenyl)-1-(4-(cyanomethyl)-4...)Show SMILES CC1(CC#N)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C27H31ClN4O2/c1-27(10-13-29)11-14-32(15-12-27)26(34)24(16-19-6-8-22(28)9-7-19)31-25(33)23-17-20-4-2-3-5-21(20)18-30-23/h2-9,23-24,30H,10-12,14-18H2,1H3,(H,31,33)/t23-,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human MC4R |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50220706

((R)-N-((S)-1-(4-benzyl-4-((1-methyl-1H-tetrazol-5-...)Show SMILES Cn1nnnc1CC1(Cc2ccccc2)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C34H38ClN7O2/c1-41-31(38-39-40-41)22-34(21-25-7-3-2-4-8-25)15-17-42(18-16-34)33(44)30(19-24-11-13-28(35)14-12-24)37-32(43)29-20-26-9-5-6-10-27(26)23-36-29/h2-14,29-30,36H,15-23H2,1H3,(H,37,43)/t29-,30+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 840 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC3R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50220694

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-(4-chlorophenyl...)Show SMILES Cn1nnnc1CC1(CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1)c1ccc(Cl)cc1 Show InChI InChI=1S/C33H35Cl2N7O2/c1-41-30(38-39-40-41)20-33(25-8-12-27(35)13-9-25)14-16-42(17-15-33)32(44)29(18-22-6-10-26(34)11-7-22)37-31(43)28-19-23-4-2-3-5-24(23)21-36-28/h2-13,28-29,36H,14-21H2,1H3,(H,37,43)/t28-,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 434 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC3R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220691

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-isobutyl-4-((1-...)Show SMILES CC(C)CC1(Cc2nnnn2C)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C31H40ClN7O2/c1-21(2)18-31(19-28-35-36-37-38(28)3)12-14-39(15-13-31)30(41)27(16-22-8-10-25(32)11-9-22)34-29(40)26-17-23-6-4-5-7-24(23)20-33-26/h4-11,21,26-27,33H,12-20H2,1-3H3,(H,34,40)/t26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC4R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220711

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-(cyanomethyl)-4...)Show SMILES Clc1ccc(C[C@H](NC(=O)[C@H]2Cc3ccccc3CN2)C(=O)N2CCC(CC#N)(CC2)C2CCCC2)cc1 Show InChI InChI=1S/C31H37ClN4O2/c32-26-11-9-22(10-12-26)19-28(35-29(37)27-20-23-5-1-2-6-24(23)21-34-27)30(38)36-17-14-31(13-16-33,15-18-36)25-7-3-4-8-25/h1-2,5-6,9-12,25,27-28,34H,3-4,7-8,13-15,17-21H2,(H,35,37)/t27-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 196 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC4R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220703

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-cyclohexyl-4-((...)Show SMILES Cn1nnnc1CC1(CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1)C1CCCCC1 Show InChI InChI=1S/C33H42ClN7O2/c1-40-30(37-38-39-40)21-33(26-9-3-2-4-10-26)15-17-41(18-16-33)32(43)29(19-23-11-13-27(34)14-12-23)36-31(42)28-20-24-7-5-6-8-25(24)22-35-28/h5-8,11-14,26,28-29,35H,2-4,9-10,15-22H2,1H3,(H,36,42)/t28-,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC4R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50220688

((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-(4,4-dimet...)Show SMILES CN1CCN[C@H](C1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C1CCC(C)(C)CC1)C(=O)NC(C)(C)C Show InChI InChI=1S/C33H52FN5O3/c1-31(2,3)37-30(42)33(24-11-13-32(4,5)14-12-24)15-18-39(19-16-33)29(41)26(21-23-7-9-25(34)10-8-23)36-28(40)27-22-38(6)20-17-35-27/h7-10,24,26-27,35H,11-22H2,1-6H3,(H,36,40)(H,37,42)/t26-,27+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC5R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50220702

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-((1-methyl-1H-t...)Show SMILES Cn1nnnc1CC1(CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1)C1CCOCC1 Show InChI InChI=1S/C32H40ClN7O3/c1-39-29(36-37-38-39)20-32(25-10-16-43-17-11-25)12-14-40(15-13-32)31(42)28(18-22-6-8-26(33)9-7-22)35-30(41)27-19-23-4-2-3-5-24(23)21-34-27/h2-9,25,27-28,34H,10-21H2,1H3,(H,35,41)/t27-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC5R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50220693

