

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224496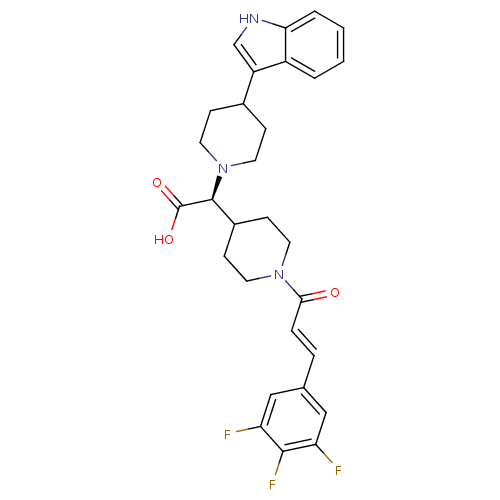 ((S,E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at CCR2 expressed in THP1 cells assessed as MCP1-induced calcium flux | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224501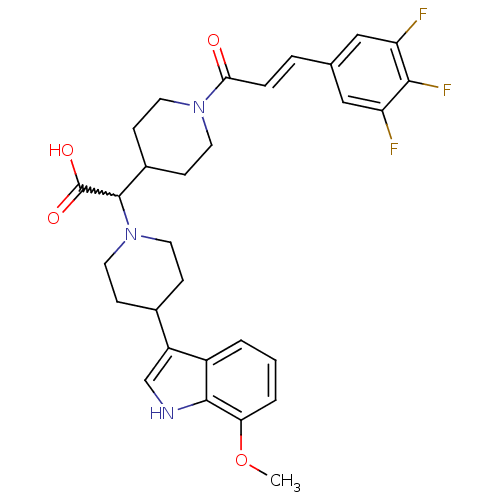 ((E)-2-(4-(7-methoxy-1H-indol-3-yl)piperidin-1-yl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224496 ((S,E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224500 ((E)-2-(4-(5-amino-1H-indol-3-yl)piperidin-1-yl)-2-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224523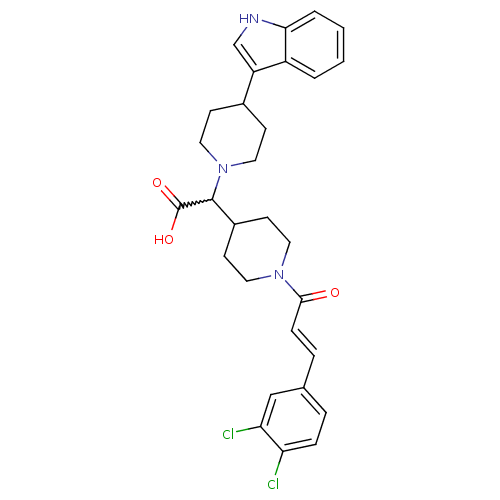 ((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(3...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224502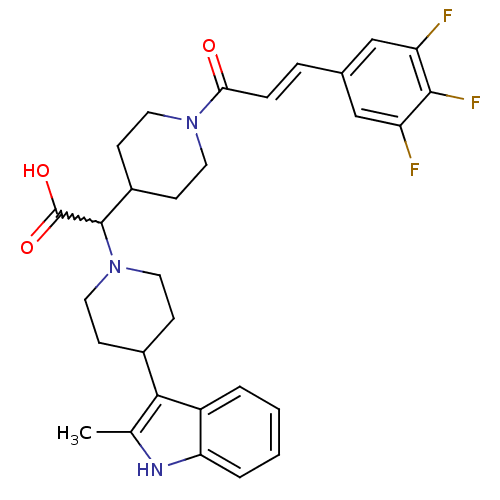 ((E)-2-(4-(2-methyl-1H-indol-3-yl)piperidin-1-yl)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224511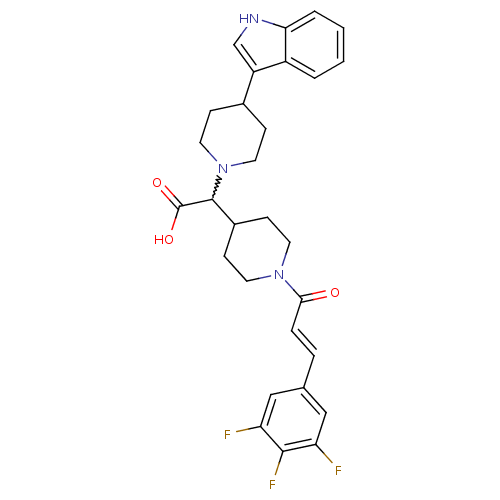 ((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(3...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224524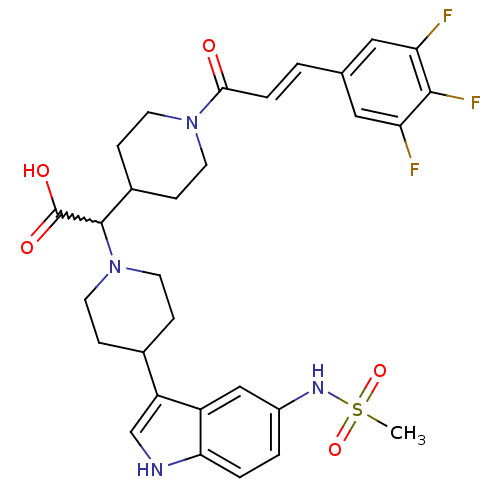 ((E)-2-(4-(5-(methylsulfonamido)-1H-indol-3-yl)pipe...