 Found 66 hits of Enzyme Inhibition Constant Data
Found 66 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227654
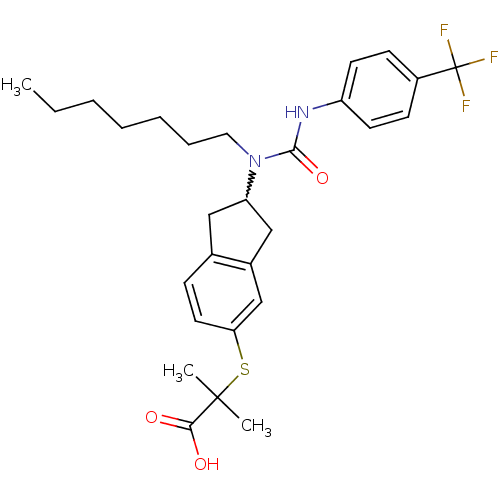
(2-(2-(1-heptyl-3-(4-(trifluoromethyl)phenyl)ureido...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(cc1)C(F)(F)F |w:8.7| Show InChI InChI=1S/C28H35F3N2O3S/c1-4-5-6-7-8-15-33(26(36)32-22-12-10-21(11-13-22)28(29,30)31)23-16-19-9-14-24(18-20(19)17-23)37-27(2,3)25(34)35/h9-14,18,23H,4-8,15-17H2,1-3H3,(H,32,36)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227657
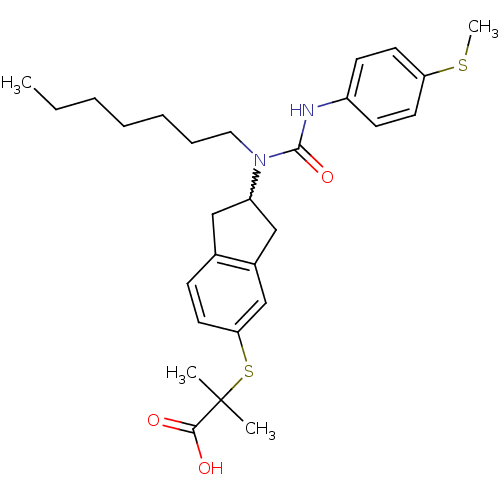
(2-(2-(1-heptyl-3-(4-(methylthio)phenyl)ureido)-2,3...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(SC)cc1 |w:8.7| Show InChI InChI=1S/C28H38N2O3S2/c1-5-6-7-8-9-16-30(27(33)29-22-11-14-24(34-4)15-12-22)23-17-20-10-13-25(19-21(20)18-23)35-28(2,3)26(31)32/h10-15,19,23H,5-9,16-18H2,1-4H3,(H,29,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227664

(2-(2-(1-ethyl-3-(4-(trifluoromethoxy)phenyl)ureido...)Show SMILES CCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |w:3.2| Show InChI InChI=1S/C23H25F3N2O4S/c1-4-28(21(31)27-16-6-8-18(9-7-16)32-23(24,25)26)17-11-14-5-10-19(13-15(14)12-17)33-22(2,3)20(29)30/h5-10,13,17H,4,11-12H2,1-3H3,(H,27,31)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227665
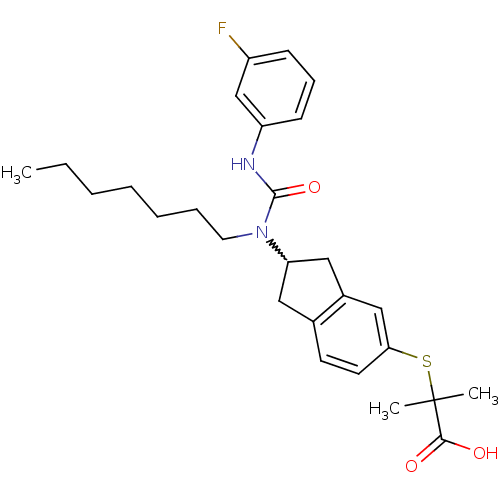
(2-(2-(3-(3-fluorophenyl)-1-heptylureido)-2,3-dihyd...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1cccc(F)c1 |w:8.7| Show InChI InChI=1S/C27H35FN2O3S/c1-4-5-6-7-8-14-30(26(33)29-22-11-9-10-21(28)18-22)23-15-19-12-13-24(17-20(19)16-23)34-27(2,3)25(31)32/h9-13,17-18,23H,4-8,14-16H2,1-3H3,(H,29,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.67E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227673
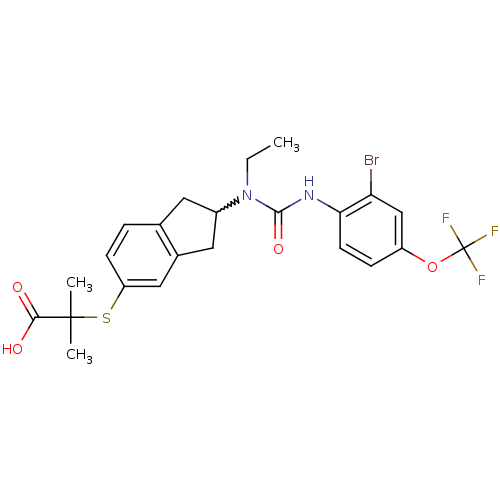
(2-(2-(3-(2-bromo-4-(trifluoromethoxy)phenyl)-1-eth...)Show SMILES CCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1Br |w:3.2| Show InChI InChI=1S/C23H24BrF3N2O4S/c1-4-29(21(32)28-19-8-6-16(12-18(19)24)33-23(25,26)27)15-9-13-5-7-17(11-14(13)10-15)34-22(2,3)20(30)31/h5-8,11-12,15H,4,9-10H2,1-3H3,(H,28,32)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227678
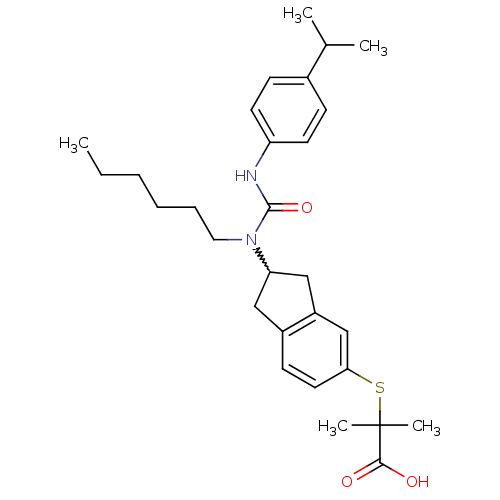
(2-(2-(1-hexyl-3-(4-isopropylphenyl)ureido)-2,3-dih...)Show SMILES CCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(cc1)C(C)C |w:7.6| Show InChI InChI=1S/C29H40N2O3S/c1-6-7-8-9-16-31(28(34)30-24-13-10-21(11-14-24)20(2)3)25-17-22-12-15-26(19-23(22)18-25)35-29(4,5)27(32)33/h10-15,19-20,25H,6-9,16-18H2,1-5H3,(H,30,34)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227687
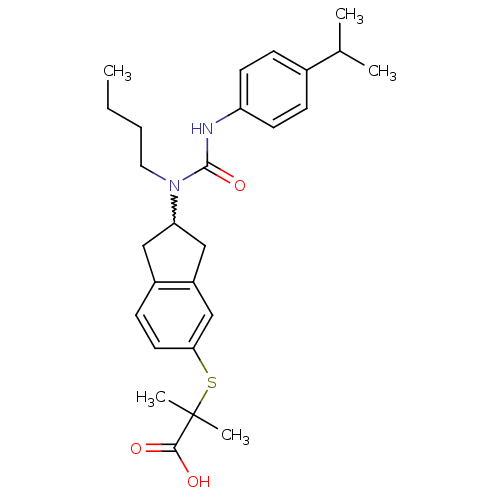
(2-(2-(1-butyl-3-(4-isopropylphenyl)ureido)-2,3-dih...)Show SMILES CCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(cc1)C(C)C |w:5.4| Show InChI InChI=1S/C27H36N2O3S/c1-6-7-14-29(26(32)28-22-11-8-19(9-12-22)18(2)3)23-15-20-10-13-24(17-21(20)16-23)33-27(4,5)25(30)31/h8-13,17-18,23H,6-7,14-16H2,1-5H3,(H,28,32)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227701
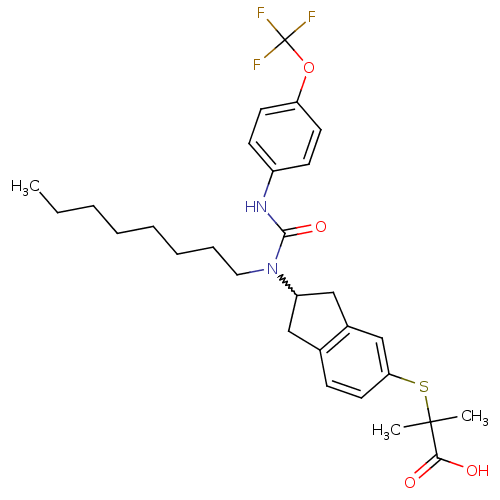
(2-methyl-2-(2-(1-octyl-3-(4-(trifluoromethoxy)phen...)Show SMILES CCCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |w:9.8| Show InChI InChI=1S/C29H37F3N2O4S/c1-4-5-6-7-8-9-16-34(27(37)33-22-11-13-24(14-12-22)38-29(30,31)32)23-17-20-10-15-25(19-21(20)18-23)39-28(2,3)26(35)36/h10-15,19,23H,4-9,16-18H2,1-3H3,(H,33,37)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227700
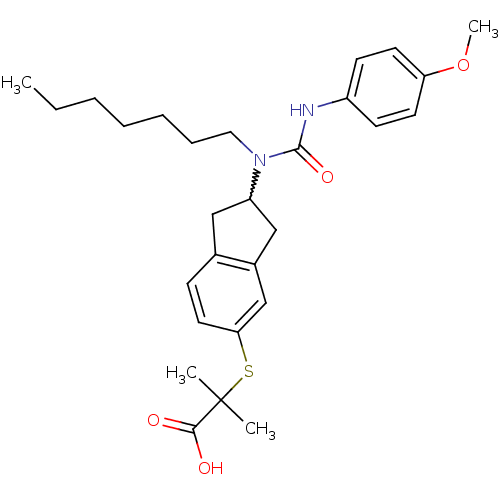
