 Found 66 hits of Enzyme Inhibition Constant Data
Found 66 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228014
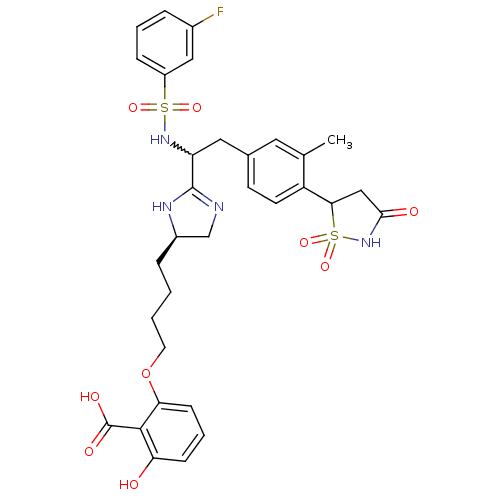
(2-[4-((R)-2-{1-(3-fluoro-benzenesulfonylamino)-2-[...)Show SMILES Cc1cc(CC(NS(=O)(=O)c2cccc(F)c2)C2=NC[C@@H](CCCCOc3cccc(O)c3C(O)=O)N2)ccc1C1CC(=O)NS1(=O)=O |w:5.5,t:18| Show InChI InChI=1S/C32H35FN4O9S2/c1-19-14-20(11-12-24(19)28-17-29(39)37-48(28,44)45)15-25(36-47(42,43)23-8-4-6-21(33)16-23)31-34-18-22(35-31)7-2-3-13-46-27-10-5-9-26(38)30(27)32(40)41/h4-6,8-12,14,16,22,25,28,36,38H,2-3,7,13,15,17-18H2,1H3,(H,34,35)(H,37,39)(H,40,41)/t22-,25?,28?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228013
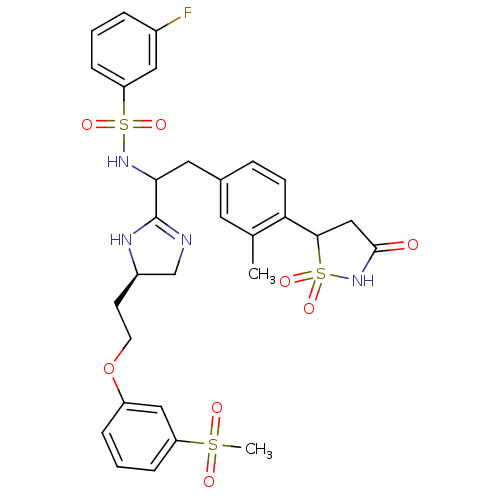
(3-fluoro-N-{1-{(R)-5-[2-(3-methanesulfonyl-phenoxy...)Show SMILES Cc1cc(CC(NS(=O)(=O)c2cccc(F)c2)C2=NC[C@@H](CCOc3cccc(c3)S(C)(=O)=O)N2)ccc1C1CC(=O)NS1(=O)=O |t:18| Show InChI InChI=1S/C30H33FN4O8S3/c1-19-13-20(9-10-26(19)28-17-29(36)35-46(28,41)42)14-27(34-45(39,40)25-8-3-5-21(31)15-25)30-32-18-22(33-30)11-12-43-23-6-4-7-24(16-23)44(2,37)38/h3-10,13,15-16,22,27-28,34H,11-12,14,17-18H2,1-2H3,(H,32,33)(H,35,36)/t22-,27?,28?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228024
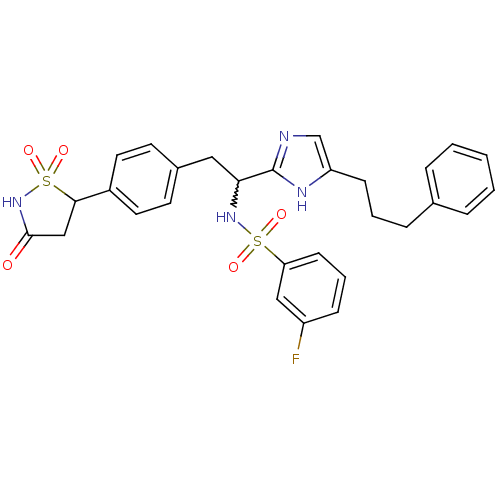
(3-fluoro-N-{1-[5-(3-phenyl-propyl)-1H-imidazol-2-y...)Show SMILES Fc1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1ncc(CCCc2ccccc2)[nH]1 |w:11.11| Show InChI InChI=1S/C29H29FN4O5S2/c30-23-9-5-11-25(17-23)40(36,37)33-26(29-31-19-24(32-29)10-4-8-20-6-2-1-3-7-20)16-21-12-14-22(15-13-21)27-18-28(35)34-41(27,38)39/h1-3,5-7,9,11-15,17,19,26-27,33H,4,8,10,16,18H2,(H,31,32)(H,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228013