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-(cyanomethyl)-4...)Show SMILES Clc1ccc(C[C@H](NC(=O)[C@H]2Cc3ccccc3CN2)C(=O)N2CCC(CC#N)(CC2)C2CCCCC2)cc1 Show InChI InChI=1S/C32H39ClN4O2/c33-27-12-10-23(11-13-27)20-29(36-30(38)28-21-24-6-4-5-7-25(24)22-35-28)31(39)37-18-15-32(14-17-34,16-19-37)26-8-2-1-3-9-26/h4-7,10-13,26,28-29,35H,1-3,8-9,14-16,18-22H2,(H,36,38)/t28-,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 640 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC5R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220707

((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-(4-oxocycl...)Show SMILES CN1CCN[C@H](C1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C1CCC(=O)C=C1)C(=O)NC(C)(C)C |c:35| Show InChI InChI=1S/C31H44FN5O4/c1-30(2,3)35-29(41)31(22-7-11-24(38)12-8-22)13-16-37(17-14-31)28(40)25(19-21-5-9-23(32)10-6-21)34-27(39)26-20-36(4)18-15-33-26/h5-7,9-11,22,25-26,33H,8,12-20H2,1-4H3,(H,34,39)(H,35,41)/t22?,25-,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC4R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50220691

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-isobutyl-4-((1-...)Show SMILES CC(C)CC1(Cc2nnnn2C)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C31H40ClN7O2/c1-21(2)18-31(19-28-35-36-37-38(28)3)12-14-39(15-13-31)30(41)27(16-22-8-10-25(32)11-9-22)34-29(40)26-17-23-6-4-5-7-24(23)20-33-26/h4-11,21,26-27,33H,12-20H2,1-3H3,(H,34,40)/t26-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 662 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC5R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50220699

((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-(4-methylc...)Show SMILES CC1CCC(CC1)C1(CCN(CC1)C(=O)[C@H](Cc1ccc(F)cc1)NC(=O)[C@H]1CN(C)CCN1)C(=O)NC(C)(C)C |wU:27.29,wD:15.26,(5.43,-14.96,;4.11,-15.74,;4.11,-17.28,;2.79,-18.06,;1.45,-17.3,;1.43,-15.76,;2.77,-14.98,;.13,-18.08,;1.46,-18.85,;1.46,-20.39,;.12,-21.15,;-1.21,-20.4,;-1.2,-18.85,;.13,-22.69,;1.46,-23.46,;-1.2,-23.47,;-1.2,-25.01,;.14,-25.77,;.13,-27.31,;1.47,-28.08,;2.8,-27.3,;4.14,-28.07,;2.79,-25.75,;1.45,-25,;-2.54,-22.7,;-3.87,-23.47,;-3.87,-25.01,;-5.21,-22.71,;-6.55,-23.49,;-7.89,-22.72,;-9.22,-23.49,;-7.9,-21.16,;-6.56,-20.38,;-5.21,-21.15,;-1.2,-17.31,;-2.54,-18.08,;-1.2,-15.77,;-2.54,-15,;-1.78,-13.66,;-3.32,-16.33,;-3.88,-14.24,)| Show InChI InChI=1S/C32H50FN5O3/c1-22-6-10-24(11-7-22)32(30(41)36-31(2,3)4)14-17-38(18-15-32)29(40)26(20-23-8-12-25(33)13-9-23)35-28(39)27-21-37(5)19-16-34-27/h8-9,12-13,22,24,26-27,34H,6-7,10-11,14-21H2,1-5H3,(H,35,39)(H,36,41)/t22?,24?,26-,27+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 888 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC5R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50220700

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-hexyl-4-((1-met...)Show SMILES CCCCCCC1(Cc2nnnn2C)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C33H44ClN7O2/c1-3-4-5-8-15-33(22-30-37-38-39-40(30)2)16-18-41(19-17-33)32(43)29(20-24-11-13-27(34)14-12-24)36-31(42)28-21-25-9-6-7-10-26(25)23-35-28/h6-7,9-14,28-29,35H,3-5,8,15-23H2,1-2H3,(H,36,42)/t28-,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 684 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC5R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220690