(2-(2-(1-heptyl-3-(4-methoxyphenyl)ureido)-2,3-dihy...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC)cc1 |w:8.7| Show InChI InChI=1S/C28H38N2O4S/c1-5-6-7-8-9-16-30(27(33)29-22-11-13-24(34-4)14-12-22)23-17-20-10-15-25(19-21(20)18-23)35-28(2,3)26(31)32/h10-15,19,23H,5-9,16-18H2,1-4H3,(H,29,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 910 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227697
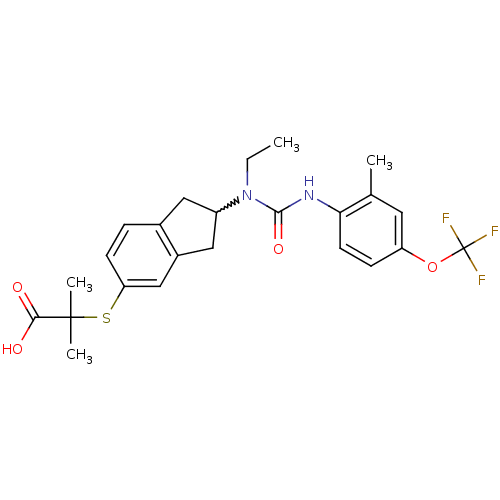
(2-(2-(1-ethyl-3-(2-methyl-4-(trifluoromethoxy)phen...)Show SMILES CCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1C |w:3.2| Show InChI InChI=1S/C24H27F3N2O4S/c1-5-29(22(32)28-20-9-7-18(10-14(20)2)33-24(25,26)27)17-11-15-6-8-19(13-16(15)12-17)34-23(3,4)21(30)31/h6-10,13,17H,5,11-12H2,1-4H3,(H,28,32)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 598 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227663
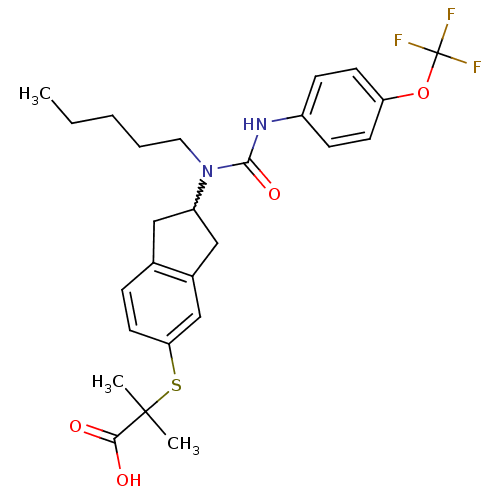
(2-methyl-2-(2-(1-pentyl-3-(4-(trifluoromethoxy)phe...)Show SMILES CCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |w:6.5| Show InChI InChI=1S/C26H31F3N2O4S/c1-4-5-6-13-31(24(34)30-19-8-10-21(11-9-19)35-26(27,28)29)20-14-17-7-12-22(16-18(17)15-20)36-25(2,3)23(32)33/h7-12,16,20H,4-6,13-15H2,1-3H3,(H,30,34)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 105 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227672
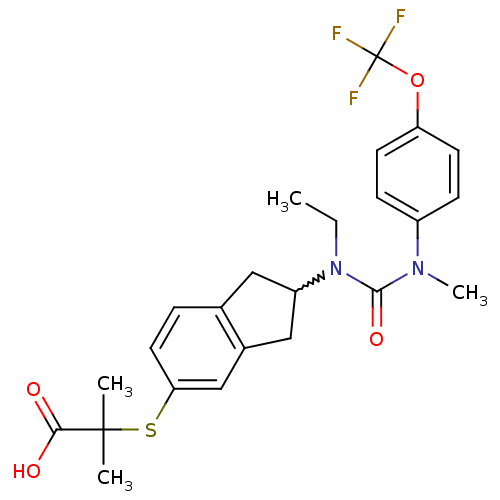
(2-(2-(1-ethyl-3-methyl-3-(4-(trifluoromethoxy)phen...)Show SMILES CCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)N(C)c1ccc(OC(F)(F)F)cc1 |w:3.2| Show InChI InChI=1S/C24H27F3N2O4S/c1-5-29(22(32)28(4)17-7-9-19(10-8-17)33-24(25,26)27)18-12-15-6-11-20(14-16(15)13-18)34-23(2,3)21(30)31/h6-11,14,18H,5,12-13H2,1-4H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227683
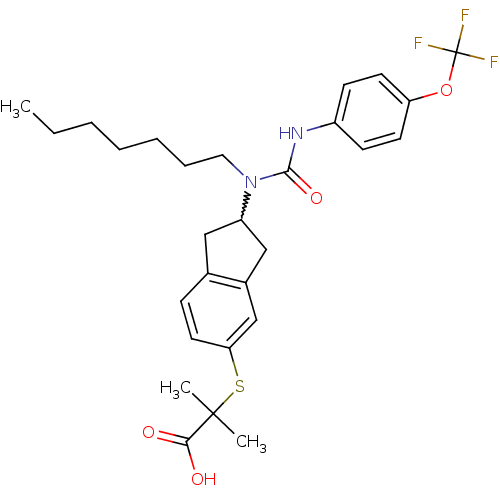
(2-(2-(1-heptyl-3-(4-(trifluoromethoxy)phenyl)ureid...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |w:8.7| Show InChI InChI=1S/C28H35F3N2O4S/c1-4-5-6-7-8-15-33(26(36)32-21-10-12-23(13-11-21)37-28(29,30)31)22-16-19-9-14-24(18-20(19)17-22)38-27(2,3)25(34)35/h9-14,18,22H,4-8,15-17H2,1-3H3,(H,32,36)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 294 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 190 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma expressed in HEK293 cells by GAL4 transactivation assay |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227695
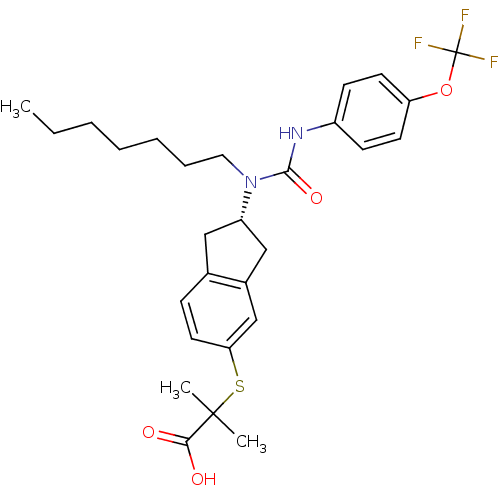
((S)-2-(2-(1-heptyl-3-(4-(trifluoromethoxy)phenyl)u...)Show SMILES CCCCCCCN([C@H]1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C28H35F3N2O4S/c1-4-5-6-7-8-15-33(26(36)32-21-10-12-23(13-11-21)37-28(29,30)31)22-16-19-9-14-24(18-20(19)17-22)38-27(2,3)25(34)35/h9-14,18,22H,4-8,15-17H2,1-3H3,(H,32,36)(H,34,35)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.30E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM28799
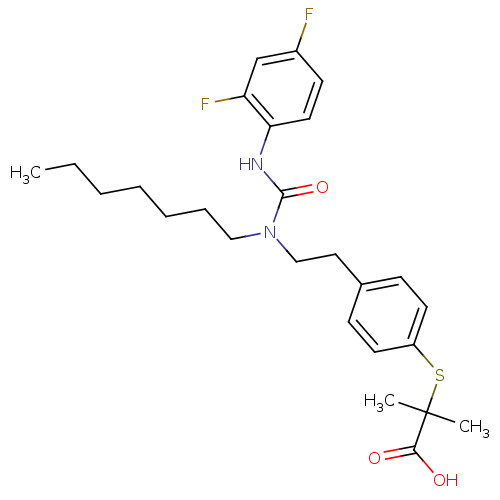
(2-{[4-(2-{[(2,4-difluorophenyl)carbamoyl](heptyl)a...)Show SMILES CCCCCCCN(CCc1ccc(SC(C)(C)C(O)=O)cc1)C(=O)Nc1ccc(F)cc1F Show InChI InChI=1S/C26H34F2N2O3S/c1-4-5-6-7-8-16-30(25(33)29-23-14-11-20(27)18-22(23)28)17-15-19-9-12-21(13-10-19)34-26(2,3)24(31)32/h9-14,18H,4-8,15-17H2,1-3H3,(H,29,33)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha expressed in HEK293 cells by GAL4 transactivation assay |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227659
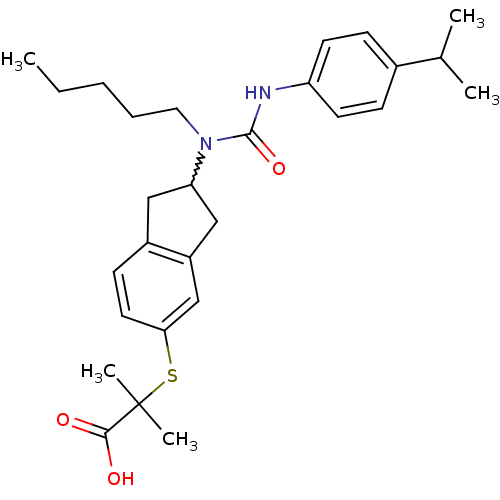
(2-(2-(3-(4-isopropylphenyl)-1-pentylureido)-2,3-di...)Show SMILES CCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(cc1)C(C)C |w:6.5| Show InChI InChI=1S/C28H38N2O3S/c1-6-7-8-15-30(27(33)29-23-12-9-20(10-13-23)19(2)3)24-16-21-11-14-25(18-22(21)17-24)34-28(4,5)26(31)32/h9-14,18-19,24H,6-8,15-17H2,1-5H3,(H,29,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 159 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227668
