 Found 66 hits of Enzyme Inhibition Constant Data
Found 66 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221914
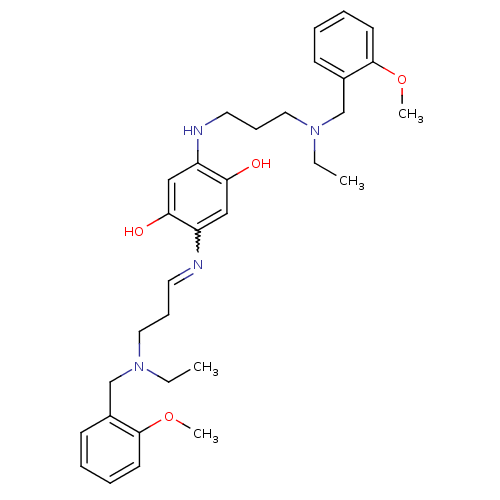
(2,5-bis{3-[ethyl(2-methoxybenzyl)amino]propylamino...)Show SMILES CCN(CCCNc1cc(O)c(cc1O)N=CCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |w:15.15| Show InChI InChI=1S/C32H44N4O4/c1-5-35(23-25-13-7-9-15-31(25)39-3)19-11-17-33-27-21-30(38)28(22-29(27)37)34-18-12-20-36(6-2)24-26-14-8-10-16-32(26)40-4/h7-10,13-17,21-22,34,37-38H,5-6,11-12,18-20,23-24H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221909

(2,5-bis{5-[ethyl(2-methoxybenzyl)amino]pentylamino...)Show SMILES CCN(CCCCCNc1cc(O)c(cc1O)N=CCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |w:17.17| Show InChI InChI=1S/C36H52N4O4/c1-5-39(27-29-17-9-11-19-35(29)43-3)23-15-7-13-21-37-31-25-34(42)32(26-33(31)41)38-22-14-8-16-24-40(6-2)28-30-18-10-12-20-36(30)44-4/h9-12,17-21,25-26,38,41-42H,5-8,13-16,22-24,27-28H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221918

(2,5-bis{6-[ethyl(2-methoxybenzyl)amino]hexylamino}...)Show SMILES CCN(CCCCCCNc1c(O)c(Br)c(N=CCCCCCN(CC)Cc2ccccc2OC)c(O)c1Br)Cc1ccccc1OC |w:16.15| Show InChI InChI=1S/C38H54Br2N4O4/c1-5-43(27-29-19-11-13-21-31(29)47-3)25-17-9-7-15-23-41-35-33(39)38(46)36(34(40)37(35)45)42-24-16-8-10-18-26-44(6-2)28-30-20-12-14-22-32(30)48-4/h11-14,19-23,42,45-46H,5-10,15-18,24-28H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221908