((R)-N-((S)-1-(4-butyl-4-(cyanomethyl)piperidin-1-y...)Show SMILES CCCCC1(CC#N)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C30H37ClN4O2/c1-2-3-12-30(13-16-32)14-17-35(18-15-30)29(37)27(19-22-8-10-25(31)11-9-22)34-28(36)26-20-23-6-4-5-7-24(23)21-33-26/h4-11,26-27,33H,2-3,12-15,17-21H2,1H3,(H,34,36)/t26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 281 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC4R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50220706

((R)-N-((S)-1-(4-benzyl-4-((1-methyl-1H-tetrazol-5-...)Show SMILES Cn1nnnc1CC1(Cc2ccccc2)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C34H38ClN7O2/c1-41-31(38-39-40-41)22-34(21-25-7-3-2-4-8-25)15-17-42(18-16-34)33(44)30(19-24-11-13-28(35)14-12-24)37-32(43)29-20-26-9-5-6-10-27(26)23-36-29/h2-14,29-30,36H,15-23H2,1H3,(H,37,43)/t29-,30+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC5R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220699

((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-(4-methylc...)Show SMILES CC1CCC(CC1)C1(CCN(CC1)C(=O)[C@H](Cc1ccc(F)cc1)NC(=O)[C@H]1CN(C)CCN1)C(=O)NC(C)(C)C |wU:27.29,wD:15.26,(5.43,-14.96,;4.11,-15.74,;4.11,-17.28,;2.79,-18.06,;1.45,-17.3,;1.43,-15.76,;2.77,-14.98,;.13,-18.08,;1.46,-18.85,;1.46,-20.39,;.12,-21.15,;-1.21,-20.4,;-1.2,-18.85,;.13,-22.69,;1.46,-23.46,;-1.2,-23.47,;-1.2,-25.01,;.14,-25.77,;.13,-27.31,;1.47,-28.08,;2.8,-27.3,;4.14,-28.07,;2.79,-25.75,;1.45,-25,;-2.54,-22.7,;-3.87,-23.47,;-3.87,-25.01,;-5.21,-22.71,;-6.55,-23.49,;-7.89,-22.72,;-9.22,-23.49,;-7.9,-21.16,;-6.56,-20.38,;-5.21,-21.15,;-1.2,-17.31,;-2.54,-18.08,;-1.2,-15.77,;-2.54,-15,;-1.78,-13.66,;-3.32,-16.33,;-3.88,-14.24,)| Show InChI InChI=1S/C32H50FN5O3/c1-22-6-10-24(11-7-22)32(30(41)36-31(2,3)4)14-17-38(18-15-32)29(40)26(20-23-8-12-25(33)13-9-23)35-28(39)27-21-37(5)19-16-34-27/h8-9,12-13,22,24,26-27,34H,6-7,10-11,14-21H2,1-5H3,(H,35,39)(H,36,41)/t22?,24?,26-,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC4R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50220691

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-isobutyl-4-((1-...)Show SMILES CC(C)CC1(Cc2nnnn2C)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C31H40ClN7O2/c1-21(2)18-31(19-28-35-36-37-38(28)3)12-14-39(15-13-31)30(41)27(16-22-8-10-25(32)11-9-22)34-29(40)26-17-23-6-4-5-7-24(23)20-33-26/h4-11,21,26-27,33H,12-20H2,1-3H3,(H,34,40)/t26-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 680 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC3R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50220689

((R)-N-((S)-1-(4-sec-butyl-4-((1-methyl-1H-tetrazol...)Show SMILES CCC(C)C1(Cc2nnnn2C)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 |w:2.2| Show InChI InChI=1S/C31H40ClN7O2/c1-4-21(2)31(19-28-35-36-37-38(28)3)13-15-39(16-14-31)30(41)27(17-22-9-11-25(32)12-10-22)34-29(40)26-18-23-7-5-6-8-24(23)20-33-26/h5-12,21,26-27,33H,4,13-20H2,1-3H3,(H,34,40)/t21?,26-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC5R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50220694

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-(4-chlorophenyl...)Show SMILES Cn1nnnc1CC1(CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1)c1ccc(Cl)cc1 Show InChI InChI=1S/C33H35Cl2N7O2/c1-41-30(38-39-40-41)20-33(25-8-12-27(35)13-9-25)14-16-42(17-15-33)32(44)29(18-22-6-10-26(34)11-7-22)37-31(43)28-19-23-4-2-3-5-24(23)21-36-28/h2-13,28-29,36H,14-21H2,1H3,(H,37,43)/t28-,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 527 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC5R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220688