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224519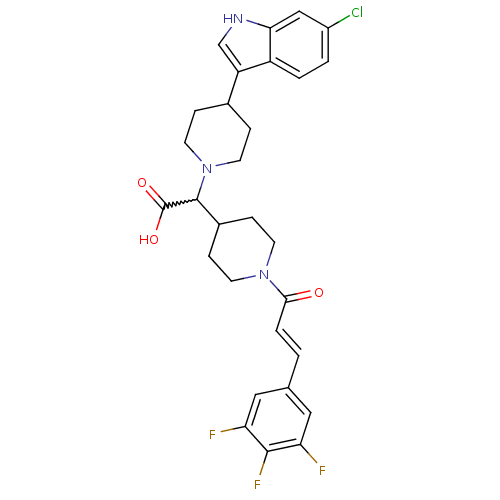 ((E)-2-(4-(6-chloro-1H-indol-3-yl)piperidin-1-yl)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224512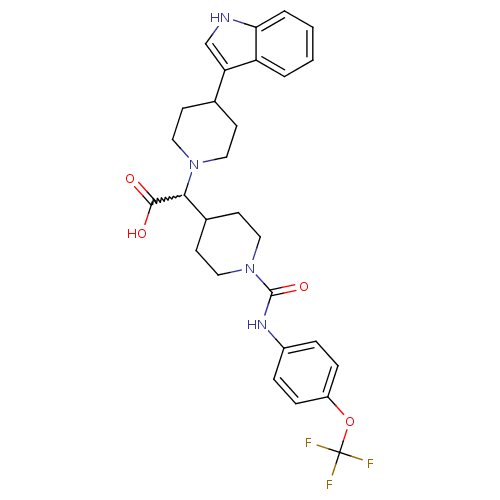 (2-(1-((4-(trifluoromethoxy)phenyl)carbamoyl)piperi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224510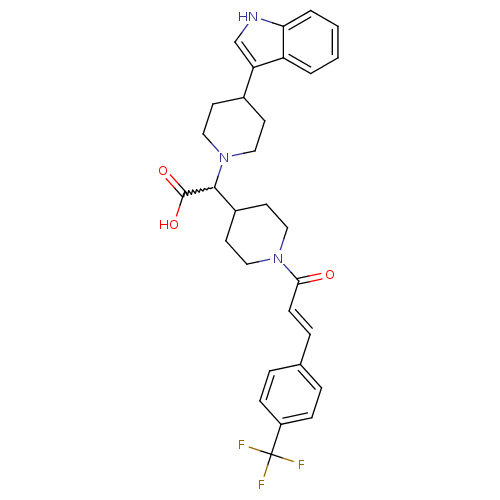 ((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(4...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224508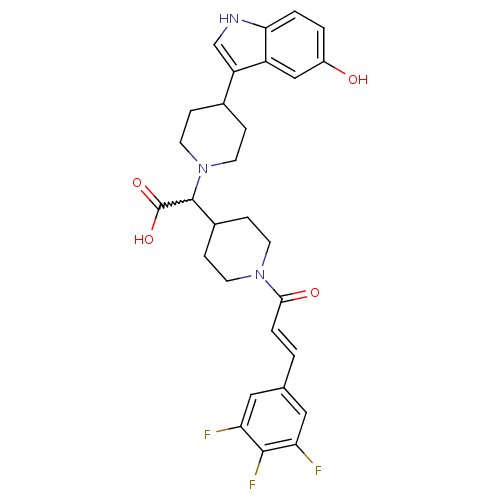 ((E)-2-(4-(5-hydroxy-1H-indol-3-yl)piperidin-1-yl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224521 ((E)-2-(4-(5-methoxy-1H-indol-3-yl)piperidin-1-yl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224506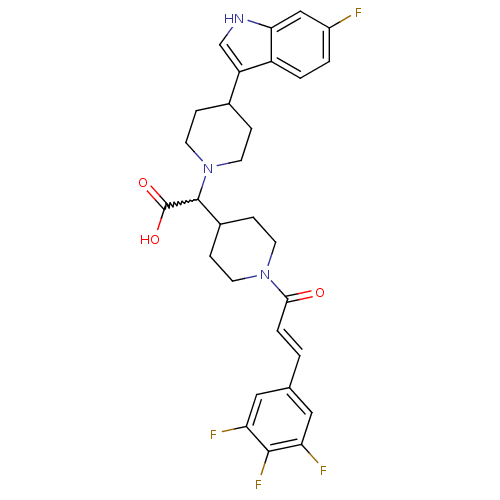 ((E)-2-(4-(6-fluoro-1H-indol-3-yl)piperidin-1-yl)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224499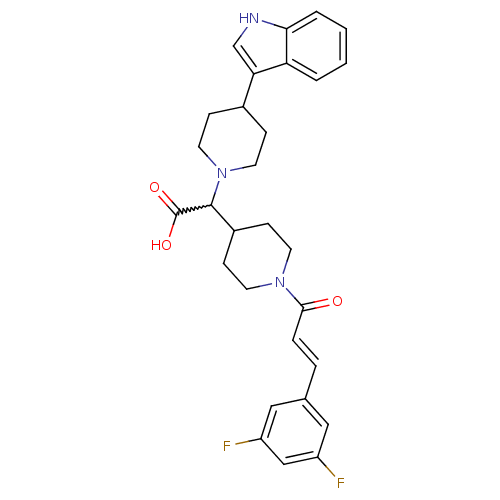 ((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(3...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224516 (2-(1-((3,4-dichlorophenyl)carbamoyl)piperidin-4-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224498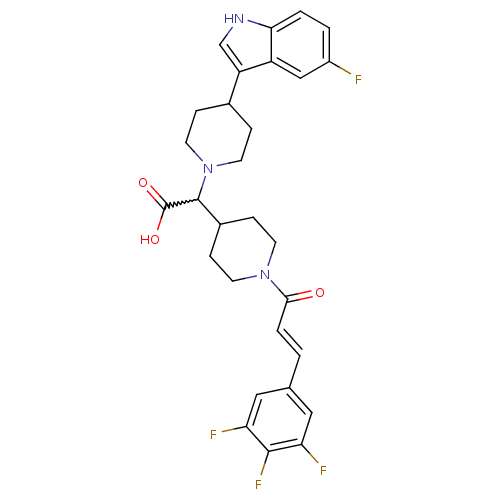 ((E)-2-(4-(5-fluoro-1H-indol-3-yl)piperidin-1-yl)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224515 ((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-cinna...