(2,5-bis{6-[ethyl(2-methoxybenzyl)amino]hexylamino}...)Show SMILES CCN(CCCCCCNc1c(O)c(Cl)c(N=CCCCCCN(CC)Cc2ccccc2OC)c(O)c1Cl)Cc1ccccc1OC |w:16.15| Show InChI InChI=1S/C38H54Cl2N4O4/c1-5-43(27-29-19-11-13-21-31(29)47-3)25-17-9-7-15-23-41-35-33(39)38(46)36(34(40)37(35)45)42-24-16-8-10-18-26-44(6-2)28-30-20-12-14-22-32(30)48-4/h11-14,19-23,42,45-46H,5-10,15-18,24-28H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221926
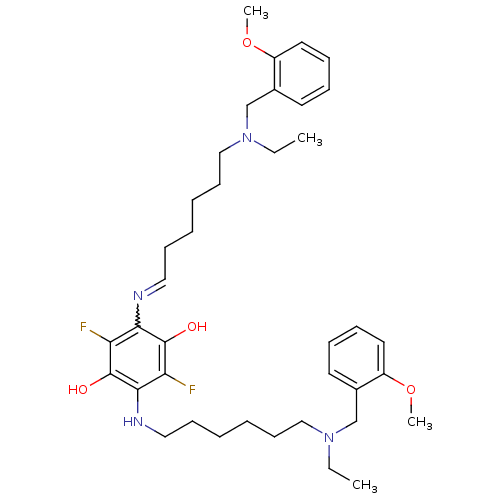
(2,5-bis{6-[ethyl(2-methoxybenzyl)amino]hexylamino}...)Show SMILES CCN(CCCCCCNc1c(O)c(F)c(N=CCCCCCN(CC)Cc2ccccc2OC)c(O)c1F)Cc1ccccc1OC |w:16.15| Show InChI InChI=1S/C38H54F2N4O4/c1-5-43(27-29-19-11-13-21-31(29)47-3)25-17-9-7-15-23-41-35-33(39)38(46)36(34(40)37(35)45)42-24-16-8-10-18-26-44(6-2)28-30-20-12-14-22-32(30)48-4/h11-14,19-23,42,45-46H,5-10,15-18,24-28H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221929
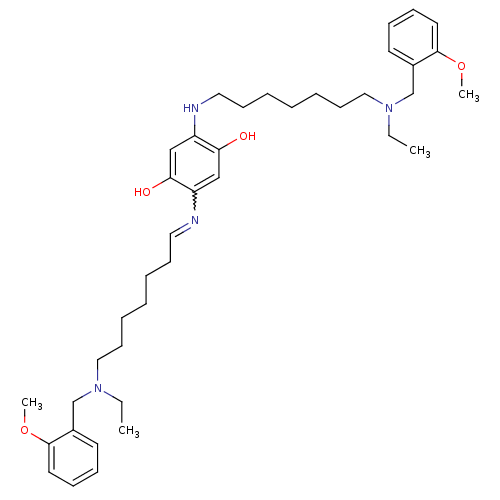
(2,5-bis{7-[ethyl(2-methoxybenzyl)amino]heptylamino...)Show SMILES CCN(CCCCCCCNc1cc(O)c(cc1O)N=CCCCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |w:19.19| Show InChI InChI=1S/C40H60N4O4/c1-5-43(31-33-21-13-15-23-39(33)47-3)27-19-11-7-9-17-25-41-35-29-38(46)36(30-37(35)45)42-26-18-10-8-12-20-28-44(6-2)32-34-22-14-16-24-40(34)48-4/h13-16,21-25,29-30,42,45-46H,5-12,17-20,26-28,31-32H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221922
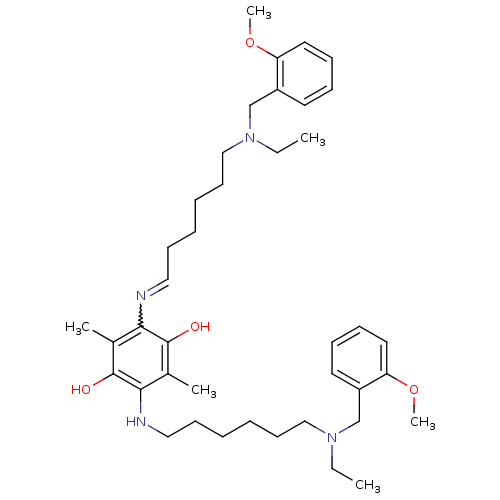
(2,5-bis{6-[ethyl(2-methoxybenzyl)amino]hexylamino}...)Show SMILES CCN(CCCCCCNc1c(C)c(O)c(N=CCCCCCN(CC)Cc2ccccc2OC)c(C)c1O)Cc1ccccc1OC |w:16.15| Show InChI InChI=1S/C40H60N4O4/c1-7-43(29-33-21-13-15-23-35(33)47-5)27-19-11-9-17-25-41-37-31(3)40(46)38(32(4)39(37)45)42-26-18-10-12-20-28-44(8-2)30-34-22-14-16-24-36(34)48-6/h13-16,21-25,42,45-46H,7-12,17-20,26-30H2,1-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221905
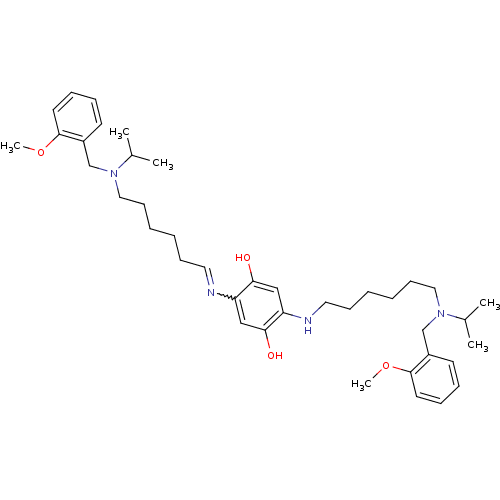
(2,5-bis{6-[isopropyl(2-methoxybenzyl)amino]hexylam...)Show SMILES COc1ccccc1CN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(Cc1ccccc1OC)C(C)C)C(C)C |w:25.26| Show InChI InChI=1S/C40H60N4O4/c1-31(2)43(29-33-19-11-13-21-39(33)47-5)25-17-9-7-15-23-41-35-27-38(46)36(28-37(35)45)42-24-16-8-10-18-26-44(32(3)4)30-34-20-12-14-22-40(34)48-6/h11-14,19-23,27-28,31-32,42,45-46H,7-10,15-18,24-26,29-30H2,1-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221921