((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-(4,4-dimet...)Show SMILES CN1CCN[C@H](C1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C1CCC(C)(C)CC1)C(=O)NC(C)(C)C Show InChI InChI=1S/C33H52FN5O3/c1-31(2,3)37-30(42)33(24-11-13-32(4,5)14-12-24)15-18-39(19-16-33)29(41)26(21-23-7-9-25(34)10-8-23)36-28(40)27-22-38(6)20-17-35-27/h7-10,24,26-27,35H,11-22H2,1-6H3,(H,36,40)(H,37,42)/t26-,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 546 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC4R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50220698

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-(cyanomethyl)-4...)Show SMILES CC(C)C1(CC#N)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C29H35ClN4O2/c1-20(2)29(11-14-31)12-15-34(16-13-29)28(36)26(17-21-7-9-24(30)10-8-21)33-27(35)25-18-22-5-3-4-6-23(22)19-32-25/h3-10,20,25-26,32H,11-13,15-19H2,1-2H3,(H,33,35)/t25-,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC5R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50220711

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-(cyanomethyl)-4...)Show SMILES Clc1ccc(C[C@H](NC(=O)[C@H]2Cc3ccccc3CN2)C(=O)N2CCC(CC#N)(CC2)C2CCCC2)cc1 Show InChI InChI=1S/C31H37ClN4O2/c32-26-11-9-22(10-12-26)19-28(35-29(37)27-20-23-5-1-2-6-24(23)21-34-27)30(38)36-17-14-31(13-16-33,15-18-36)25-7-3-4-8-25/h1-2,5-6,9-12,25,27-28,34H,3-4,7-8,13-15,17-21H2,(H,35,37)/t27-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 630 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC5R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50220689

((R)-N-((S)-1-(4-sec-butyl-4-((1-methyl-1H-tetrazol...)Show SMILES CCC(C)C1(Cc2nnnn2C)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 |w:2.2| Show InChI InChI=1S/C31H40ClN7O2/c1-4-21(2)31(19-28-35-36-37-38(28)3)13-15-39(16-14-31)30(41)27(17-22-9-11-25(32)12-10-22)34-29(40)26-18-23-7-5-6-8-24(23)20-33-26/h5-12,21,26-27,33H,4,13-20H2,1-3H3,(H,34,40)/t21?,26-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 263 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC3R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220706

((R)-N-((S)-1-(4-benzyl-4-((1-methyl-1H-tetrazol-5-...)Show SMILES Cn1nnnc1CC1(Cc2ccccc2)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C34H38ClN7O2/c1-41-31(38-39-40-41)22-34(21-25-7-3-2-4-8-25)15-17-42(18-16-34)33(44)30(19-24-11-13-28(35)14-12-24)37-32(43)29-20-26-9-5-6-10-27(26)23-36-29/h2-14,29-30,36H,15-23H2,1H3,(H,37,43)/t29-,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 111 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC4R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50220702

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-((1-methyl-1H-t...)Show SMILES Cn1nnnc1CC1(CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1)C1CCOCC1 Show InChI InChI=1S/C32H40ClN7O3/c1-39-29(36-37-38-39)20-32(25-10-16-43-17-11-25)12-14-40(15-13-32)31(42)28(18-22-6-8-26(33)9-7-22)35-30(41)27-19-23-4-2-3-5-24(23)21-34-27/h2-9,25,27-28,34H,10-21H2,1H3,(H,35,41)/t27-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC3R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220697

((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-(cyclohex-...)Show SMILES CN1CCN[C@H](C1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C1CCCC=C1)C(=O)NC(C)(C)C |w:27.29,c:34| Show InChI InChI=1S/C31H46FN5O3/c1-30(2,3)35-29(40)31(23-8-6-5-7-9-23)14-17-37(18-15-31)28(39)25(20-22-10-12-24(32)13-11-22)34-27(38)26-21-36(4)19-16-33-26/h6,8,10-13,23,25-26,33H,5,7,9,14-21H2,1-4H3,(H,34,38)(H,35,40)/t23?,25-,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 85 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC4R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220702