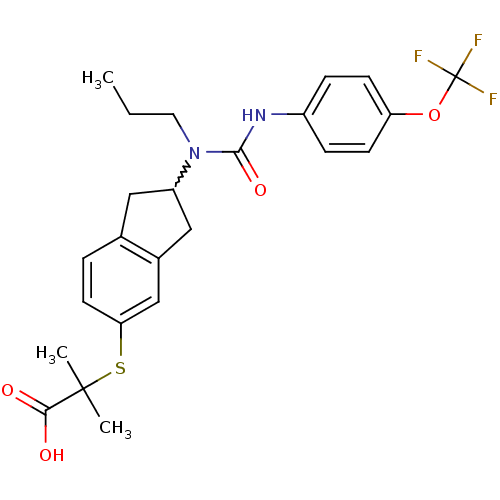
(2-methyl-2-(2-(1-propyl-3-(4-(trifluoromethoxy)phe...)Show SMILES CCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |w:4.3| Show InChI InChI=1S/C24H27F3N2O4S/c1-4-11-29(22(32)28-17-6-8-19(9-7-17)33-24(25,26)27)18-12-15-5-10-20(14-16(15)13-18)34-23(2,3)21(30)31/h5-10,14,18H,4,11-13H2,1-3H3,(H,28,32)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 158 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50227669

((R)-2-(2-(1-ethyl-3-(4-(trifluoromethoxy)phenyl)ur...)Show SMILES CCN([C@@H]1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C23H25F3N2O4S/c1-4-28(21(31)27-16-6-8-18(9-7-16)32-23(24,25)26)17-11-14-5-10-19(13-15(14)12-17)33-22(2,3)20(29)30/h5-10,13,17H,4,11-12H2,1-3H3,(H,27,31)(H,29,30)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta expressed in HEK293 cells by GAL4 transactivation assay |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227670
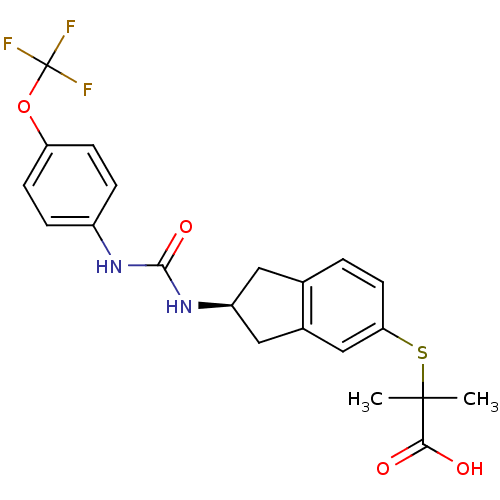
((S)-2-methyl-2-(2-(3-(4-(trifluoromethoxy)phenyl)u...)Show SMILES CC(C)(Sc1ccc2C[C@@H](Cc2c1)NC(=O)Nc1ccc(OC(F)(F)F)cc1)C(O)=O Show InChI InChI=1S/C21H21F3N2O4S/c1-20(2,18(27)28)31-17-8-3-12-9-15(10-13(12)11-17)26-19(29)25-14-4-6-16(7-5-14)30-21(22,23)24/h3-8,11,15H,9-10H2,1-2H3,(H,27,28)(H2,25,26,29)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227669

((R)-2-(2-(1-ethyl-3-(4-(trifluoromethoxy)phenyl)ur...)Show SMILES CCN([C@@H]1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C23H25F3N2O4S/c1-4-28(21(31)27-16-6-8-18(9-7-16)32-23(24,25)26)17-11-14-5-10-19(13-15(14)12-17)33-22(2,3)20(29)30/h5-10,13,17H,4,11-12H2,1-3H3,(H,27,31)(H,29,30)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227686
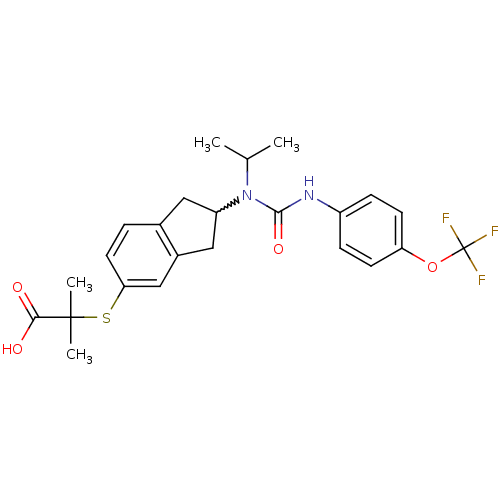
(2-(2-(1-isopropyl-3-(4-(trifluoromethoxy)phenyl)ur...)Show SMILES CC(C)N(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |w:4.3| Show InChI InChI=1S/C24H27F3N2O4S/c1-14(2)29(22(32)28-17-6-8-19(9-7-17)33-24(25,26)27)18-11-15-5-10-20(13-16(15)12-18)34-23(3,4)21(30)31/h5-10,13-14,18H,11-12H2,1-4H3,(H,28,32)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227698
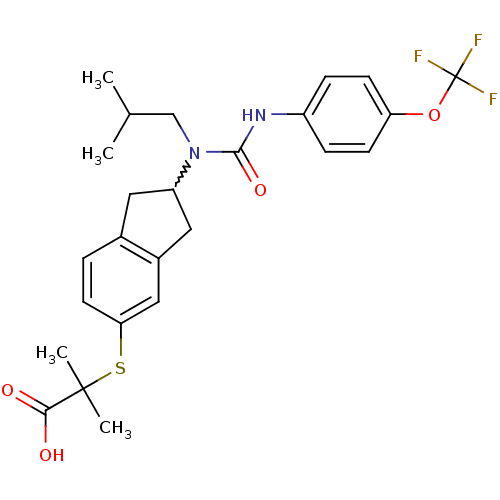
(2-(2-(1-isobutyl-3-(4-(trifluoromethoxy)phenyl)ure...)Show SMILES CC(C)CN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |w:5.4| Show InChI InChI=1S/C25H29F3N2O4S/c1-15(2)14-30(23(33)29-18-6-8-20(9-7-18)34-25(26,27)28)19-11-16-5-10-21(13-17(16)12-19)35-24(3,4)22(31)32/h5-10,13,15,19H,11-12,14H2,1-4H3,(H,29,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227699
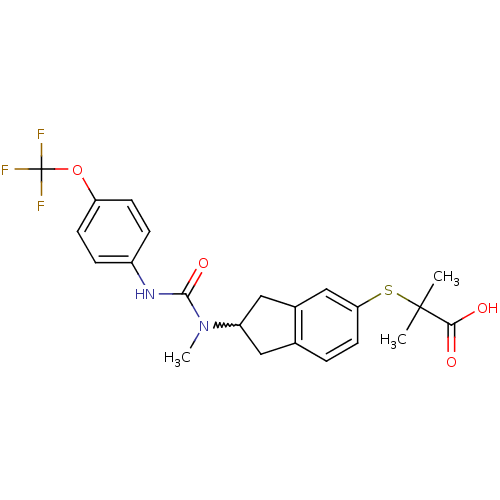
(2-methyl-2-(2-(1-methyl-3-(4-(trifluoromethoxy)phe...)Show SMILES CN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |w:2.1| Show InChI InChI=1S/C22H23F3N2O4S/c1-21(2,19(28)29)32-18-9-4-13-10-16(11-14(13)12-18)27(3)20(30)26-15-5-7-17(8-6-15)31-22(23,24)25/h4-9,12,16H,10-11H2,1-3H3,(H,26,30)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.85E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227703
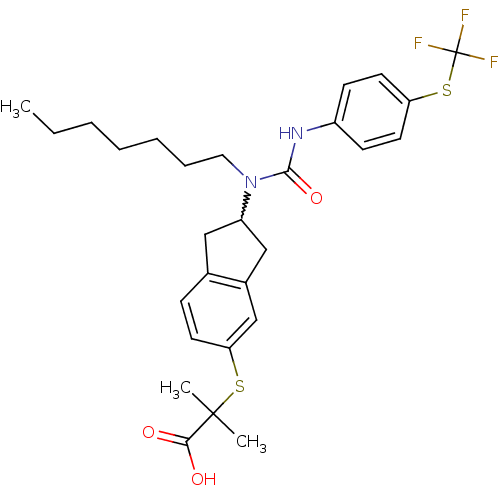
(2-(2-(1-heptyl-3-(4-(trifluoromethylthio)phenyl)ur...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(SC(F)(F)F)cc1 |w:8.7| Show InChI InChI=1S/C28H35F3N2O3S2/c1-4-5-6-7-8-15-33(26(36)32-21-10-13-23(14-11-21)38-28(29,30)31)22-16-19-9-12-24(18-20(19)17-22)37-27(2,3)25(34)35/h9-14,18,22H,4-8,15-17H2,1-3H3,(H,32,36)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 229 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227679
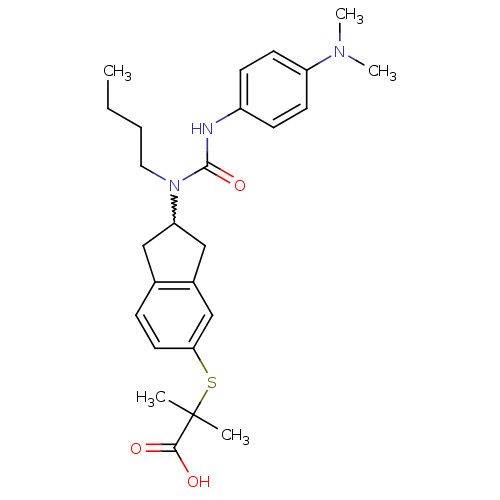