(2,5-bis{4-[ethyl(2-methoxybenzyl)amino]butylamino}...)Show SMILES CCN(CCCCNc1cc(O)c(cc1O)N=CCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |w:16.16| Show InChI InChI=1S/C34H48N4O4/c1-5-37(25-27-15-7-9-17-33(27)41-3)21-13-11-19-35-29-23-32(40)30(24-31(29)39)36-20-12-14-22-38(6-2)26-28-16-8-10-18-34(28)42-4/h7-10,15-19,23-24,36,39-40H,5-6,11-14,20-22,25-26H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221910
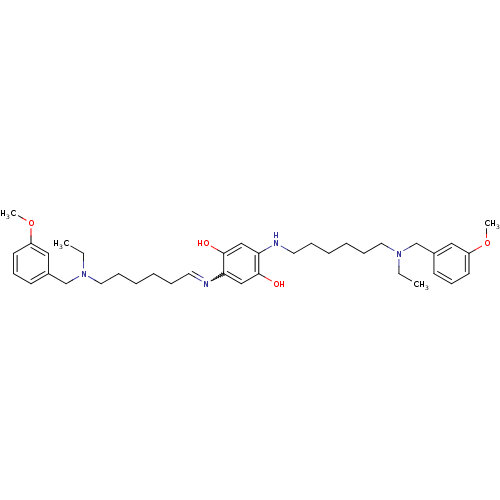
(2,5-bis{6-[ethyl(3-methoxybenzyl)amino]hexylamino}...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1cccc(OC)c1)Cc1cccc(OC)c1 |w:18.18| Show InChI InChI=1S/C38H56N4O4/c1-5-41(29-31-17-15-19-33(25-31)45-3)23-13-9-7-11-21-39-35-27-38(44)36(28-37(35)43)40-22-12-8-10-14-24-42(6-2)30-32-18-16-20-34(26-32)46-4/h15-21,25-28,40,43-44H,5-14,22-24,29-30H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221932
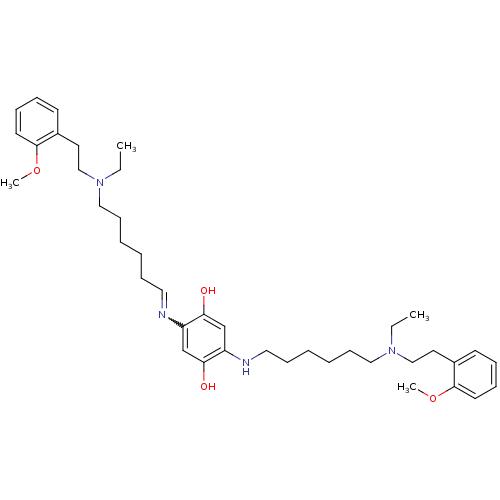
(2,5-bis(6-{ethyl[2-(2-methoxyphenyl)ethyl]amino}he...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)CCc1ccccc1OC)CCc1ccccc1OC |w:18.18| Show InChI InChI=1S/C40H60N4O4/c1-5-43(29-23-33-19-11-13-21-39(33)47-3)27-17-9-7-15-25-41-35-31-38(46)36(32-37(35)45)42-26-16-8-10-18-28-44(6-2)30-24-34-20-12-14-22-40(34)48-4/h11-14,19-22,25,31-32,42,45-46H,5-10,15-18,23-24,26-30H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM10620

((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...)Show InChI InChI=1S/C14H22N2O2/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4/h7-11H,6H2,1-5H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221920
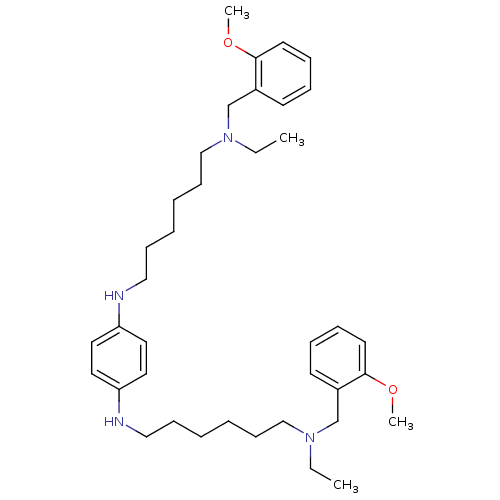
(CHEMBL230434 | N,N'-bis{6-[ethyl(2-methoxybenzyl)a...)Show SMILES CCN(CCCCCCNc1ccc(NCCCCCCN(CC)Cc2ccccc2OC)cc1)Cc1ccccc1OC Show InChI InChI=1S/C38H58N4O2/c1-5-41(31-33-19-11-13-21-37(33)43-3)29-17-9-7-15-27-39-35-23-25-36(26-24-35)40-28-16-8-10-18-30-42(6-2)32-34-20-12-14-22-38(34)44-4/h11-14,19-26,39-40H,5-10,15-18,27-32H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221912

(2,5-bis{6-[ethyl(4-methoxybenzyl)amino]hexylamino}...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1ccc(OC)cc1)Cc1ccc(OC)cc1 |w:18.18| Show InChI InChI=1S/C38H56N4O4/c1-5-41(29-31-15-19-33(45-3)20-16-31)25-13-9-7-11-23-39-35-27-38(44)36(28-37(35)43)40-24-12-8-10-14-26-42(6-2)30-32-17-21-34(46-4)22-18-32/h15-23,27-28,40,43-44H,5-14,24-26,29-30H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221930
(2,5-bis{6-[ethyl(thiophen-2-ylmethyl)amino]hexylam...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1cccs1)Cc1cccs1 |w:18.18| Show InChI InChI=1S/C32H48N4O2S2/c1-3-35(25-27-15-13-21-39-27)19-11-7-5-9-17-33-29-23-32(38)30(24-31(29)37)34-18-10-6-8-12-20-36(4-2)26-28-16-14-22-40-28/h13-17,21-24,34,37-38H,3-12,18-20,25-26H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221915
(2,5-bis{2-[ethyl(2-methoxybenzyl)amino]ethylamino}...)Show SMILES CCN(CCNc1cc(O)c(cc1O)N=CCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |w:14.14| Show InChI InChI=1S/C30H40N4O4/c1-5-33(21-23-11-7-9-13-29(23)37-3)17-15-31-25-19-28(36)26(20-27(25)35)32-16-18-34(6-2)22-24-12-8-10-14-30(24)38-4/h7-15,19-20,32,35-36H,5-6,16-18,21-22H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221928