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-((1-methyl-1H-t...)Show SMILES Cn1nnnc1CC1(CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1)C1CCOCC1 Show InChI InChI=1S/C32H40ClN7O3/c1-39-29(36-37-38-39)20-32(25-10-16-43-17-11-25)12-14-40(15-13-32)31(42)28(18-22-6-8-26(33)9-7-22)35-30(41)27-19-23-4-2-3-5-24(23)21-34-27/h2-9,25,27-28,34H,10-21H2,1H3,(H,35,41)/t27-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 157 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC4R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220705

((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-(1-methylc...)Show SMILES CN1CCN[C@H](C1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C(=O)NC(C)(C)C)C1(C)CCCCC1 Show InChI InChI=1S/C32H50FN5O3/c1-30(2,3)36-29(41)32(31(4)13-7-6-8-14-31)15-18-38(19-16-32)28(40)25(21-23-9-11-24(33)12-10-23)35-27(39)26-22-37(5)20-17-34-26/h9-12,25-26,34H,6-8,13-22H2,1-5H3,(H,35,39)(H,36,41)/t25-,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 58 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC4R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50220699

((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-(4-methylc...)Show SMILES CC1CCC(CC1)C1(CCN(CC1)C(=O)[C@H](Cc1ccc(F)cc1)NC(=O)[C@H]1CN(C)CCN1)C(=O)NC(C)(C)C |wU:27.29,wD:15.26,(5.43,-14.96,;4.11,-15.74,;4.11,-17.28,;2.79,-18.06,;1.45,-17.3,;1.43,-15.76,;2.77,-14.98,;.13,-18.08,;1.46,-18.85,;1.46,-20.39,;.12,-21.15,;-1.21,-20.4,;-1.2,-18.85,;.13,-22.69,;1.46,-23.46,;-1.2,-23.47,;-1.2,-25.01,;.14,-25.77,;.13,-27.31,;1.47,-28.08,;2.8,-27.3,;4.14,-28.07,;2.79,-25.75,;1.45,-25,;-2.54,-22.7,;-3.87,-23.47,;-3.87,-25.01,;-5.21,-22.71,;-6.55,-23.49,;-7.89,-22.72,;-9.22,-23.49,;-7.9,-21.16,;-6.56,-20.38,;-5.21,-21.15,;-1.2,-17.31,;-2.54,-18.08,;-1.2,-15.77,;-2.54,-15,;-1.78,-13.66,;-3.32,-16.33,;-3.88,-14.24,)| Show InChI InChI=1S/C32H50FN5O3/c1-22-6-10-24(11-7-22)32(30(41)36-31(2,3)4)14-17-38(18-15-32)29(40)26(20-23-8-12-25(33)13-9-23)35-28(39)27-21-37(5)19-16-34-27/h8-9,12-13,22,24,26-27,34H,6-7,10-11,14-21H2,1-5H3,(H,35,39)(H,36,41)/t22?,24?,26-,27+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC3R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50220688

((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-(4,4-dimet...)Show SMILES CN1CCN[C@H](C1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C1CCC(C)(C)CC1)C(=O)NC(C)(C)C Show InChI InChI=1S/C33H52FN5O3/c1-31(2,3)37-30(42)33(24-11-13-32(4,5)14-12-24)15-18-39(19-16-33)29(41)26(21-23-7-9-25(34)10-8-23)36-28(40)27-22-38(6)20-17-35-27/h7-10,24,26-27,35H,11-22H2,1-6H3,(H,36,40)(H,37,42)/t26-,27+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 241 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC3R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220710

((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-cyclohexyl...)Show SMILES CN1CCN[C@H](C1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C1CCCCC1)C(=O)NC(C)(C)C Show InChI InChI=1S/C31H48FN5O3/c1-30(2,3)35-29(40)31(23-8-6-5-7-9-23)14-17-37(18-15-31)28(39)25(20-22-10-12-24(32)13-11-22)34-27(38)26-21-36(4)19-16-33-26/h10-13,23,25-26,33H,5-9,14-21H2,1-4H3,(H,34,38)(H,35,40)/t25-,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC4R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50220703

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-cyclohexyl-4-((...)Show SMILES Cn1nnnc1CC1(CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1)C1CCCCC1 Show InChI InChI=1S/C33H42ClN7O2/c1-40-30(37-38-39-40)21-33(26-9-3-2-4-10-26)15-17-41(18-16-33)32(43)29(19-23-11-13-27(34)14-12-23)36-31(42)28-20-24-7-5-6-8-25(24)22-35-28/h5-8,11-14,26,28-29,35H,2-4,9-10,15-22H2,1H3,(H,36,42)/t28-,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 222 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC5R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220696