(3-fluoro-N-{1-{(R)-5-[2-(3-methanesulfonyl-phenoxy...)Show SMILES Cc1cc(CC(NS(=O)(=O)c2cccc(F)c2)C2=NC[C@@H](CCOc3cccc(c3)S(C)(=O)=O)N2)ccc1C1CC(=O)NS1(=O)=O |t:18| Show InChI InChI=1S/C30H33FN4O8S3/c1-19-13-20(9-10-26(19)28-17-29(36)35-46(28,41)42)14-27(34-45(39,40)25-8-3-5-21(31)15-25)30-32-18-22(33-30)11-12-43-23-6-4-7-24(16-23)44(2,37)38/h3-10,13,15-16,22,27-28,34H,11-12,14,17-18H2,1-2H3,(H,32,33)(H,35,36)/t22-,27?,28?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228008

(2-[4-(2-{1-(3-fluoro-benzenesulfonylamino)-2-[3-me...)Show SMILES Cc1cc(CC(NS(=O)(=O)c2cccc(F)c2)c2ncc(CCCCOc3cccc(O)c3C(O)=O)[nH]2)ccc1C1CC(=O)NS1(=O)=O |w:5.5| Show InChI InChI=1S/C32H33FN4O9S2/c1-19-14-20(11-12-24(19)28-17-29(39)37-48(28,44)45)15-25(36-47(42,43)23-8-4-6-21(33)16-23)31-34-18-22(35-31)7-2-3-13-46-27-10-5-9-26(38)30(27)32(40)41/h4-6,8-12,14,16,18,25,28,36,38H,2-3,7,13,15,17H2,1H3,(H,34,35)(H,37,39)(H,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228038

(3-fluoro-N-{1-[(R)-5-(2-fluoro-benzyl)-4,5-dihydro...)Show SMILES Fc1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)C1=NC[C@@H](Cc2ccccc2F)N1 |w:11.11,t:30| Show InChI InChI=1S/C27H26F2N4O5S2/c28-20-5-3-6-22(14-20)39(35,36)32-24(27-30-16-21(31-27)13-19-4-1-2-7-23(19)29)12-17-8-10-18(11-9-17)25-15-26(34)33-40(25,37)38/h1-11,14,21,24-25,32H,12-13,15-16H2,(H,30,31)(H,33,34)/t21-,24?,25?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228024

(3-fluoro-N-{1-[5-(3-phenyl-propyl)-1H-imidazol-2-y...)Show SMILES Fc1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1ncc(CCCc2ccccc2)[nH]1 |w:11.11| Show InChI InChI=1S/C29H29FN4O5S2/c30-23-9-5-11-25(17-23)40(36,37)33-26(29-31-19-24(32-29)10-4-8-20-6-2-1-3-7-20)16-21-12-14-22(15-13-21)27-18-28(35)34-41(27,38)39/h1-3,5-7,9,11-15,17,19,26-27,33H,4,8,10,16,18H2,(H,31,32)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50189675

(CHEMBL436933 | N-{1-(1H-benzoimidazol-2-yl)-2-[4-(...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C25H21F3N4O5S2/c26-25(27,28)17-4-3-5-18(13-17)38(34,35)31-21(24-29-19-6-1-2-7-20(19)30-24)12-15-8-10-16(11-9-15)22-14-23(33)32-39(22,36)37/h1-11,13,21-22,31H,12,14H2,(H,29,30)(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228014