(2,5-bis{6-[ethyl(2-methylbenzyl)amino]hexylamino}-...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1ccccc1C)Cc1ccccc1C |w:18.18| Show InChI InChI=1S/C38H56N4O2/c1-5-41(29-33-21-13-11-19-31(33)3)25-17-9-7-15-23-39-35-27-38(44)36(28-37(35)43)40-24-16-8-10-18-26-42(6-2)30-34-22-14-12-20-32(34)4/h11-14,19-23,27-28,40,43-44H,5-10,15-18,24-26,29-30H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221924
(2,5-bis[6-(benzylethylamino)hexylamino]-[1,4]-benz...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1ccccc1)Cc1ccccc1 |w:18.18| Show InChI InChI=1S/C36H52N4O2/c1-3-39(29-31-19-11-9-12-20-31)25-17-7-5-15-23-37-33-27-36(42)34(28-35(33)41)38-24-16-6-8-18-26-40(4-2)30-32-21-13-10-14-22-32/h9-14,19-23,27-28,38,41-42H,3-8,15-18,24-26,29-30H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221933
(2,5-bis{6-[(2-methoxybenzyl)methylamino]hexylamino...)Show SMILES COc1ccccc1CN(C)CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(C)Cc1ccccc1OC |w:26.27| Show InChI InChI=1S/C36H52N4O4/c1-39(27-29-17-9-11-19-35(29)43-3)23-15-7-5-13-21-37-31-25-34(42)32(26-33(31)41)38-22-14-6-8-16-24-40(2)28-30-18-10-12-20-36(30)44-4/h9-12,17-21,25-26,38,41-42H,5-8,13-16,22-24,27-28H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221907

(2,5-bis{6-[ethyl(2-methoxybenzyl)amino]hexylamino}...)Show SMILES CCN(CCCCCCNc1c(O)c(-c2ccccc2)c(N=CCCCCCN(CC)Cc2ccccc2OC)c(O)c1-c1ccccc1)Cc1ccccc1OC |w:21.21| Show InChI InChI=1S/C50H64N4O4/c1-5-53(37-41-29-17-19-31-43(41)57-3)35-23-9-7-21-33-51-47-45(39-25-13-11-14-26-39)50(56)48(46(49(47)55)40-27-15-12-16-28-40)52-34-22-8-10-24-36-54(6-2)38-42-30-18-20-32-44(42)58-4/h11-20,25-33,52,55-56H,5-10,21-24,34-38H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221906
(2,5-bis[6-(2-methoxybenzylamino)hexylamino]-[1,4]-...)Show SMILES COc1ccccc1CNCCCCCCNc1cc(O)c(cc1O)N=CCCCCCNCc1ccccc1OC |w:25.26| Show InChI InChI=1S/C34H48N4O4/c1-41-33-17-9-7-15-27(33)25-35-19-11-3-5-13-21-37-29-23-32(40)30(24-31(29)39)38-22-14-6-4-12-20-36-26-28-16-8-10-18-34(28)42-2/h7-10,15-18,21,23-24,35-36,38-40H,3-6,11-14,19-20,22,25-26H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221923

(2,5-bis{6-[ethyl(pyridin-2-ylmethyl)amino]hexylami...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1ccccn1)Cc1ccccn1 |w:18.18| Show InChI InChI=1S/C34H50N6O2/c1-3-39(27-29-17-9-13-19-35-29)23-15-7-5-11-21-37-31-25-34(42)32(26-33(31)41)38-22-12-6-8-16-24-40(4-2)28-30-18-10-14-20-36-30/h9-10,13-14,17-21,25-26,38,41-42H,3-8,11-12,15-16,22-24,27-28H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221916
(2,5-bis{6-[ethyl(pyridin-3-ylmethyl)amino]hexylami...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1cccnc1)Cc1cccnc1 |w:18.18| Show InChI InChI=1S/C34H50N6O2/c1-3-39(27-29-15-13-17-35-25-29)21-11-7-5-9-19-37-31-23-34(42)32(24-33(31)41)38-20-10-6-8-12-22-40(4-2)28-30-16-14-18-36-26-30/h13-19,23-26,38,41-42H,3-12,20-22,27-28H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221931

(2,5-bis{6-[ethyl(furan-2-ylmethyl)amino]hexylamino...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1ccco1)Cc1ccco1 |w:18.18| Show InChI InChI=1S/C32H48N4O4/c1-3-35(25-27-15-13-21-39-27)19-11-7-5-9-17-33-29-23-32(38)30(24-31(29)37)34-18-10-6-8-12-20-36(4-2)26-28-16-14-22-40-28/h13-17,21-24,34,37-38H,3-12,18-20,25-26H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50067482
(6-[(2-Methoxy-benzyl)-methyl-amino]-hexanoic acid ...)Show SMILES COc1ccccc1CN(C)CCCCCC(=O)N(C)CCCCCCCCN(C)C(=O)CCCCCN(C)Cc1ccccc1OC Show InChI InChI=1S/C40H66N4O4/c1-41(33-35-23-15-17-25-37(35)47-5)29-19-11-13-27-39(45)43(3)31-21-9-7-8-10-22-32-44(4)40(46)28-14-12-20-30-42(2)34-36-24-16-18-26-38(36)48-6/h15-18,23-26H,7-14,19-22,27-34H2,1-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221911
(2,5-bis{6-[ethyl(pyridin-4-ylmethyl)amino]hexylami...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1ccncc1)Cc1ccncc1 |w:18.18| Show InChI InChI=1S/C34H50N6O2/c1-3-39(27-29-13-19-35-20-14-29)23-11-7-5-9-17-37-31-25-34(42)32(26-33(31)41)38-18-10-6-8-12-24-40(4-2)28-30-15-21-36-22-16-30/h13-17,19-22,25-26,38,41-42H,3-12,18,23-24,27-28H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 192 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 424 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221922