(2-(2-(1-butyl-3-(4-(dimethylamino)phenyl)ureido)-2...)Show SMILES CCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(cc1)N(C)C |w:5.4| Show InChI InChI=1S/C26H35N3O3S/c1-6-7-14-29(25(32)27-20-9-11-21(12-10-20)28(4)5)22-15-18-8-13-23(17-19(18)16-22)33-26(2,3)24(30)31/h8-13,17,22H,6-7,14-16H2,1-5H3,(H,27,32)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 561 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM28799

(2-{[4-(2-{[(2,4-difluorophenyl)carbamoyl](heptyl)a...)Show SMILES CCCCCCCN(CCc1ccc(SC(C)(C)C(O)=O)cc1)C(=O)Nc1ccc(F)cc1F Show InChI InChI=1S/C26H34F2N2O3S/c1-4-5-6-7-8-16-30(25(33)29-23-14-11-20(27)18-22(23)28)17-15-19-9-12-21(13-10-19)34-26(2,3)24(31)32/h9-14,18H,4-8,15-17H2,1-3H3,(H,29,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 166 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227680
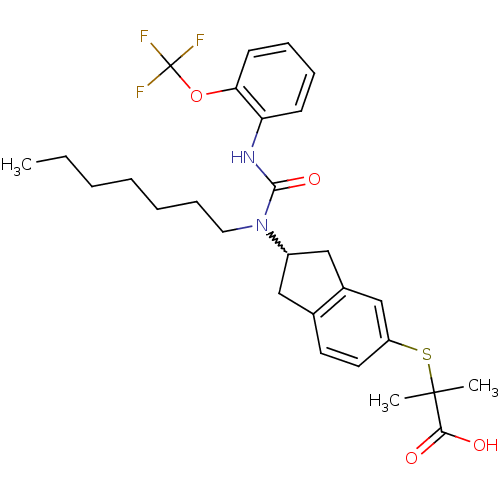
(2-(2-(1-heptyl-3-(2-(trifluoromethoxy)phenyl)ureid...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccccc1OC(F)(F)F |w:8.7| Show InChI InChI=1S/C28H35F3N2O4S/c1-4-5-6-7-10-15-33(26(36)32-23-11-8-9-12-24(23)37-28(29,30)31)21-16-19-13-14-22(18-20(19)17-21)38-27(2,3)25(34)35/h8-9,11-14,18,21H,4-7,10,15-17H2,1-3H3,(H,32,36)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.99E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50371235
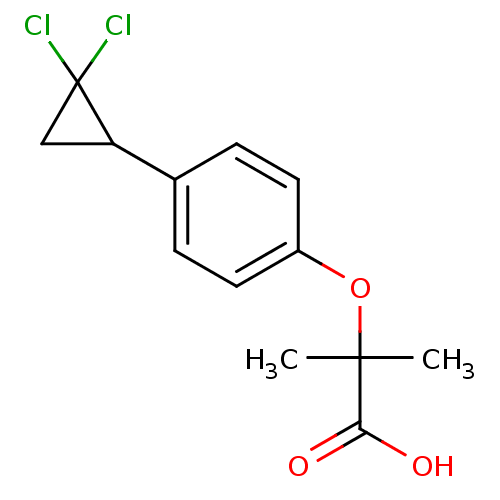
(CIPROFIBRATE)Show InChI InChI=1S/C13H14Cl2O3/c1-12(2,11(16)17)18-9-5-3-8(4-6-9)10-7-13(10,14)15/h3-6,10H,7H2,1-2H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227708
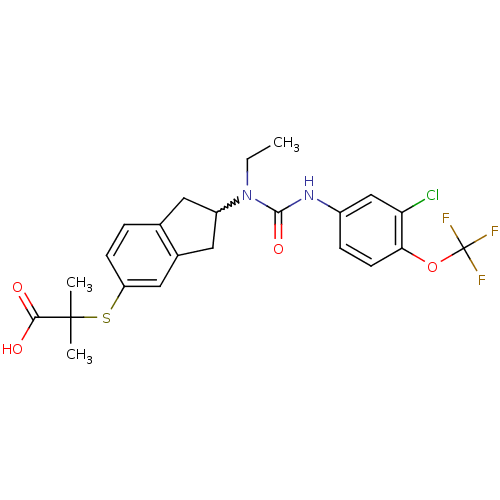
(2-(2-(3-(3-chloro-4-(trifluoromethoxy)phenyl)-1-et...)Show SMILES CCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)c(Cl)c1 |w:3.2| Show InChI InChI=1S/C23H24ClF3N2O4S/c1-4-29(21(32)28-15-6-8-19(18(24)12-15)33-23(25,26)27)16-9-13-5-7-17(11-14(13)10-16)34-22(2,3)20(30)31/h5-8,11-12,16H,4,9-10H2,1-3H3,(H,28,32)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50227669

((R)-2-(2-(1-ethyl-3-(4-(trifluoromethoxy)phenyl)ur...)Show SMILES CCN([C@@H]1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C23H25F3N2O4S/c1-4-28(21(31)27-16-6-8-18(9-7-16)32-23(24,25)26)17-11-14-5-10-19(13-15(14)12-17)33-22(2,3)20(29)30/h5-10,13,17H,4,11-12H2,1-3H3,(H,27,31)(H,29,30)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha expressed in HEK293 cells by GAL4 transactivation assay |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227662
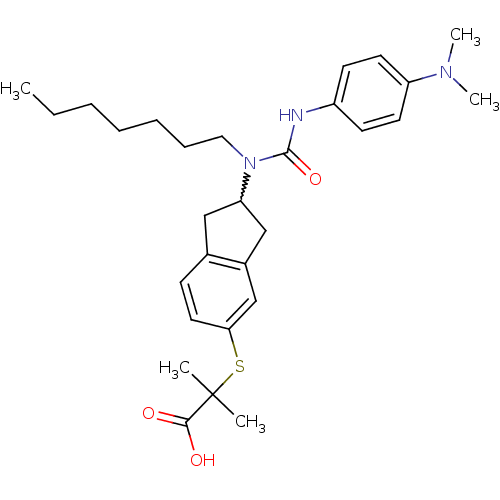
(2-(2-(3-(4-(dimethylamino)phenyl)-1-heptylureido)-...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(cc1)N(C)C |w:8.7| Show InChI InChI=1S/C29H41N3O3S/c1-6-7-8-9-10-17-32(28(35)30-23-12-14-24(15-13-23)31(4)5)25-18-21-11-16-26(20-22(21)19-25)36-29(2,3)27(33)34/h11-16,20,25H,6-10,17-19H2,1-5H3,(H,30,35)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 252 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227677
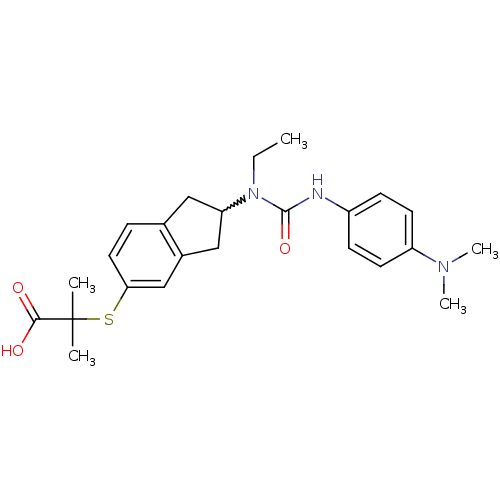
(2-(2-(3-(4-(dimethylamino)phenyl)-1-ethylureido)-2...)Show SMILES CCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(cc1)N(C)C |w:3.2| Show InChI InChI=1S/C24H31N3O3S/c1-6-27(23(30)25-18-8-10-19(11-9-18)26(4)5)20-13-16-7-12-21(15-17(16)14-20)31-24(2,3)22(28)29/h7-12,15,20H,6,13-14H2,1-5H3,(H,25,30)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 117 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28799

(2-{[4-(2-{[(2,4-difluorophenyl)carbamoyl](heptyl)a...)Show SMILES CCCCCCCN(CCc1ccc(SC(C)(C)C(O)=O)cc1)C(=O)Nc1ccc(F)cc1F Show InChI InChI=1S/C26H34F2N2O3S/c1-4-5-6-7-8-16-30(25(33)29-23-14-11-20(27)18-22(23)28)17-15-19-9-12-21(13-10-19)34-26(2,3)24(31)32/h9-14,18H,4-8,15-17H2,1-3H3,(H,29,33)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma expressed in HEK293 cells by GAL4 transactivation assay |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227688