(2-[4-((R)-2-{1-(3-fluoro-benzenesulfonylamino)-2-[...)Show SMILES Cc1cc(CC(NS(=O)(=O)c2cccc(F)c2)C2=NC[C@@H](CCCCOc3cccc(O)c3C(O)=O)N2)ccc1C1CC(=O)NS1(=O)=O |w:5.5,t:18| Show InChI InChI=1S/C32H35FN4O9S2/c1-19-14-20(11-12-24(19)28-17-29(39)37-48(28,44)45)15-25(36-47(42,43)23-8-4-6-21(33)16-23)31-34-18-22(35-31)7-2-3-13-46-27-10-5-9-26(38)30(27)32(40)41/h4-6,8-12,14,16,22,25,28,36,38H,2-3,7,13,15,17-18H2,1H3,(H,34,35)(H,37,39)(H,40,41)/t22-,25?,28?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228008

(2-[4-(2-{1-(3-fluoro-benzenesulfonylamino)-2-[3-me...)Show SMILES Cc1cc(CC(NS(=O)(=O)c2cccc(F)c2)c2ncc(CCCCOc3cccc(O)c3C(O)=O)[nH]2)ccc1C1CC(=O)NS1(=O)=O |w:5.5| Show InChI InChI=1S/C32H33FN4O9S2/c1-19-14-20(11-12-24(19)28-17-29(39)37-48(28,44)45)15-25(36-47(42,43)23-8-4-6-21(33)16-23)31-34-18-22(35-31)7-2-3-13-46-27-10-5-9-26(38)30(27)32(40)41/h4-6,8-12,14,16,18,25,28,36,38H,2-3,7,13,15,17H2,1H3,(H,34,35)(H,37,39)(H,40,41) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228036

(CHEMBL253269 | N-{1-[5-(3-phenyl-propyl)-1H-imidaz...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1ncc(CCCc2ccccc2)[nH]1 |w:14.14| Show InChI InChI=1S/C30H29F3N4O5S2/c31-30(32,33)23-9-5-11-25(17-23)43(39,40)36-26(29-34-19-24(35-29)10-4-8-20-6-2-1-3-7-20)16-21-12-14-22(15-13-21)27-18-28(38)37-44(27,41)42/h1-3,5-7,9,11-15,17,19,26-27,36H,4,8,10,16,18H2,(H,34,35)(H,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228036

(CHEMBL253269 | N-{1-[5-(3-phenyl-propyl)-1H-imidaz...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1ncc(CCCc2ccccc2)[nH]1 |w:14.14| Show InChI InChI=1S/C30H29F3N4O5S2/c31-30(32,33)23-9-5-11-25(17-23)43(39,40)36-26(29-34-19-24(35-29)10-4-8-20-6-2-1-3-7-20)16-21-12-14-22(15-13-21)27-18-28(38)37-44(27,41)42/h1-3,5-7,9,11-15,17,19,26-27,36H,4,8,10,16,18H2,(H,34,35)(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228032

(CHEMBL403908 | N-{1-(5-phenyl-1H-imidazol-2-yl)-2-...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1nc(c[nH]1)-c1ccccc1 |w:14.14| Show InChI InChI=1S/C27H23F3N4O5S2/c28-27(29,30)20-7-4-8-21(14-20)40(36,37)33-22(26-31-16-23(32-26)18-5-2-1-3-6-18)13-17-9-11-19(12-10-17)24-15-25(35)34-41(24,38)39/h1-12,14,16,22,24,33H,13,15H2,(H,31,32)(H,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228038

(3-fluoro-N-{1-[(R)-5-(2-fluoro-benzyl)-4,5-dihydro...)Show SMILES Fc1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)C1=NC[C@@H](Cc2ccccc2F)N1 |w:11.11,t:30| Show InChI InChI=1S/C27H26F2N4O5S2/c28-20-5-3-6-22(14-20)39(35,36)32-24(27-30-16-21(31-27)13-19-4-1-2-7-23(19)29)12-17-8-10-18(11-9-17)25-15-26(34)33-40(25,37)38/h1-11,14,21,24-25,32H,12-13,15-16H2,(H,30,31)(H,33,34)/t21-,24?,25?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228025