(2,5-bis{6-[ethyl(2-methoxybenzyl)amino]hexylamino}...)Show SMILES CCN(CCCCCCNc1c(C)c(O)c(N=CCCCCCN(CC)Cc2ccccc2OC)c(C)c1O)Cc1ccccc1OC |w:16.15| Show InChI InChI=1S/C40H60N4O4/c1-7-43(29-33-21-13-15-23-35(33)47-5)27-19-11-9-17-25-41-37-31(3)40(46)38(32(4)39(37)45)42-26-18-10-12-20-28-44(8-2)30-34-22-14-16-24-36(34)48-6/h13-16,21-25,42,45-46H,7-12,17-20,26-30H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 462 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221920

(CHEMBL230434 | N,N'-bis{6-[ethyl(2-methoxybenzyl)a...)Show SMILES CCN(CCCCCCNc1ccc(NCCCCCCN(CC)Cc2ccccc2OC)cc1)Cc1ccccc1OC Show InChI InChI=1S/C38H58N4O2/c1-5-41(31-33-19-11-13-21-37(33)43-3)29-17-9-7-15-27-39-35-23-25-36(26-24-35)40-28-16-8-10-18-30-42(6-2)32-34-20-12-14-22-38(34)44-4/h11-14,19-26,39-40H,5-10,15-18,27-32H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 505 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221932

(2,5-bis(6-{ethyl[2-(2-methoxyphenyl)ethyl]amino}he...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)CCc1ccccc1OC)CCc1ccccc1OC |w:18.18| Show InChI InChI=1S/C40H60N4O4/c1-5-43(29-23-33-19-11-13-21-39(33)47-3)27-17-9-7-15-25-41-35-31-38(46)36(32-37(35)45)42-26-16-8-10-18-28-44(6-2)30-24-34-20-12-14-22-40(34)48-4/h11-14,19-22,25,31-32,42,45-46H,5-10,15-18,23-24,26-30H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 518 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221917

(2,5-bis{6-[(2-chlorobenzyl)ethylamino]hexylamino}-...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1ccccc1Cl)Cc1ccccc1Cl |w:18.18| Show InChI InChI=1S/C36H50Cl2N4O2/c1-3-41(27-29-17-9-11-19-31(29)37)23-15-7-5-13-21-39-33-25-36(44)34(26-35(33)43)40-22-14-6-8-16-24-42(4-2)28-30-18-10-12-20-32(30)38/h9-12,17-21,25-26,40,43-44H,3-8,13-16,22-24,27-28H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 593 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221924

(2,5-bis[6-(benzylethylamino)hexylamino]-[1,4]-benz...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1ccccc1)Cc1ccccc1 |w:18.18| Show InChI InChI=1S/C36H52N4O2/c1-3-39(29-31-19-11-9-12-20-31)25-17-7-5-15-23-37-33-27-36(42)34(28-35(33)41)38-24-16-6-8-18-26-40(4-2)30-32-21-13-10-14-22-32/h9-14,19-23,27-28,38,41-42H,3-8,15-18,24-26,29-30H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 645 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221905

(2,5-bis{6-[isopropyl(2-methoxybenzyl)amino]hexylam...)Show SMILES COc1ccccc1CN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(Cc1ccccc1OC)C(C)C)C(C)C |w:25.26| Show InChI InChI=1S/C40H60N4O4/c1-31(2)43(29-33-19-11-13-21-39(33)47-5)25-17-9-7-15-23-41-35-27-38(46)36(28-37(35)45)42-24-16-8-10-18-26-44(32(3)4)30-34-20-12-14-22-40(34)48-6/h11-14,19-23,27-28,31-32,42,45-46H,7-10,15-18,24-26,29-30H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221918

(2,5-bis{6-[ethyl(2-methoxybenzyl)amino]hexylamino}...)Show SMILES CCN(CCCCCCNc1c(O)c(Br)c(N=CCCCCCN(CC)Cc2ccccc2OC)c(O)c1Br)Cc1ccccc1OC |w:16.15| Show InChI InChI=1S/C38H54Br2N4O4/c1-5-43(27-29-19-11-13-21-31(29)47-3)25-17-9-7-15-23-41-35-33(39)38(46)36(34(40)37(35)45)42-24-16-8-10-18-26-44(6-2)28-30-20-12-14-22-32(30)48-4/h11-14,19-23,42,45-46H,5-10,15-18,24-28H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 696 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221908

(2,5-bis{6-[ethyl(2-methoxybenzyl)amino]hexylamino}...)Show SMILES CCN(CCCCCCNc1c(O)c(Cl)c(N=CCCCCCN(CC)Cc2ccccc2OC)c(O)c1Cl)Cc1ccccc1OC |w:16.15| Show InChI InChI=1S/C38H54Cl2N4O4/c1-5-43(27-29-19-11-13-21-31(29)47-3)25-17-9-7-15-23-41-35-33(39)38(46)36(34(40)37(35)45)42-24-16-8-10-18-26-44(6-2)28-30-20-12-14-22-32(30)48-4/h11-14,19-23,42,45-46H,5-10,15-18,24-28H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221929