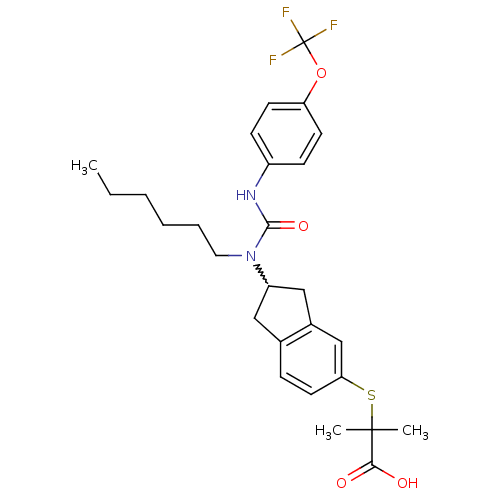
(2-(2-(1-hexyl-3-(4-(trifluoromethoxy)phenyl)ureido...)Show SMILES CCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |w:7.6| Show InChI InChI=1S/C27H33F3N2O4S/c1-4-5-6-7-14-32(25(35)31-20-9-11-22(12-10-20)36-27(28,29)30)21-15-18-8-13-23(17-19(18)16-21)37-26(2,3)24(33)34/h8-13,17,21H,4-7,14-16H2,1-3H3,(H,31,35)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 166 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227690
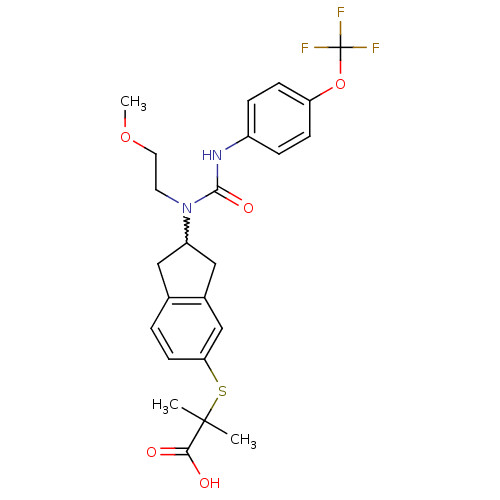
(2-(2-(1-(2-methoxyethyl)-3-(4-(trifluoromethoxy)ph...)Show SMILES COCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |w:5.4| Show InChI InChI=1S/C24H27F3N2O5S/c1-23(2,21(30)31)35-20-9-4-15-12-18(13-16(15)14-20)29(10-11-33-3)22(32)28-17-5-7-19(8-6-17)34-24(25,26)27/h4-9,14,18H,10-13H2,1-3H3,(H,28,32)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 425 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227689
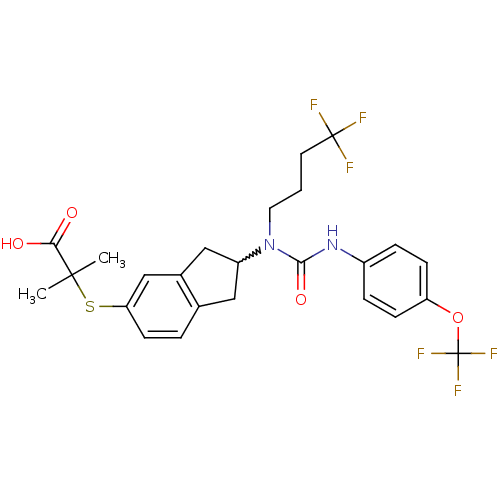
(2-methyl-2-(2-(1-(4,4,4-trifluorobutyl)-3-(4-(trif...)Show SMILES CC(C)(Sc1ccc2CC(Cc2c1)N(CCCC(F)(F)F)C(=O)Nc1ccc(OC(F)(F)F)cc1)C(O)=O |w:9.14| Show InChI InChI=1S/C25H26F6N2O4S/c1-23(2,21(34)35)38-20-9-4-15-12-18(13-16(15)14-20)33(11-3-10-24(26,27)28)22(36)32-17-5-7-19(8-6-17)37-25(29,30)31/h4-9,14,18H,3,10-13H2,1-2H3,(H,32,36)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 707 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma expressed in HEK293 cells assessed as aP2 gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227704

(2-methyl-2-(2-(1-pentyl-3-(4-(trifluoromethylthio)...)Show SMILES CCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(SC(F)(F)F)cc1 |w:6.5| Show InChI InChI=1S/C26H31F3N2O3S2/c1-4-5-6-13-31(24(34)30-19-8-11-21(12-9-19)36-26(27,28)29)20-14-17-7-10-22(16-18(17)15-20)35-25(2,3)23(32)33/h7-12,16,20H,4-6,13-15H2,1-3H3,(H,30,34)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 46 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227707
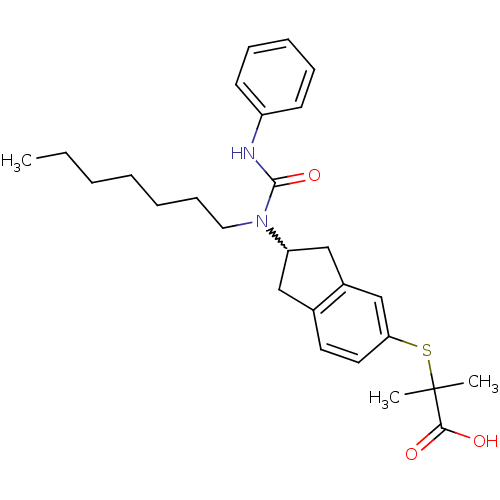
(2-(2-(1-heptyl-3-phenylureido)-2,3-dihydro-1H-inde...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccccc1 |w:8.7| Show InChI InChI=1S/C27H36N2O3S/c1-4-5-6-7-11-16-29(26(32)28-22-12-9-8-10-13-22)23-17-20-14-15-24(19-21(20)18-23)33-27(2,3)25(30)31/h8-10,12-15,19,23H,4-7,11,16-18H2,1-3H3,(H,28,32)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227655
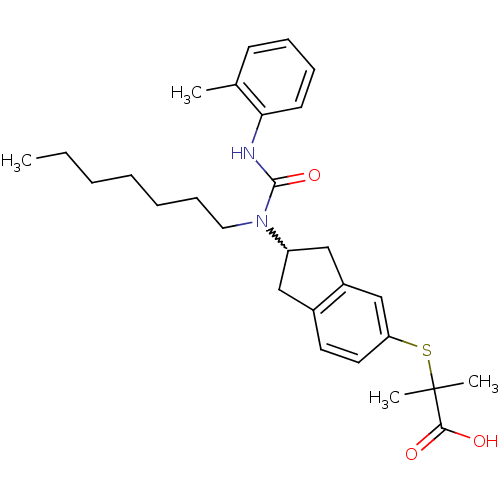
(2-(2-(1-heptyl-3-o-tolylureido)-2,3-dihydro-1H-ind...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccccc1C |w:8.7| Show InChI InChI=1S/C28H38N2O3S/c1-5-6-7-8-11-16-30(27(33)29-25-13-10-9-12-20(25)2)23-17-21-14-15-24(19-22(21)18-23)34-28(3,4)26(31)32/h9-10,12-15,19,23H,5-8,11,16-18H2,1-4H3,(H,29,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.54E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227661
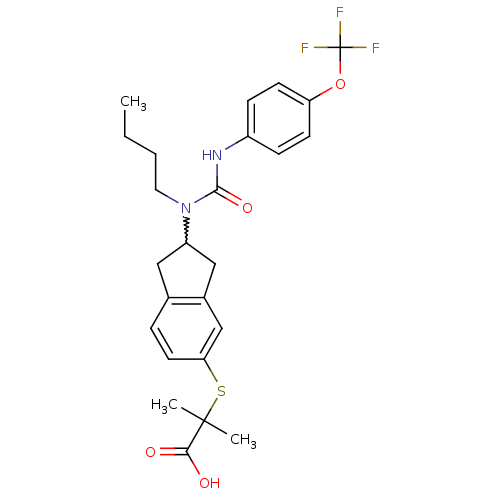
(2-(2-(1-butyl-3-(4-(trifluoromethoxy)phenyl)ureido...)Show SMILES CCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |w:5.4| Show InChI InChI=1S/C25H29F3N2O4S/c1-4-5-12-30(23(33)29-18-7-9-20(10-8-18)34-25(26,27)28)19-13-16-6-11-21(15-17(16)14-19)35-24(2,3)22(31)32/h6-11,15,19H,4-5,12-14H2,1-3H3,(H,29,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 195 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227666
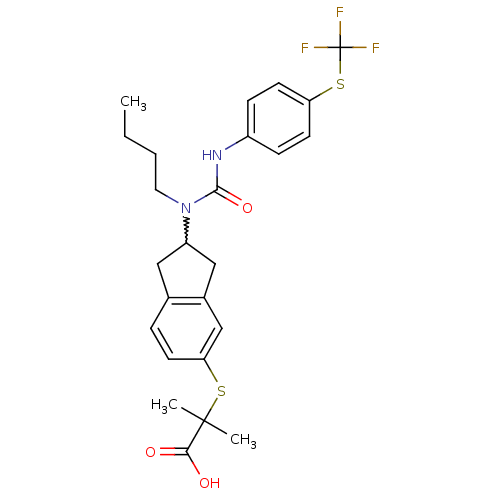
(2-(2-(1-butyl-3-(4-(trifluoromethylthio)phenyl)ure...)Show SMILES CCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(SC(F)(F)F)cc1 |w:5.4| Show InChI InChI=1S/C25H29F3N2O3S2/c1-4-5-12-30(23(33)29-18-7-10-20(11-8-18)35-25(26,27)28)19-13-16-6-9-21(15-17(16)14-19)34-24(2,3)22(31)32/h6-11,15,19H,4-5,12-14H2,1-3H3,(H,29,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227667