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-((1-methyl-1H-t...)Show SMILES Cn1nnnc1CC1(CC(C)(C)C)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C32H42ClN7O2/c1-31(2,3)21-32(19-28-36-37-38-39(28)4)13-15-40(16-14-32)30(42)27(17-22-9-11-25(33)12-10-22)35-29(41)26-18-23-7-5-6-8-24(23)20-34-26/h5-12,26-27,34H,13-21H2,1-4H3,(H,35,41)/t26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC4R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220693

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-(cyanomethyl)-4...)Show SMILES Clc1ccc(C[C@H](NC(=O)[C@H]2Cc3ccccc3CN2)C(=O)N2CCC(CC#N)(CC2)C2CCCCC2)cc1 Show InChI InChI=1S/C32H39ClN4O2/c33-27-12-10-23(11-13-27)20-29(36-30(38)28-21-24-6-4-5-7-25(24)22-35-28)31(39)37-18-15-32(14-17-34,16-19-37)26-8-2-1-3-9-26/h4-7,10-13,26,28-29,35H,1-3,8-9,14-16,18-22H2,(H,36,38)/t28-,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 189 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC4R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220692

((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-(cycloprop...)Show SMILES CN1CCN[C@H](C1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC2CC2)(CC1)C(=O)NC(C)(C)C Show InChI InChI=1S/C29H44FN5O3/c1-28(2,3)33-27(38)29(18-21-5-6-21)11-14-35(15-12-29)26(37)23(17-20-7-9-22(30)10-8-20)32-25(36)24-19-34(4)16-13-31-24/h7-10,21,23-24,31H,5-6,11-19H2,1-4H3,(H,32,36)(H,33,38)/t23-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.17E+3 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC4R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50220696

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-((1-methyl-1H-t...)Show SMILES Cn1nnnc1CC1(CC(C)(C)C)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C32H42ClN7O2/c1-31(2,3)21-32(19-28-36-37-38-39(28)4)13-15-40(16-14-32)30(42)27(17-22-9-11-25(33)12-10-22)35-29(41)26-18-23-7-5-6-8-24(23)20-34-26/h5-12,26-27,34H,13-21H2,1-4H3,(H,35,41)/t26-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 380 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC5R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220694

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-(4-chlorophenyl...)Show SMILES Cn1nnnc1CC1(CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1)c1ccc(Cl)cc1 Show InChI InChI=1S/C33H35Cl2N7O2/c1-41-30(38-39-40-41)20-33(25-8-12-27(35)13-9-25)14-16-42(17-15-33)32(44)29(18-22-6-10-26(34)11-7-22)37-31(43)28-19-23-4-2-3-5-24(23)21-36-28/h2-13,28-29,36H,14-21H2,1H3,(H,37,43)/t28-,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC4R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220689

((R)-N-((S)-1-(4-sec-butyl-4-((1-methyl-1H-tetrazol...)Show SMILES CCC(C)C1(Cc2nnnn2C)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 |w:2.2| Show InChI InChI=1S/C31H40ClN7O2/c1-4-21(2)31(19-28-35-36-37-38(28)3)13-15-39(16-14-31)30(41)27(17-22-9-11-25(32)12-10-22)34-29(40)26-18-23-7-5-6-8-24(23)20-33-26/h5-12,21,26-27,33H,4,13-20H2,1-3H3,(H,34,40)/t21?,26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC4R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220695

((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-(cyclobuty...)Show SMILES CN1CCN[C@H](C1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC2CCC2)(CC1)C(=O)NC(C)(C)C Show InChI InChI=1S/C30H46FN5O3/c1-29(2,3)34-28(39)30(19-22-6-5-7-22)12-15-36(16-13-30)27(38)24(18-21-8-10-23(31)11-9-21)33-26(37)25-20-35(4)17-14-32-25/h8-11,22,24-25,32H,5-7,12-20H2,1-4H3,(H,33,37)(H,34,39)/t24-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 563 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC4R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50220690

((R)-N-((S)-1-(4-butyl-4-(cyanomethyl)piperidin-1-y...)Show SMILES CCCCC1(CC#N)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C30H37ClN4O2/c1-2-3-12-30(13-16-32)14-17-35(18-15-30)29(37)27(19-22-8-10-25(31)11-9-22)34-28(36)26-20-23-6-4-5-7-24(23)21-33-26/h4-11,26-27,33H,2-3,12-15,17-21H2,1H3,(H,34,36)/t26-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 480 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC5R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50220705