(2-[4-((R)-2-{1-(3-fluoro-benzenesulfonylamino)-2-[...)Show SMILES COC(=O)c1c(O)cccc1OCCCC[C@@H]1CN=C(N1)C(Cc1ccc(C2CC(=O)NS2(=O)=O)c(C)c1)NS(=O)(=O)c1cccc(F)c1 |w:21.41,c:19| Show InChI InChI=1S/C33H37FN4O9S2/c1-20-15-21(12-13-25(20)29-18-30(40)38-49(29,44)45)16-26(37-48(42,43)24-9-5-7-22(34)17-24)32-35-19-23(36-32)8-3-4-14-47-28-11-6-10-27(39)31(28)33(41)46-2/h5-7,9-13,15,17,23,26,29,37,39H,3-4,8,14,16,18-19H2,1-2H3,(H,35,36)(H,38,40)/t23-,26?,29?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50189675

(CHEMBL436933 | N-{1-(1H-benzoimidazol-2-yl)-2-[4-(...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C25H21F3N4O5S2/c26-25(27,28)17-4-3-5-18(13-17)38(34,35)31-21(24-29-19-6-1-2-7-20(19)30-24)12-15-8-10-16(11-9-15)22-14-23(33)32-39(22,36)37/h1-11,13,21-22,31H,12,14H2,(H,29,30)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228039
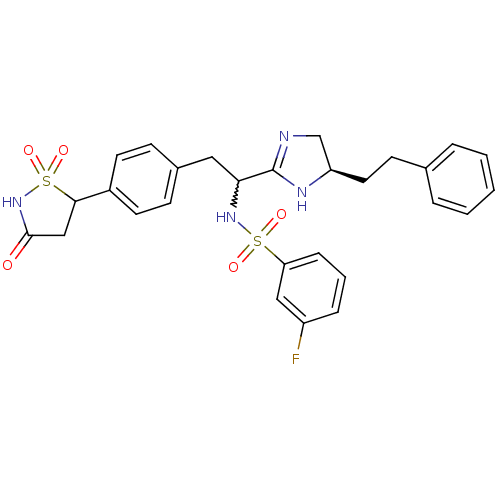
(3-fluoro-N-{1-((R)-5-phenethyl-4,5-dihydro-1H-imid...)Show SMILES Fc1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)C1=NC[C@@H](CCc2ccccc2)N1 |w:11.11,t:30| Show InChI InChI=1S/C28H29FN4O5S2/c29-22-7-4-8-24(16-22)39(35,36)32-25(28-30-18-23(31-28)14-11-19-5-2-1-3-6-19)15-20-9-12-21(13-10-20)26-17-27(34)33-40(26,37)38/h1-10,12-13,16,23,25-26,32H,11,14-15,17-18H2,(H,30,31)(H,33,34)/t23-,25?,26?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228012

(3-fluoro-N-{1-{(R)-5-[3-(3-methanesulfonyl-phenoxy...)Show SMILES Cc1cc(CC(NS(=O)(=O)c2cccc(F)c2)C2=NC[C@@H](CCCOc3cccc(c3)S(C)(=O)=O)N2)ccc1C1CC(=O)NS1(=O)=O |w:5.5,t:18| Show InChI InChI=1S/C31H35FN4O8S3/c1-20-14-21(11-12-27(20)29-18-30(37)36-47(29,42)43)15-28(35-46(40,41)26-10-3-6-22(32)16-26)31-33-19-23(34-31)7-5-13-44-24-8-4-9-25(17-24)45(2,38)39/h3-4,6,8-12,14,16-17,23,28-29,35H,5,7,13,15,18-19H2,1-2H3,(H,33,34)(H,36,37)/t23-,28?,29?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228021

(3-fluoro-N-{1-(5-phenyl-1H-imidazol-2-yl)-2-[4-(1,...)Show SMILES Fc1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1nc(c[nH]1)-c1ccccc1 |w:11.11| Show InChI InChI=1S/C26H23FN4O5S2/c27-20-7-4-8-21(14-20)37(33,34)30-22(26-28-16-23(29-26)18-5-2-1-3-6-18)13-17-9-11-19(12-10-17)24-15-25(32)31-38(24,35)36/h1-12,14,16,22,24,30H,13,15H2,(H,28,29)(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228028