(2,5-bis{7-[ethyl(2-methoxybenzyl)amino]heptylamino...)Show SMILES CCN(CCCCCCCNc1cc(O)c(cc1O)N=CCCCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |w:19.19| Show InChI InChI=1S/C40H60N4O4/c1-5-43(31-33-21-13-15-23-39(33)47-3)27-19-11-7-9-17-25-41-35-29-38(46)36(30-37(35)45)42-26-18-10-8-12-20-28-44(6-2)32-34-22-14-16-24-40(34)48-4/h13-16,21-25,29-30,42,45-46H,5-12,17-20,26-28,31-32H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221926

(2,5-bis{6-[ethyl(2-methoxybenzyl)amino]hexylamino}...)Show SMILES CCN(CCCCCCNc1c(O)c(F)c(N=CCCCCCN(CC)Cc2ccccc2OC)c(O)c1F)Cc1ccccc1OC |w:16.15| Show InChI InChI=1S/C38H54F2N4O4/c1-5-43(27-29-19-11-13-21-31(29)47-3)25-17-9-7-15-23-41-35-33(39)38(46)36(34(40)37(35)45)42-24-16-8-10-18-26-44(6-2)28-30-20-12-14-22-32(30)48-4/h11-14,19-23,42,45-46H,5-10,15-18,24-28H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221910

(2,5-bis{6-[ethyl(3-methoxybenzyl)amino]hexylamino}...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1cccc(OC)c1)Cc1cccc(OC)c1 |w:18.18| Show InChI InChI=1S/C38H56N4O4/c1-5-41(29-31-17-15-19-33(25-31)45-3)23-13-9-7-11-21-39-35-27-38(44)36(28-37(35)43)40-22-12-8-10-14-24-42(6-2)30-32-18-16-20-34(26-32)46-4/h15-21,25-28,40,43-44H,5-14,22-24,29-30H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221928

(2,5-bis{6-[ethyl(2-methylbenzyl)amino]hexylamino}-...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1ccccc1C)Cc1ccccc1C |w:18.18| Show InChI InChI=1S/C38H56N4O2/c1-5-41(29-33-21-13-11-19-31(33)3)25-17-9-7-15-23-39-35-27-38(44)36(28-37(35)43)40-24-16-8-10-18-26-42(6-2)30-34-22-14-12-20-32(34)4/h11-14,19-23,27-28,40,43-44H,5-10,15-18,24-26,29-30H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221907

(2,5-bis{6-[ethyl(2-methoxybenzyl)amino]hexylamino}...)Show SMILES CCN(CCCCCCNc1c(O)c(-c2ccccc2)c(N=CCCCCCN(CC)Cc2ccccc2OC)c(O)c1-c1ccccc1)Cc1ccccc1OC |w:21.21| Show InChI InChI=1S/C50H64N4O4/c1-5-53(37-41-29-17-19-31-43(41)57-3)35-23-9-7-21-33-51-47-45(39-25-13-11-14-26-39)50(56)48(46(49(47)55)40-27-15-12-16-28-40)52-34-22-8-10-24-36-54(6-2)38-42-30-18-20-32-44(42)58-4/h11-20,25-33,52,55-56H,5-10,21-24,34-38H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221927

(2,5-bis{6-[ethyl(2-nitrobenzyl)amino]hexylamino}-[...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1ccccc1[N+]([O-])=O)Cc1ccccc1[N+]([O-])=O |w:18.18| Show InChI InChI=1S/C36H50N6O6/c1-3-39(27-29-17-9-11-19-33(29)41(45)46)23-15-7-5-13-21-37-31-25-36(44)32(26-35(31)43)38-22-14-6-8-16-24-40(4-2)28-30-18-10-12-20-34(30)42(47)48/h9-12,17-21,25-26,38,43-44H,3-8,13-16,22-24,27-28H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221912

(2,5-bis{6-[ethyl(4-methoxybenzyl)amino]hexylamino}...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1ccc(OC)cc1)Cc1ccc(OC)cc1 |w:18.18| Show InChI InChI=1S/C38H56N4O4/c1-5-41(29-31-15-19-33(45-3)20-16-31)25-13-9-7-11-23-39-35-27-38(44)36(28-37(35)43)40-24-12-8-10-14-26-42(6-2)30-32-17-21-34(46-4)22-18-32/h15-23,27-28,40,43-44H,5-14,24-26,29-30H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221923

(2,5-bis{6-[ethyl(pyridin-2-ylmethyl)amino]hexylami...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1ccccn1)Cc1ccccn1 |w:18.18| Show InChI InChI=1S/C34H50N6O2/c1-3-39(27-29-17-9-13-19-35-29)23-15-7-5-11-21-37-31-25-34(42)32(26-33(31)41)38-22-12-6-8-16-24-40(4-2)28-30-18-10-14-20-36-30/h9-10,13-14,17-21,25-26,38,41-42H,3-8,11-12,15-16,22-24,27-28H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221930

(2,5-bis{6-[ethyl(thiophen-2-ylmethyl)amino]hexylam...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1cccs1)Cc1cccs1 |w:18.18| Show InChI InChI=1S/C32H48N4O2S2/c1-3-35(25-27-15-13-21-39-27)19-11-7-5-9-17-33-29-23-32(38)30(24-31(29)37)34-18-10-6-8-12-20-36(4-2)26-28-16-14-22-40-28/h13-17,21-24,34,37-38H,3-12,18-20,25-26H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10620