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224505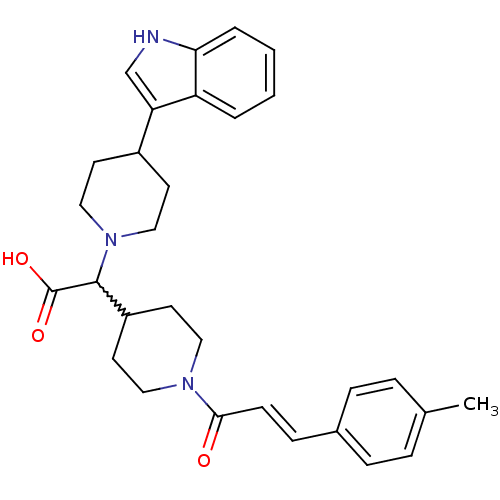 ((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-p-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224517 (2-(1-((4-(trifluoromethyl)phenyl)carbamoyl)piperid...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224514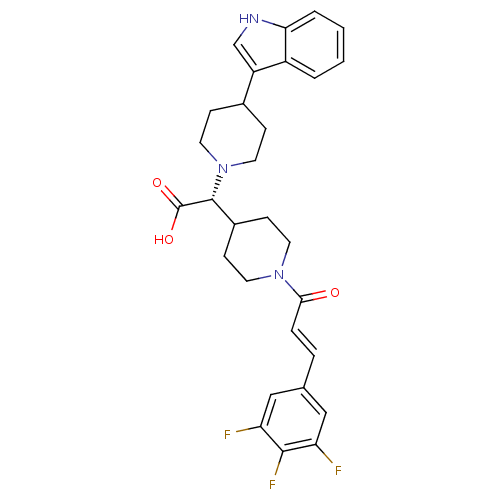 ((R,E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224507 ((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(4...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224503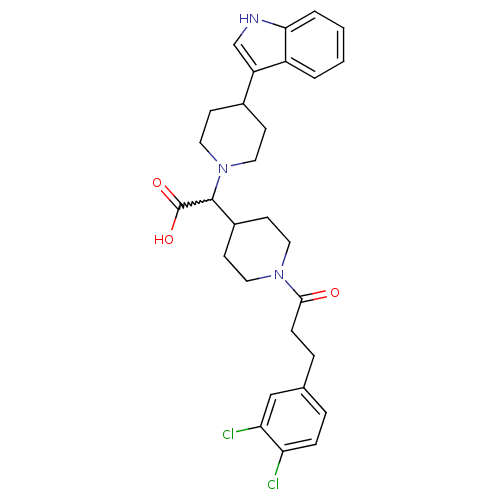 (2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(3,4-d...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224520 ((E)-1-(4-((4-(1H-indol-3-yl)piperidin-1-yl)methyl)...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224513 ((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(4...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224495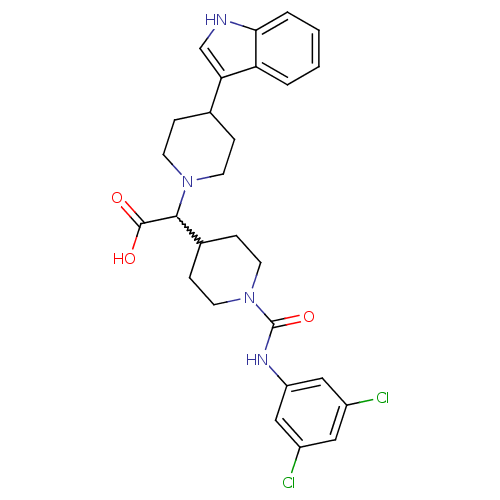 (2-(1-((3,5-dichlorophenyl)carbamoyl)piperidin-4-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224522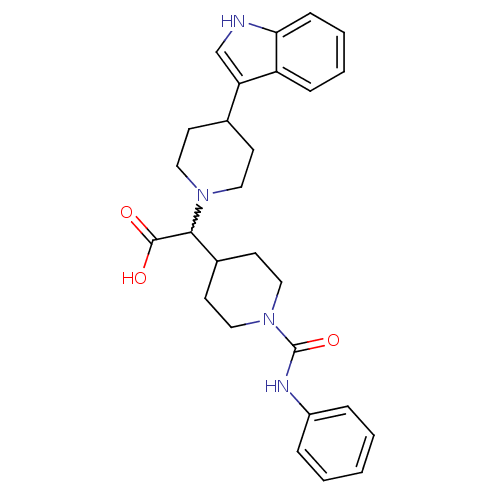 (2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(phenylca...