(3-fluoro-N-{1-(5-phenethyl-1H-imidazol-2-yl)-2-[4-...)Show SMILES Fc1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1ncc(CCc2ccccc2)[nH]1 |w:11.11| Show InChI InChI=1S/C28H27FN4O5S2/c29-22-7-4-8-24(16-22)39(35,36)32-25(28-30-18-23(31-28)14-11-19-5-2-1-3-6-19)15-20-9-12-21(13-10-20)26-17-27(34)33-40(26,37)38/h1-10,12-13,16,18,25-26,32H,11,14-15,17H2,(H,30,31)(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228032

(CHEMBL403908 | N-{1-(5-phenyl-1H-imidazol-2-yl)-2-...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1nc(c[nH]1)-c1ccccc1 |w:14.14| Show InChI InChI=1S/C27H23F3N4O5S2/c28-27(29,30)20-7-4-8-21(14-20)40(36,37)33-22(26-31-16-23(32-26)18-5-2-1-3-6-18)13-17-9-11-19(12-10-17)24-15-25(35)34-41(24,38)39/h1-12,14,16,22,24,33H,13,15H2,(H,31,32)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228029
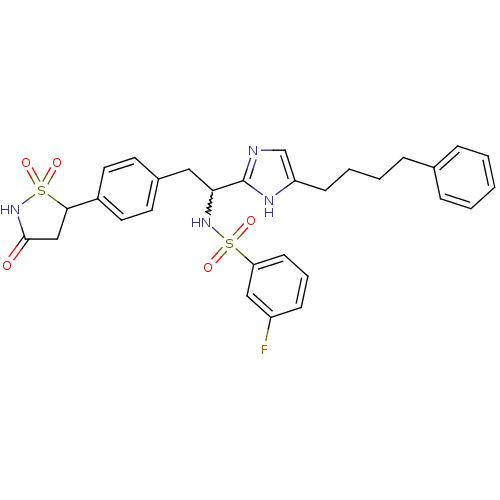
(3-fluoro-N-{1-[5-(4-phenyl-butyl)-1H-imidazol-2-yl...)Show SMILES Fc1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1ncc(CCCCc2ccccc2)[nH]1 |w:11.11| Show InChI InChI=1S/C30H31FN4O5S2/c31-24-10-6-12-26(18-24)41(37,38)34-27(17-22-13-15-23(16-14-22)28-19-29(36)35-42(28,39)40)30-32-20-25(33-30)11-5-4-9-21-7-2-1-3-8-21/h1-3,6-8,10,12-16,18,20,27-28,34H,4-5,9,11,17,19H2,(H,32,33)(H,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228025

(2-[4-((R)-2-{1-(3-fluoro-benzenesulfonylamino)-2-[...)Show SMILES COC(=O)c1c(O)cccc1OCCCC[C@@H]1CN=C(N1)C(Cc1ccc(C2CC(=O)NS2(=O)=O)c(C)c1)NS(=O)(=O)c1cccc(F)c1 |w:21.41,c:19| Show InChI InChI=1S/C33H37FN4O9S2/c1-20-15-21(12-13-25(20)29-18-30(40)38-49(29,44)45)16-26(37-48(42,43)24-9-5-7-22(34)17-24)32-35-19-23(36-32)8-3-4-14-47-28-11-6-10-27(39)31(28)33(41)46-2/h5-7,9-13,15,17,23,26,29,37,39H,3-4,8,14,16,18-19H2,1-2H3,(H,35,36)(H,38,40)/t23-,26?,29?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228020