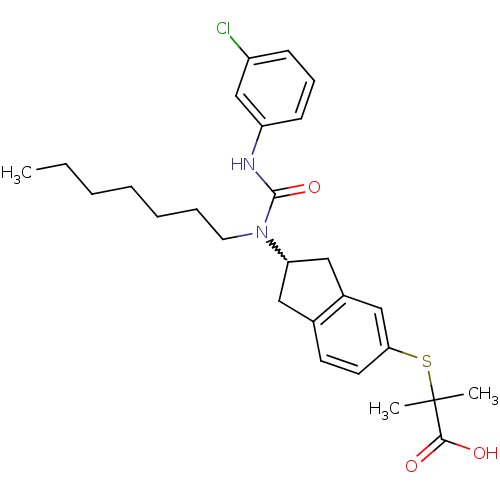
(2-(2-(3-(3-chlorophenyl)-1-heptylureido)-2,3-dihyd...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1cccc(Cl)c1 |w:8.7| Show InChI InChI=1S/C27H35ClN2O3S/c1-4-5-6-7-8-14-30(26(33)29-22-11-9-10-21(28)18-22)23-15-19-12-13-24(17-20(19)16-23)34-27(2,3)25(31)32/h9-13,17-18,23H,4-8,14-16H2,1-3H3,(H,29,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227675
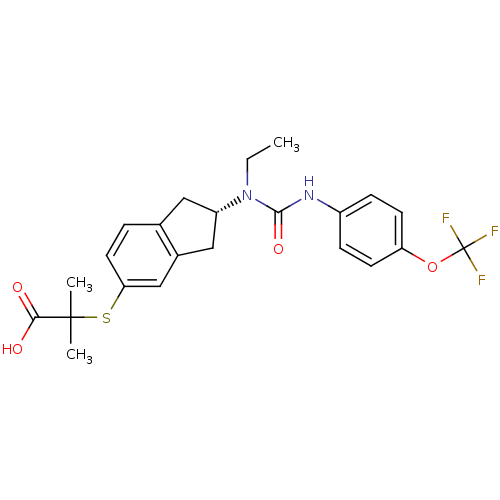
((S)-2-(2-(1-ethyl-3-(4-(trifluoromethoxy)phenyl)ur...)Show SMILES CCN([C@H]1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C23H25F3N2O4S/c1-4-28(21(31)27-16-6-8-18(9-7-16)32-23(24,25)26)17-11-14-5-10-19(13-15(14)12-17)33-22(2,3)20(29)30/h5-10,13,17H,4,11-12H2,1-3H3,(H,27,31)(H,29,30)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 165 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM28799

(2-{[4-(2-{[(2,4-difluorophenyl)carbamoyl](heptyl)a...)Show SMILES CCCCCCCN(CCc1ccc(SC(C)(C)C(O)=O)cc1)C(=O)Nc1ccc(F)cc1F Show InChI InChI=1S/C26H34F2N2O3S/c1-4-5-6-7-8-16-30(25(33)29-23-14-11-20(27)18-22(23)28)17-15-19-9-12-21(13-10-19)34-26(2,3)24(31)32/h9-14,18H,4-8,15-17H2,1-3H3,(H,29,33)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta expressed in HEK293 cells by GAL4 transactivation assay |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227682
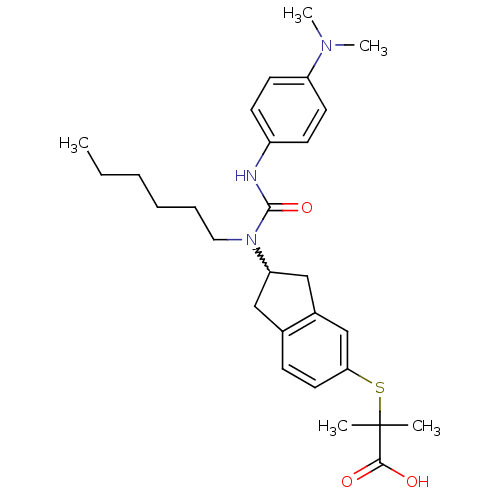
(2-(2-(3-(4-(dimethylamino)phenyl)-1-hexylureido)-2...)Show SMILES CCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(cc1)N(C)C |w:7.6| Show InChI InChI=1S/C28H39N3O3S/c1-6-7-8-9-16-31(27(34)29-22-11-13-23(14-12-22)30(4)5)24-17-20-10-15-25(19-21(20)18-24)35-28(2,3)26(32)33/h10-15,19,24H,6-9,16-18H2,1-5H3,(H,29,34)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 219 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227684
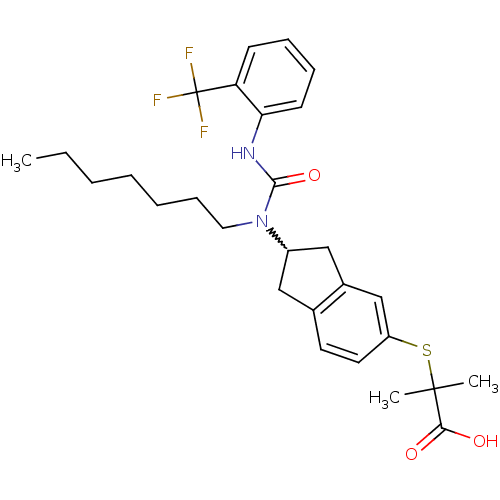
(2-(2-(1-heptyl-3-(2-(trifluoromethyl)phenyl)ureido...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccccc1C(F)(F)F |w:8.7| Show InChI InChI=1S/C28H35F3N2O3S/c1-4-5-6-7-10-15-33(26(36)32-24-12-9-8-11-23(24)28(29,30)31)21-16-19-13-14-22(18-20(19)17-21)37-27(2,3)25(34)35/h8-9,11-14,18,21H,4-7,10,15-17H2,1-3H3,(H,32,36)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.39E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227685
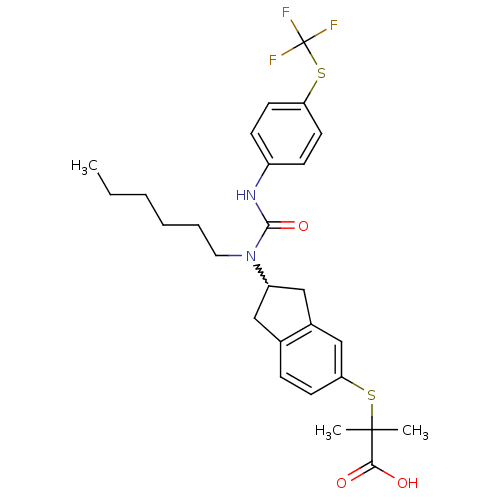
(2-(2-(1-hexyl-3-(4-(trifluoromethylthio)phenyl)ure...)Show SMILES CCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(SC(F)(F)F)cc1 |w:7.6| Show InChI InChI=1S/C27H33F3N2O3S2/c1-4-5-6-7-14-32(25(35)31-20-9-12-22(13-10-20)37-27(28,29)30)21-15-18-8-11-23(17-19(18)16-21)36-26(2,3)24(33)34/h8-13,17,21H,4-7,14-16H2,1-3H3,(H,31,35)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 249 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227692
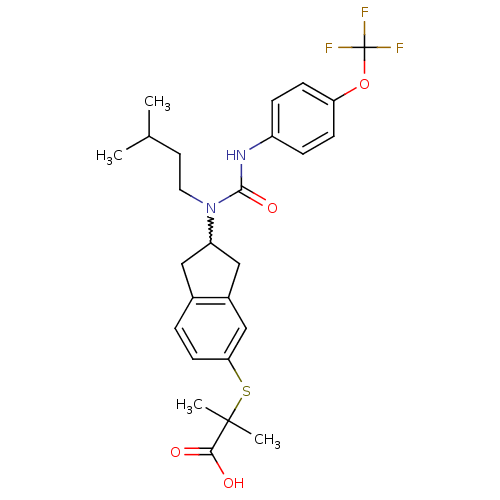
(2-(2-(1-isopentyl-3-(4-(trifluoromethoxy)phenyl)ur...)Show SMILES CC(C)CCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |w:6.5| Show InChI InChI=1S/C26H31F3N2O4S/c1-16(2)11-12-31(24(34)30-19-6-8-21(9-7-19)35-26(27,28)29)20-13-17-5-10-22(15-18(17)14-20)36-25(3,4)23(32)33/h5-10,15-16,20H,11-14H2,1-4H3,(H,30,34)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 386 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227693
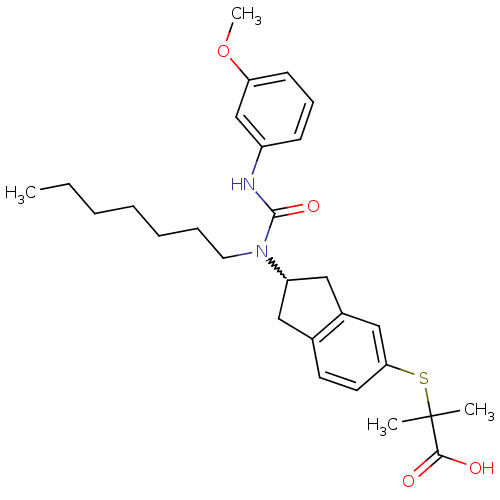