((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...)Show InChI InChI=1S/C14H22N2O2/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4/h7-11H,6H2,1-5H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221906

(2,5-bis[6-(2-methoxybenzylamino)hexylamino]-[1,4]-...)Show SMILES COc1ccccc1CNCCCCCCNc1cc(O)c(cc1O)N=CCCCCCNCc1ccccc1OC |w:25.26| Show InChI InChI=1S/C34H48N4O4/c1-41-33-17-9-7-15-27(33)25-35-19-11-3-5-13-21-37-29-23-32(40)30(24-31(29)39)38-22-14-6-4-12-20-36-26-28-16-8-10-18-34(28)42-2/h7-10,15-18,21,23-24,35-36,38-40H,3-6,11-14,19-20,22,25-26H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221931

(2,5-bis{6-[ethyl(furan-2-ylmethyl)amino]hexylamino...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1ccco1)Cc1ccco1 |w:18.18| Show InChI InChI=1S/C32H48N4O4/c1-3-35(25-27-15-13-21-39-27)19-11-7-5-9-17-33-29-23-32(38)30(24-31(29)37)34-18-10-6-8-12-20-36(4-2)26-28-16-14-22-40-28/h13-17,21-24,34,37-38H,3-12,18-20,25-26H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221909

(2,5-bis{5-[ethyl(2-methoxybenzyl)amino]pentylamino...)Show SMILES CCN(CCCCCNc1cc(O)c(cc1O)N=CCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |w:17.17| Show InChI InChI=1S/C36H52N4O4/c1-5-39(27-29-17-9-11-19-35(29)43-3)23-15-7-13-21-37-31-25-34(42)32(26-33(31)41)38-22-14-8-16-24-40(6-2)28-30-18-10-12-20-36(30)44-4/h9-12,17-21,25-26,38,41-42H,5-8,13-16,22-24,27-28H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221933

(2,5-bis{6-[(2-methoxybenzyl)methylamino]hexylamino...)Show SMILES COc1ccccc1CN(C)CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(C)Cc1ccccc1OC |w:26.27| Show InChI InChI=1S/C36H52N4O4/c1-39(27-29-17-9-11-19-35(29)43-3)23-15-7-5-13-21-37-31-25-34(42)32(26-33(31)41)38-22-14-6-8-16-24-40(2)28-30-18-10-12-20-36(30)44-4/h9-12,17-21,25-26,38,41-42H,5-8,13-16,22-24,27-28H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221914

(2,5-bis{3-[ethyl(2-methoxybenzyl)amino]propylamino...)Show SMILES CCN(CCCNc1cc(O)c(cc1O)N=CCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |w:15.15| Show InChI InChI=1S/C32H44N4O4/c1-5-35(23-25-13-7-9-15-31(25)39-3)19-11-17-33-27-21-30(38)28(22-29(27)37)34-18-12-20-36(6-2)24-26-14-8-10-16-32(26)40-4/h7-10,13-17,21-22,34,37-38H,5-6,11-12,18-20,23-24H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50067482

(6-[(2-Methoxy-benzyl)-methyl-amino]-hexanoic acid ...)Show SMILES COc1ccccc1CN(C)CCCCCC(=O)N(C)CCCCCCCCN(C)C(=O)CCCCCN(C)Cc1ccccc1OC Show InChI InChI=1S/C40H66N4O4/c1-41(33-35-23-15-17-25-37(35)47-5)29-19-11-13-27-39(45)43(3)31-21-9-7-8-10-22-32-44(4)40(46)28-14-12-20-30-42(2)34-36-24-16-18-26-38(36)48-6/h15-18,23-26H,7-14,19-22,27-34H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221913

(6-(4-{5-[ethyl(2-methoxybenzyl)carbamoyl]pentylami...)Show SMILES CCN(Cc1ccccc1OC)C(=O)CCCCCNc1cc(O)c(cc1O)N=CCCCCC(=O)N(CC)Cc1ccccc1OC |w:28.29| Show InChI InChI=1S/C38H52N4O6/c1-5-41(27-29-17-11-13-19-35(29)47-3)37(45)21-9-7-15-23-39-31-25-34(44)32(26-33(31)43)40-24-16-8-10-22-38(46)42(6-2)28-30-18-12-14-20-36(30)48-4/h11-14,17-20,23,25-26,40,43-44H,5-10,15-16,21-22,24,27-28H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221916

(2,5-bis{6-[ethyl(pyridin-3-ylmethyl)amino]hexylami...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1cccnc1)Cc1cccnc1 |w:18.18| Show InChI InChI=1S/C34H50N6O2/c1-3-39(27-29-15-13-17-35-25-29)21-11-7-5-9-19-37-31-23-34(42)32(24-33(31)41)38-20-10-6-8-12-22-40(4-2)28-30-16-14-18-36-26-30/h13-19,23-26,38,41-42H,3-12,20-22,27-28H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221921