(CHEMBL252710 | N-{1-((R)-5-benzyl-4,5-dihydro-1H-i...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)C1=NC[C@@H](Cc2ccccc2)N1 |w:14.14,t:33| Show InChI InChI=1S/C28H27F3N4O5S2/c29-28(30,31)21-7-4-8-23(15-21)41(37,38)34-24(27-32-17-22(33-27)13-18-5-2-1-3-6-18)14-19-9-11-20(12-10-19)25-16-26(36)35-42(25,39)40/h1-12,15,22,24-25,34H,13-14,16-17H2,(H,32,33)(H,35,36)/t22-,24?,25?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228009
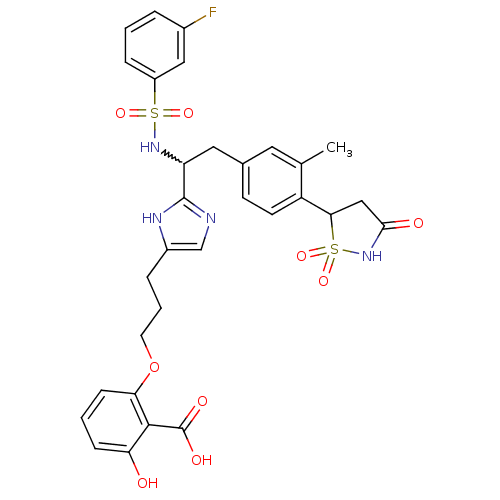
(2-[3-(2-{1-(3-fluoro-benzenesulfonylamino)-2-[3-me...)Show SMILES Cc1cc(CC(NS(=O)(=O)c2cccc(F)c2)c2ncc(CCCOc3cccc(O)c3C(O)=O)[nH]2)ccc1C1CC(=O)NS1(=O)=O |w:5.5| Show InChI InChI=1S/C31H31FN4O9S2/c1-18-13-19(10-11-23(18)27-16-28(38)36-47(27,43)44)14-24(35-46(41,42)22-7-2-5-20(32)15-22)30-33-17-21(34-30)6-4-12-45-26-9-3-8-25(37)29(26)31(39)40/h2-3,5,7-11,13,15,17,24,27,35,37H,4,6,12,14,16H2,1H3,(H,33,34)(H,36,38)(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228039

(3-fluoro-N-{1-((R)-5-phenethyl-4,5-dihydro-1H-imid...)Show SMILES Fc1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)C1=NC[C@@H](CCc2ccccc2)N1 |w:11.11,t:30| Show InChI InChI=1S/C28H29FN4O5S2/c29-22-7-4-8-24(16-22)39(35,36)32-25(28-30-18-23(31-28)14-11-19-5-2-1-3-6-19)15-20-9-12-21(13-10-20)26-17-27(34)33-40(26,37)38/h1-10,12-13,16,23,25-26,32H,11,14-15,17-18H2,(H,30,31)(H,33,34)/t23-,25?,26?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228033
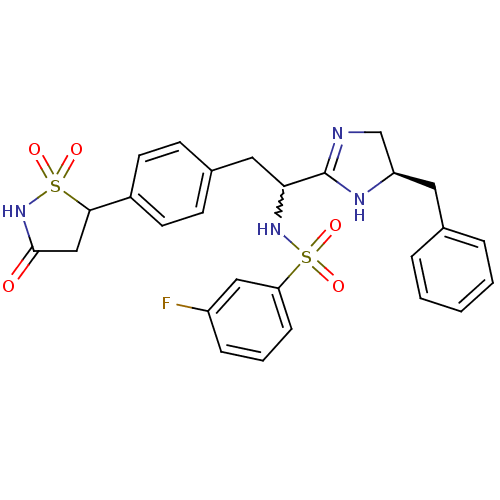
(CHEMBL253475 | N-{1-((R)-5-benzyl-4,5-dihydro-1H-i...)Show SMILES Fc1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)C1=NC[C@@H](Cc2ccccc2)N1 |w:11.11,t:30| Show InChI InChI=1S/C27H27FN4O5S2/c28-21-7-4-8-23(15-21)38(34,35)31-24(27-29-17-22(30-27)13-18-5-2-1-3-6-18)14-19-9-11-20(12-10-19)25-16-26(33)32-39(25,36)37/h1-12,15,22,24-25,31H,13-14,16-17H2,(H,29,30)(H,32,33)/t22-,24?,25?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228020

(CHEMBL252710 | N-{1-((R)-5-benzyl-4,5-dihydro-1H-i...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)C1=NC[C@@H](Cc2ccccc2)N1 |w:14.14,t:33| Show InChI InChI=1S/C28H27F3N4O5S2/c29-28(30,31)21-7-4-8-23(15-21)41(37,38)34-24(27-32-17-22(33-27)13-18-5-2-1-3-6-18)14-19-9-11-20(12-10-19)25-16-26(36)35-42(25,39)40/h1-12,15,22,24-25,34H,13-14,16-17H2,(H,32,33)(H,35,36)/t22-,24?,25?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228021