(2-(2-(1-heptyl-3-(3-methoxyphenyl)ureido)-2,3-dihy...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1cccc(OC)c1 |w:8.7| Show InChI InChI=1S/C28H38N2O4S/c1-5-6-7-8-9-15-30(27(33)29-22-11-10-12-24(19-22)34-4)23-16-20-13-14-25(18-21(20)17-23)35-28(2,3)26(31)32/h10-14,18-19,23H,5-9,15-17H2,1-4H3,(H,29,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227694
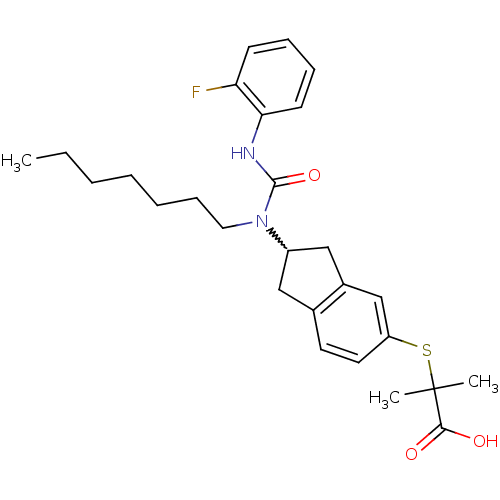
(2-(2-(3-(2-fluorophenyl)-1-heptylureido)-2,3-dihyd...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccccc1F |w:8.7| Show InChI InChI=1S/C27H35FN2O3S/c1-4-5-6-7-10-15-30(26(33)29-24-12-9-8-11-23(24)28)21-16-19-13-14-22(18-20(19)17-21)34-27(2,3)25(31)32/h8-9,11-14,18,21H,4-7,10,15-17H2,1-3H3,(H,29,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227656
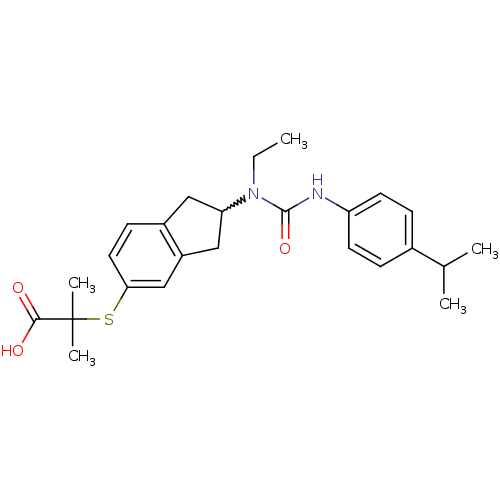
(2-(2-(1-ethyl-3-(4-isopropylphenyl)ureido)-2,3-dih...)Show SMILES CCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(cc1)C(C)C |w:3.2| Show InChI InChI=1S/C25H32N2O3S/c1-6-27(24(30)26-20-10-7-17(8-11-20)16(2)3)21-13-18-9-12-22(15-19(18)14-21)31-25(4,5)23(28)29/h7-12,15-16,21H,6,13-14H2,1-5H3,(H,26,30)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227674
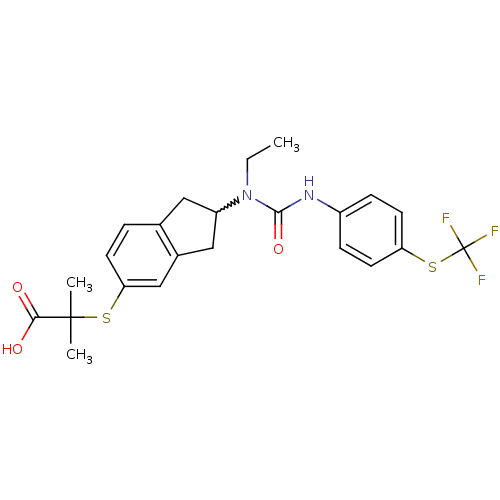
(2-(2-(1-ethyl-3-(4-(trifluoromethylthio)phenyl)ure...)Show SMILES CCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(SC(F)(F)F)cc1 |w:3.2| Show InChI InChI=1S/C23H25F3N2O3S2/c1-4-28(21(31)27-16-6-9-18(10-7-16)33-23(24,25)26)17-11-14-5-8-19(13-15(14)12-17)32-22(2,3)20(29)30/h5-10,13,17H,4,11-12H2,1-3H3,(H,27,31)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227681
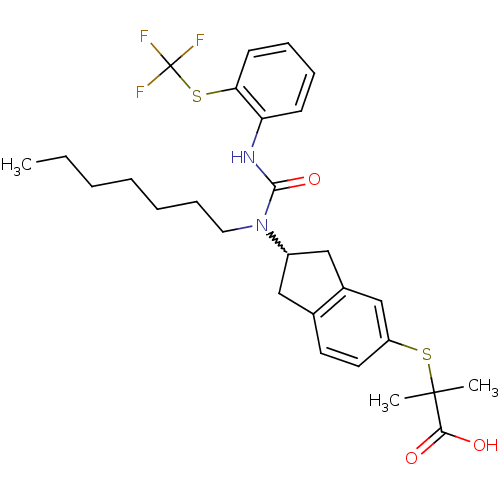
(2-(2-(1-heptyl-3-(2-(trifluoromethylthio)phenyl)ur...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccccc1SC(F)(F)F |w:8.7| Show InChI InChI=1S/C28H35F3N2O3S2/c1-4-5-6-7-10-15-33(26(36)32-23-11-8-9-12-24(23)38-28(29,30)31)21-16-19-13-14-22(18-20(19)17-21)37-27(2,3)25(34)35/h8-9,11-14,18,21H,4-7,10,15-17H2,1-3H3,(H,32,36)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.96E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50227669

((R)-2-(2-(1-ethyl-3-(4-(trifluoromethoxy)phenyl)ur...)Show SMILES CCN([C@@H]1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C23H25F3N2O4S/c1-4-28(21(31)27-16-6-8-18(9-7-16)32-23(24,25)26)17-11-14-5-10-19(13-15(14)12-17)33-22(2,3)20(29)30/h5-10,13,17H,4,11-12H2,1-3H3,(H,27,31)(H,29,30)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma expressed in HEK293 cells by GAL4 transactivation assay |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227702
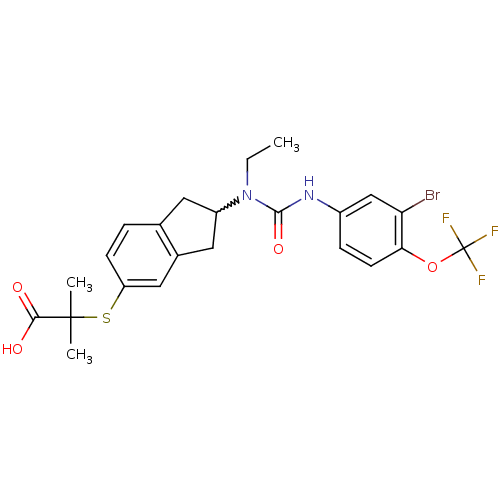
(2-(2-(3-(3-bromo-4-(trifluoromethoxy)phenyl)-1-eth...)Show SMILES CCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)c(Br)c1 |w:3.2| Show InChI InChI=1S/C23H24BrF3N2O4S/c1-4-29(21(32)28-15-6-8-19(18(24)12-15)33-23(25,26)27)16-9-13-5-7-17(11-14(13)10-16)34-22(2,3)20(30)31/h5-8,11-12,16H,4,9-10H2,1-3H3,(H,28,32)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227660
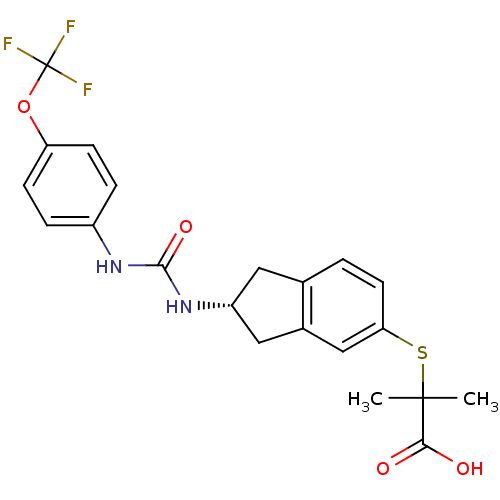
((R)-2-methyl-2-(2-(3-(4-(trifluoromethoxy)phenyl)u...)Show SMILES CC(C)(Sc1ccc2C[C@H](Cc2c1)NC(=O)Nc1ccc(OC(F)(F)F)cc1)C(O)=O Show InChI InChI=1S/C21H21F3N2O4S/c1-20(2,18(27)28)31-17-8-3-12-9-15(10-13(12)11-17)26-19(29)25-14-4-6-16(7-5-14)30-21(22,23)24/h3-8,11,15H,9-10H2,1-2H3,(H,27,28)(H2,25,26,29)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 146 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227658
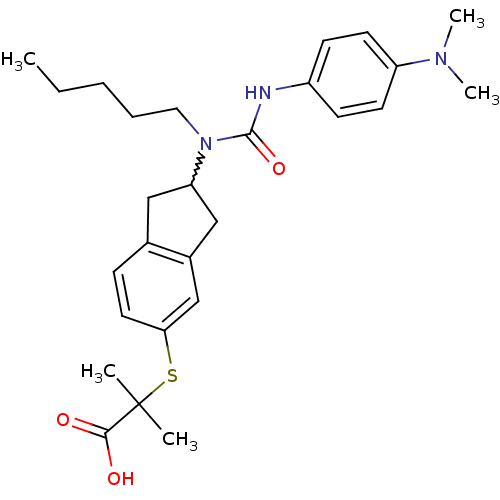
(2-(2-(3-(4-(dimethylamino)phenyl)-1-pentylureido)-...)Show SMILES CCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(cc1)N(C)C |w:6.5| Show InChI InChI=1S/C27H37N3O3S/c1-6-7-8-15-30(26(33)28-21-10-12-22(13-11-21)29(4)5)23-16-19-9-14-24(18-20(19)17-23)34-27(2,3)25(31)32/h9-14,18,23H,6-8,15-17H2,1-5H3,(H,28,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 141 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227671