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224504 ((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(3...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Mus musculus) | BDBM50224496 ((S,E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]-rat MCP1 from rat CCR2 receptor in monocytes | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Mus musculus) | BDBM50224496 ((S,E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]-mouse MCP1 from CCR2 in mouse WEHI265.1 cells | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Mus musculus) | BDBM50224496 ((S,E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]-mouse MCP1 from CCR2 in mouse peripheral blood monocytes | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224509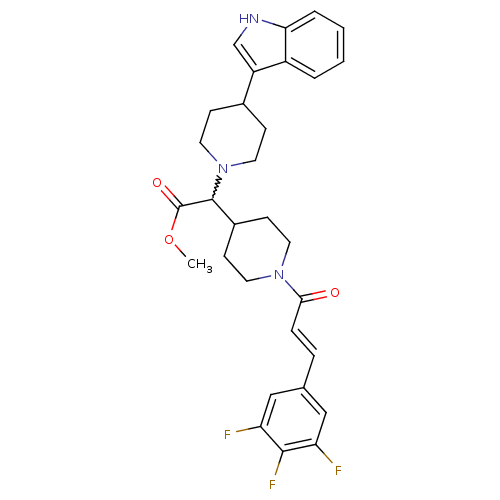 ((E)-methyl 2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224518 ((E)-2-(4-(1-acetyl-1H-indol-3-yl)piperidin-1-yl)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224497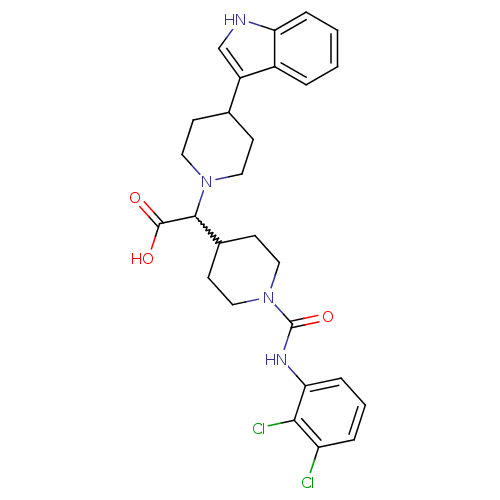 (2-(1-((2,3-dichlorophenyl)carbamoyl)piperidin-4-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||