((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-(1-methylc...)Show SMILES CN1CCN[C@H](C1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C(=O)NC(C)(C)C)C1(C)CCCCC1 Show InChI InChI=1S/C32H50FN5O3/c1-30(2,3)36-29(41)32(31(4)13-7-6-8-14-31)15-18-38(19-16-32)28(40)25(21-23-9-11-24(33)12-10-23)35-27(39)26-22-37(5)20-17-34-26/h9-12,25-26,34H,6-8,13-22H2,1-5H3,(H,35,39)(H,36,41)/t25-,26+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC5R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50220710

((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-cyclohexyl...)Show SMILES CN1CCN[C@H](C1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C1CCCCC1)C(=O)NC(C)(C)C Show InChI InChI=1S/C31H48FN5O3/c1-30(2,3)35-29(40)31(23-8-6-5-7-9-23)14-17-37(18-15-31)28(39)25(20-22-10-12-24(32)13-11-22)34-27(38)26-21-36(4)19-16-33-26/h10-13,23,25-26,33H,5-9,14-21H2,1-4H3,(H,34,38)(H,35,40)/t25-,26+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 977 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC5R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220700

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-hexyl-4-((1-met...)Show SMILES CCCCCCC1(Cc2nnnn2C)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C33H44ClN7O2/c1-3-4-5-8-15-33(22-30-37-38-39-40(30)2)16-18-41(19-17-33)32(43)29(20-24-11-13-27(34)14-12-24)36-31(42)28-21-25-9-6-7-10-26(25)23-35-28/h6-7,9-14,28-29,35H,3-5,8,15-23H2,1-2H3,(H,36,42)/t28-,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 29 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC4R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50220700

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-hexyl-4-((1-met...)Show SMILES CCCCCCC1(Cc2nnnn2C)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C33H44ClN7O2/c1-3-4-5-8-15-33(22-30-37-38-39-40(30)2)16-18-41(19-17-33)32(43)29(20-24-11-13-27(34)14-12-24)36-31(42)28-21-25-9-6-7-10-26(25)23-35-28/h6-7,9-14,28-29,35H,3-5,8,15-23H2,1-2H3,(H,36,42)/t28-,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC3R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50220703

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-cyclohexyl-4-((...)Show SMILES Cn1nnnc1CC1(CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1)C1CCCCC1 Show InChI InChI=1S/C33H42ClN7O2/c1-40-30(37-38-39-40)21-33(26-9-3-2-4-10-26)15-17-41(18-16-33)32(43)29(19-23-11-13-27(34)14-12-23)36-31(42)28-20-24-7-5-6-8-25(24)22-35-28/h5-8,11-14,26,28-29,35H,2-4,9-10,15-22H2,1H3,(H,36,42)/t28-,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 115 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC3R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220698

((R)-N-((S)-3-(4-chlorophenyl)-1-(4-(cyanomethyl)-4...)Show SMILES CC(C)C1(CC#N)CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C29H35ClN4O2/c1-20(2)29(11-14-31)12-15-34(16-13-29)28(36)26(17-21-7-9-24(30)10-8-21)33-27(35)25-18-22-5-3-4-6-23(22)19-32-25/h3-10,20,25-26,32H,11-13,15-19H2,1-2H3,(H,33,35)/t25-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 827 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC4R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50220701

((R)-N-((S)-1-(4-(tert-butylcarbamoyl)-4-tert-penty...)Show SMILES CCC(C)(C)C1(CCN(CC1)C(=O)[C@H](Cc1ccc(F)cc1)NC(=O)[C@H]1CN(C)CCN1)C(=O)NC(C)(C)C Show InChI InChI=1S/C30H48FN5O3/c1-8-29(5,6)30(27(39)34-28(2,3)4)13-16-36(17-14-30)26(38)23(19-21-9-11-22(31)12-10-21)33-25(37)24-20-35(7)18-15-32-24/h9-12,23-24,32H,8,13-20H2,1-7H3,(H,33,37)(H,34,39)/t23-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 151 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human MC4R assessed as stimulation of cAMP production |
Bioorg Med Chem Lett 17: 5720-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.084
BindingDB Entry DOI: 10.7270/Q2708141 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data