

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222089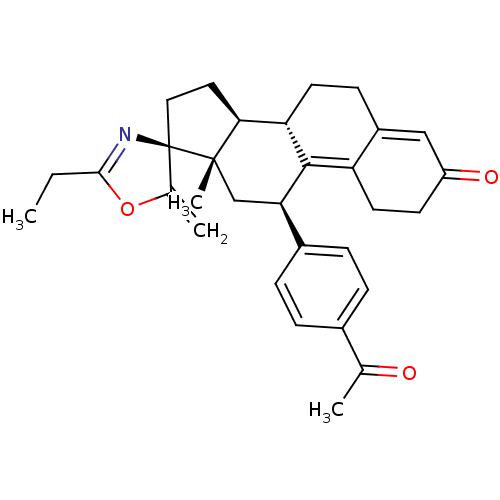 ((3S,10'S,11'S,15'S,17'R)-17'-(4-acetylphenyl)-5-et...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222091 ((3S,10'S,11'S,15'S,17'R)-17'-(4-acetylphenyl)-5,15...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222086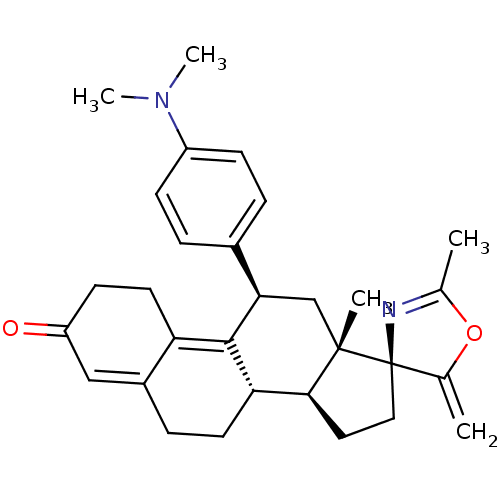 ((3S,10'S,11'S,15'S,17'R)-17'-[4-(dimethylamino)phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222092 ((3S,10'S,11'S,15'S,17'R)-17'-[4-(dimethylamino)phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222088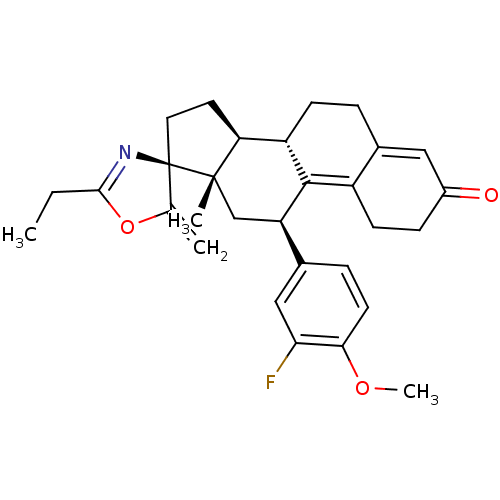 ((3S,10'S,11'S,15'S,17'R)-5-ethyl-17'-(3-fluoro-4-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222087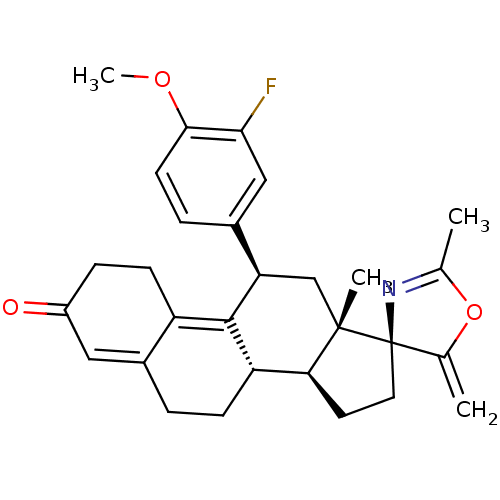 ((3S,10'S,11'S,15'S,17'R)-17'-(3-fluoro-4-methoxyph...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in A549 cells assessed as inhibition of corticoid-induced gene transcription by luciferase r... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222085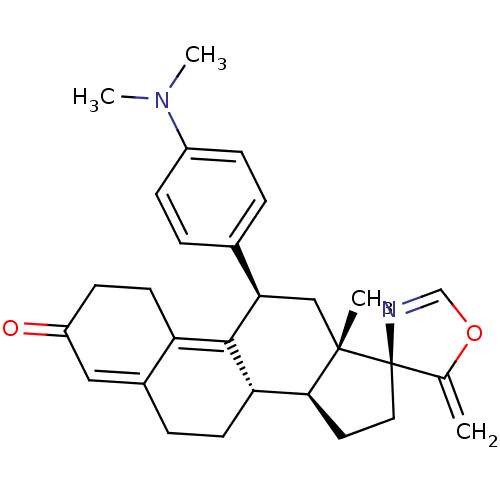 ((3S,10'S,11'S,15'S,17'R)-17'-[4-(dimethylamino)phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50222092 ((3S,10'S,11'S,15'S,17'R)-17'-[4-(dimethylamino)phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in A549 cells assessed as inhibition of corticoid-induced gene transcription by luciferase r... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50222086 ((3S,10'S,11'S,15'S,17'R)-17'-[4-(dimethylamino)phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in A549 cells assessed as inhibition of corticoid-induced gene transcription by luciferase r... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222090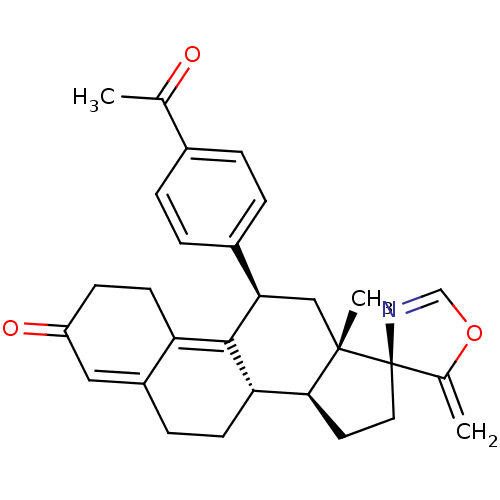 ((3S,10'S,11'S,15'S,17'R)-17'-(4-acetylphenyl)-15'-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50222089 ((3S,10'S,11'S,15'S,17'R)-17'-(4-acetylphenyl)-5-et...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in A549 cells assessed as inhibition of corticoid-induced gene transcription by luciferase r... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50222091 ((3S,10'S,11'S,15'S,17'R)-17'-(4-acetylphenyl)-5,15...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in A549 cells assessed as inhibition of corticoid-induced gene transcription by luciferase r... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50222085 ((3S,10'S,11'S,15'S,17'R)-17'-[4-(dimethylamino)phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in A549 cells assessed as inhibition of corticoid-induced gene transcription by luciferase r... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50222087 ((3S,10'S,11'S,15'S,17'R)-17'-(3-fluoro-4-methoxyph...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in A549 cells assessed as inhibition of corticoid-induced gene transcription by luciferase r... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50222088 ((3S,10'S,11'S,15'S,17'R)-5-ethyl-17'-(3-fluoro-4-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in A549 cells assessed as inhibition of corticoid-induced gene transcription by luciferase r... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50222090 ((3S,10'S,11'S,15'S,17'R)-17'-(4-acetylphenyl)-15'-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in A549 cells assessed as inhibition of corticoid-induced gene transcription by luciferase r... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||