

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50116067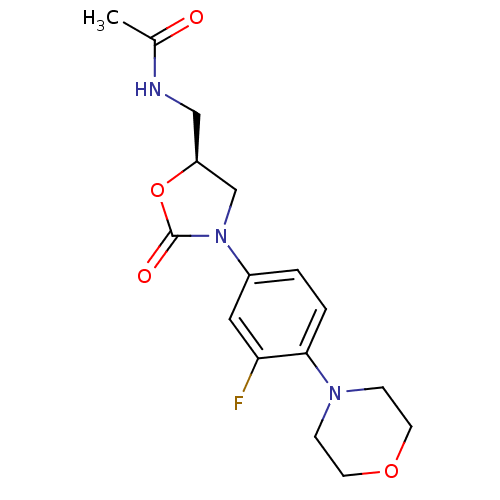 ((Linezolid)N-[3-(3-Fluoro-4-morpholin-4-yl-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA | J Med Chem 50: 5886-9 (2007) Article DOI: 10.1021/jm070708p BindingDB Entry DOI: 10.7270/Q22J6BM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50226485 ((R)-3-(3-fluoro-4-(tetrahydro-2H-pyran-4-yl)phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA | J Med Chem 50: 5886-9 (2007) Article DOI: 10.1021/jm070708p BindingDB Entry DOI: 10.7270/Q22J6BM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50226479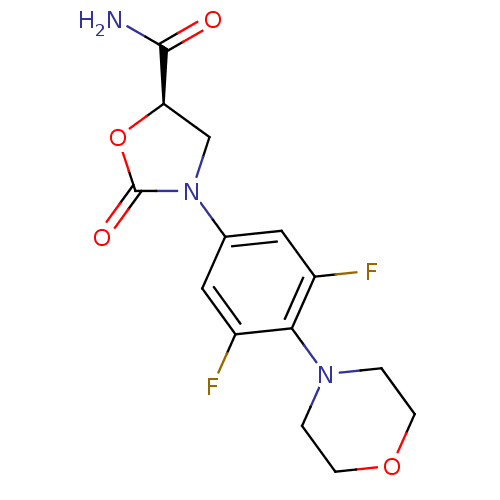 ((R)-3-(3,5-difluoro-4-morpholinophenyl)-2-oxooxazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA | J Med Chem 50: 5886-9 (2007) Article DOI: 10.1021/jm070708p BindingDB Entry DOI: 10.7270/Q22J6BM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50226484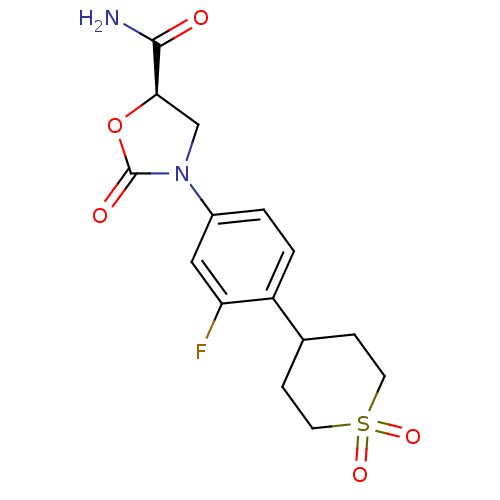 ((5R)-3-[4-(1,1-dioxidotetrahydro-2H-thiopyran-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA | J Med Chem 50: 5886-9 (2007) Article DOI: 10.1021/jm070708p BindingDB Entry DOI: 10.7270/Q22J6BM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50226480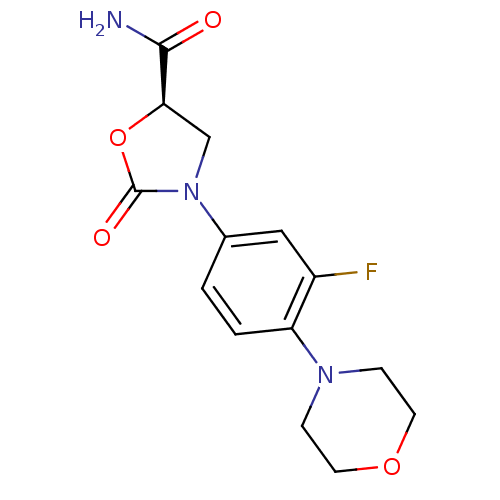 ((R)-3-(3-fluoro-4-morpholinophenyl)-2-oxooxazolidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.46E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA | J Med Chem 50: 5886-9 (2007) Article DOI: 10.1021/jm070708p BindingDB Entry DOI: 10.7270/Q22J6BM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50226481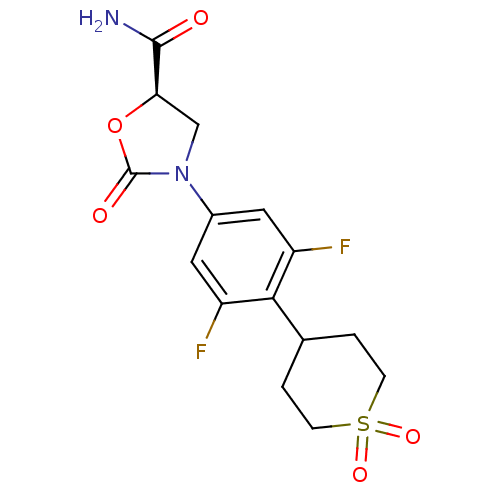 ((5R)-3-[4-(1,1-dioxidotetrahydro-2H-thiopyran-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA | J Med Chem 50: 5886-9 (2007) Article DOI: 10.1021/jm070708p BindingDB Entry DOI: 10.7270/Q22J6BM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50226482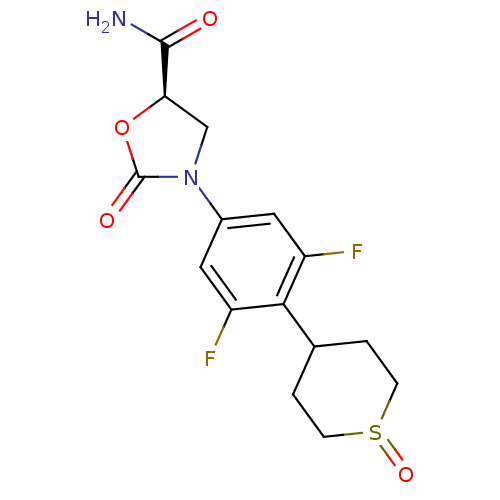 ((5R)-3-[3,5-difluoro-4-(1-oxidotetrahydro-2H-thiop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.55E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA | J Med Chem 50: 5886-9 (2007) Article DOI: 10.1021/jm070708p BindingDB Entry DOI: 10.7270/Q22J6BM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50226483 ((5R)-3-[3-fluoro-4-(1-oxidotetrahydro-2H-thiopyran...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human liver microsome CYP2D6 | J Med Chem 50: 5886-9 (2007) Article DOI: 10.1021/jm070708p BindingDB Entry DOI: 10.7270/Q22J6BM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50226483 ((5R)-3-[3-fluoro-4-(1-oxidotetrahydro-2H-thiopyran...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human liver microsome CYP1A2 | J Med Chem 50: 5886-9 (2007) Article DOI: 10.1021/jm070708p BindingDB Entry DOI: 10.7270/Q22J6BM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50226483 ((5R)-3-[3-fluoro-4-(1-oxidotetrahydro-2H-thiopyran...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human liver microsome CYP2C9 | J Med Chem 50: 5886-9 (2007) Article DOI: 10.1021/jm070708p BindingDB Entry DOI: 10.7270/Q22J6BM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50226483 ((5R)-3-[3-fluoro-4-(1-oxidotetrahydro-2H-thiopyran...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human liver microsome CYP2C19 | J Med Chem 50: 5886-9 (2007) Article DOI: 10.1021/jm070708p BindingDB Entry DOI: 10.7270/Q22J6BM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||