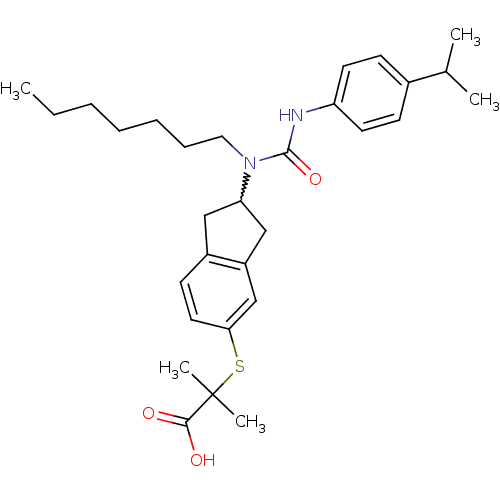
(2-(2-(1-heptyl-3-(4-isopropylphenyl)ureido)-2,3-di...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(cc1)C(C)C |w:8.7| Show InChI InChI=1S/C30H42N2O3S/c1-6-7-8-9-10-17-32(29(35)31-25-14-11-22(12-15-25)21(2)3)26-18-23-13-16-27(20-24(23)19-26)36-30(4,5)28(33)34/h11-16,20-21,26H,6-10,17-19H2,1-5H3,(H,31,35)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 252 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227676
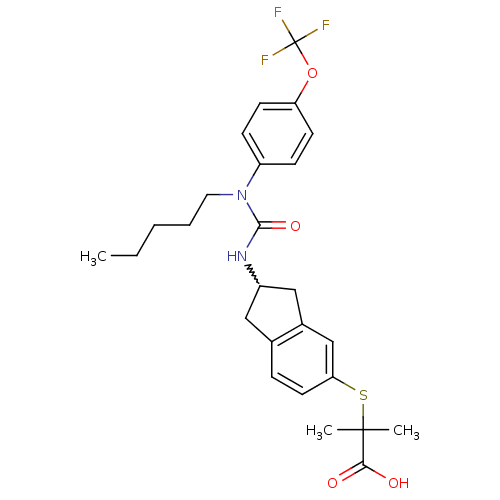
(2-methyl-2-(2-(3-pentyl-3-(4-(trifluoromethoxy)phe...)Show SMILES CCCCCN(C(=O)NC1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)c1ccc(OC(F)(F)F)cc1 |w:9.8| Show InChI InChI=1S/C26H31F3N2O4S/c1-4-5-6-13-31(20-8-10-21(11-9-20)35-26(27,28)29)24(34)30-19-14-17-7-12-22(16-18(17)15-19)36-25(2,3)23(32)33/h7-12,16,19H,4-6,13-15H2,1-3H3,(H,30,34)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50085042
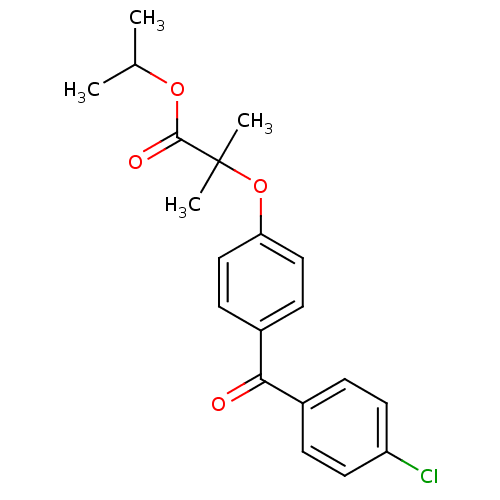
(2-(4-(4-Chlorobenzoyl)phenoxy)-2-methylpropanoic a...)Show SMILES CC(C)OC(=O)C(C)(C)Oc1ccc(cc1)C(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C20H21ClO4/c1-13(2)24-19(23)20(3,4)25-17-11-7-15(8-12-17)18(22)14-5-9-16(21)10-6-14/h5-13H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227696
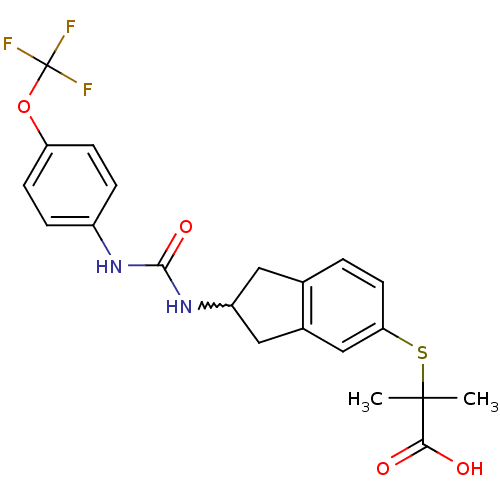
(2-methyl-2-(2-(3-(4-(trifluoromethoxy)phenyl)ureid...)Show SMILES CC(C)(Sc1ccc2CC(Cc2c1)NC(=O)Nc1ccc(OC(F)(F)F)cc1)C(O)=O |w:9.14| Show InChI InChI=1S/C21H21F3N2O4S/c1-20(2,18(27)28)31-17-8-3-12-9-15(10-13(12)11-17)26-19(29)25-14-4-6-16(7-5-14)30-21(22,23)24/h3-8,11,15H,9-10H2,1-2H3,(H,27,28)(H2,25,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 96 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227691
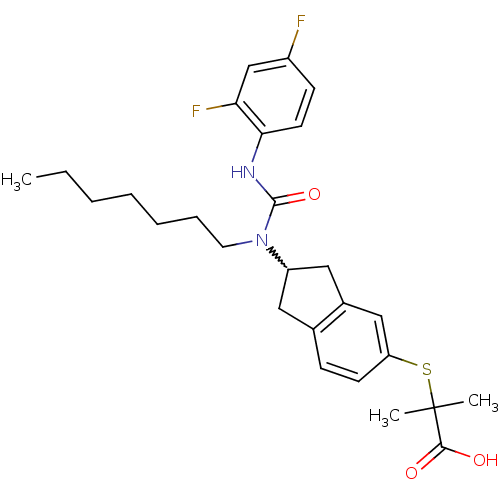
(2-(2-(3-(2,4-difluorophenyl)-1-heptylureido)-2,3-d...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(F)cc1F |w:8.7| Show InChI InChI=1S/C27H34F2N2O3S/c1-4-5-6-7-8-13-31(26(34)30-24-12-10-20(28)17-23(24)29)21-14-18-9-11-22(16-19(18)15-21)35-27(2,3)25(32)33/h9-12,16-17,21H,4-8,13-15H2,1-3H3,(H,30,34)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
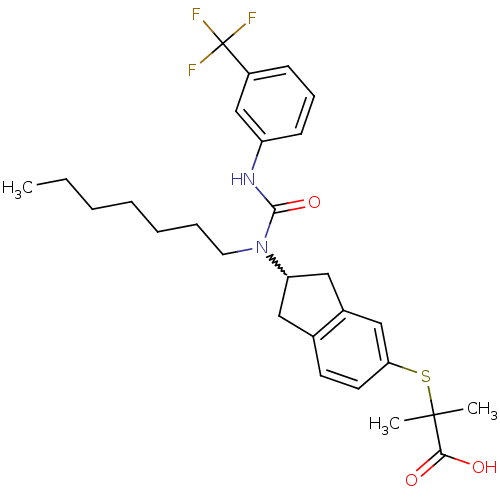
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227706
(2-(2-(1-heptyl-3-(3-(trifluoromethyl)phenyl)ureido...)Show SMILES CCCCCCCN(C1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1cccc(c1)C(F)(F)F |w:8.7| Show InChI InChI=1S/C28H35F3N2O3S/c1-4-5-6-7-8-14-33(26(36)32-22-11-9-10-21(18-22)28(29,30)31)23-15-19-12-13-24(17-20(19)16-23)37-27(2,3)25(34)35/h9-13,17-18,23H,4-8,14-16H2,1-3H3,(H,32,36)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.73E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |
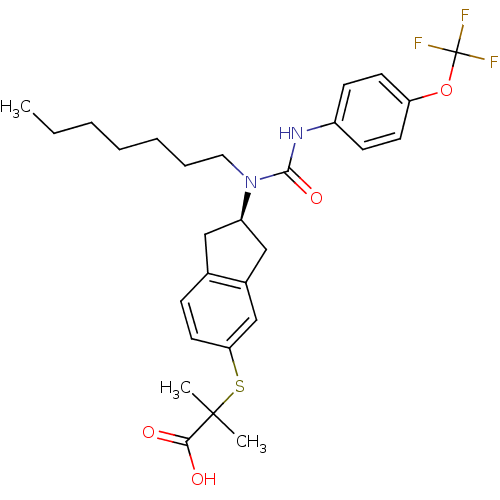
Peroxisome proliferator-activated receptor alpha
(Rattus norvegicus) | BDBM50227705
((R)-2-(2-(1-heptyl-3-(4-(trifluoromethoxy)phenyl)u...)Show SMILES CCCCCCCN([C@@H]1Cc2ccc(SC(C)(C)C(O)=O)cc2C1)C(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C28H35F3N2O4S/c1-4-5-6-7-8-15-33(26(36)32-21-10-12-23(13-11-21)37-28(29,30)31)22-16-19-9-14-24(18-20(19)17-22)38-27(2,3)25(34)35/h9-14,18,22H,4-8,15-17H2,1-3H3,(H,32,36)(H,34,35)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 169 | n/a | n/a | n/a | n/a |
Johnson& Johnson
Curated by ChEMBL
| Assay Description
Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction |
Bioorg Med Chem Lett 17: 6773-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.041
BindingDB Entry DOI: 10.7270/Q27945H6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data