(2,5-bis{4-[ethyl(2-methoxybenzyl)amino]butylamino}...)Show SMILES CCN(CCCCNc1cc(O)c(cc1O)N=CCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |w:16.16| Show InChI InChI=1S/C34H48N4O4/c1-5-37(25-27-15-7-9-17-33(27)41-3)21-13-11-19-35-29-23-32(40)30(24-31(29)39)36-20-12-14-22-38(6-2)26-28-16-8-10-18-34(28)42-4/h7-10,15-19,23-24,36,39-40H,5-6,11-14,20-22,25-26H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM31904
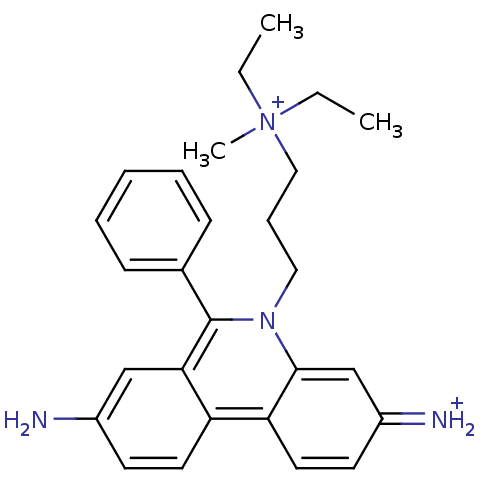
(CHEMBL345124 | Propidium | Propidium Iodide, 2 | p...)Show SMILES CC[N+](C)(CC)CCCn1c(-c2ccccc2)c2cc(N)ccc2c2ccc(=[NH2+])cc12 Show InChI InChI=1S/C27H33N4/c1-4-31(3,5-2)17-9-16-30-26-19-22(29)13-15-24(26)23-14-12-21(28)18-25(23)27(30)20-10-7-6-8-11-20/h6-8,10-15,18-19,29H,4-5,9,16-17,28H2,1-3H3/q+1/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221915

(2,5-bis{2-[ethyl(2-methoxybenzyl)amino]ethylamino}...)Show SMILES CCN(CCNc1cc(O)c(cc1O)N=CCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |w:14.14| Show InChI InChI=1S/C30H40N4O4/c1-5-33(21-23-11-7-9-13-29(23)37-3)17-15-31-25-19-28(36)26(20-27(25)35)32-16-18-34(6-2)22-24-12-8-10-14-30(24)38-4/h7-15,19-20,32,35-36H,5-6,16-18,21-22H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221913

(6-(4-{5-[ethyl(2-methoxybenzyl)carbamoyl]pentylami...)Show SMILES CCN(Cc1ccccc1OC)C(=O)CCCCCNc1cc(O)c(cc1O)N=CCCCCC(=O)N(CC)Cc1ccccc1OC |w:28.29| Show InChI InChI=1S/C38H52N4O6/c1-5-41(27-29-17-11-13-19-35(29)47-3)37(45)21-9-7-15-23-39-31-25-34(44)32(26-33(31)43)40-24-16-8-10-22-38(46)42(6-2)28-30-18-12-14-20-36(30)48-4/h11-14,17-20,23,25-26,40,43-44H,5-10,15-16,21-22,24,27-28H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 2.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM31904

(CHEMBL345124 | Propidium | Propidium Iodide, 2 | p...)Show SMILES CC[N+](C)(CC)CCCn1c(-c2ccccc2)c2cc(N)ccc2c2ccc(=[NH2+])cc12 Show InChI InChI=1S/C27H33N4/c1-4-31(3,5-2)17-9-16-30-26-19-22(29)13-15-24(26)23-14-12-21(28)18-25(23)27(30)20-10-7-6-8-11-20/h6-8,10-15,18-19,29H,4-5,9,16-17,28H2,1-3H3/q+1/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant acetylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221917

(2,5-bis{6-[(2-chlorobenzyl)ethylamino]hexylamino}-...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1ccccc1Cl)Cc1ccccc1Cl |w:18.18| Show InChI InChI=1S/C36H50Cl2N4O2/c1-3-41(27-29-17-9-11-19-31(29)37)23-15-7-5-13-21-39-33-25-36(44)34(26-35(33)43)40-22-14-6-8-16-24-42(4-2)28-30-18-10-12-20-32(30)38/h9-12,17-21,25-26,40,43-44H,3-8,13-16,22-24,27-28H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221927

(2,5-bis{6-[ethyl(2-nitrobenzyl)amino]hexylamino}-[...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1ccccc1[N+]([O-])=O)Cc1ccccc1[N+]([O-])=O |w:18.18| Show InChI InChI=1S/C36H50N6O6/c1-3-39(27-29-17-9-11-19-33(29)41(45)46)23-15-7-5-13-21-37-31-25-36(44)32(26-35(31)43)38-22-14-6-8-16-24-40(4-2)28-30-18-10-12-20-34(30)42(47)48/h9-12,17-21,25-26,38,43-44H,3-8,13-16,22-24,27-28H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50221911

(2,5-bis{6-[ethyl(pyridin-4-ylmethyl)amino]hexylami...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1ccncc1)Cc1ccncc1 |w:18.18| Show InChI InChI=1S/C34H50N6O2/c1-3-39(27-29-13-19-35-20-14-29)23-11-7-5-9-17-37-31-25-34(42)32(26-33(31)41)38-18-10-6-8-12-24-40(4-2)28-30-15-21-36-22-16-30/h13-17,19-22,25-26,38,41-42H,3-12,18,23-24,27-28H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant butyrylcholinesterase |
J Med Chem 50: 4882-97 (2007)
Article DOI: 10.1021/jm070559a
BindingDB Entry DOI: 10.7270/Q218379R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data