(3-fluoro-N-{1-(5-phenyl-1H-imidazol-2-yl)-2-[4-(1,...)Show SMILES Fc1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1nc(c[nH]1)-c1ccccc1 |w:11.11| Show InChI InChI=1S/C26H23FN4O5S2/c27-20-7-4-8-21(14-20)37(33,34)30-22(26-28-16-23(29-26)18-5-2-1-3-6-18)13-17-9-11-19(12-10-17)24-15-25(32)31-38(24,35)36/h1-12,14,16,22,24,30H,13,15H2,(H,28,29)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228026
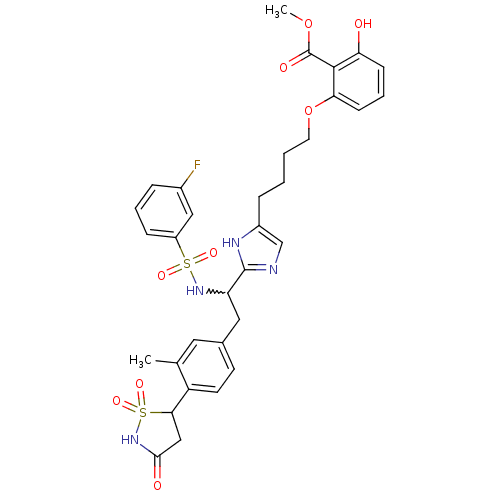
(2-[4-(2-{1-(3-fluoro-benzenesulfonylamino)-2-[3-me...)Show SMILES COC(=O)c1c(O)cccc1OCCCCc1cnc([nH]1)C(Cc1ccc(C2CC(=O)NS2(=O)=O)c(C)c1)NS(=O)(=O)c1cccc(F)c1 |w:21.41| Show InChI InChI=1S/C33H35FN4O9S2/c1-20-15-21(12-13-25(20)29-18-30(40)38-49(29,44)45)16-26(37-48(42,43)24-9-5-7-22(34)17-24)32-35-19-23(36-32)8-3-4-14-47-28-11-6-10-27(39)31(28)33(41)46-2/h5-7,9-13,15,17,19,26,29,37,39H,3-4,8,14,16,18H2,1-2H3,(H,35,36)(H,38,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228012

(3-fluoro-N-{1-{(R)-5-[3-(3-methanesulfonyl-phenoxy...)Show SMILES Cc1cc(CC(NS(=O)(=O)c2cccc(F)c2)C2=NC[C@@H](CCCOc3cccc(c3)S(C)(=O)=O)N2)ccc1C1CC(=O)NS1(=O)=O |w:5.5,t:18| Show InChI InChI=1S/C31H35FN4O8S3/c1-20-14-21(11-12-27(20)29-18-30(37)36-47(29,42)43)15-28(35-46(40,41)26-10-3-6-22(32)16-26)31-33-19-23(34-31)7-5-13-44-24-8-4-9-25(17-24)45(2,38)39/h3-4,6,8-12,14,16-17,23,28-29,35H,5,7,13,15,18-19H2,1-2H3,(H,33,34)(H,36,37)/t23-,28?,29?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228019

(CHEMBL403681 | N-{1-[5-(4-phenyl-butyl)-1H-imidazo...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1ncc(CCCCc2ccccc2)[nH]1 Show InChI InChI=1S/C31H31F3N4O5S2/c32-31(33,34)24-10-6-12-26(18-24)44(40,41)37-27(17-22-13-15-23(16-14-22)28-19-29(39)38-45(28,42)43)30-35-20-25(36-30)11-5-4-9-21-7-2-1-3-8-21/h1-3,6-8,10,12-16,18,20,27-28,37H,4-5,9,11,17,19H2,(H,35,36)(H,38,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228010

(2-[3-(2-{1-(3-fluoro-benzenesulfonylamino)-2-[3-me...)Show SMILES COC(=O)c1c(O)cccc1OCCCc1cnc([nH]1)C(Cc1ccc(C2CC(=O)NS2(=O)=O)c(C)c1)NS(=O)(=O)c1cccc(F)c1 |w:20.40| Show InChI InChI=1S/C32H33FN4O9S2/c1-19-14-20(11-12-24(19)28-17-29(39)37-48(28,43)44)15-25(36-47(41,42)23-8-3-6-21(33)16-23)31-34-18-22(35-31)7-5-13-46-27-10-4-9-26(38)30(27)32(40)45-2/h3-4,6,8-12,14,16,18,25,28,36,38H,5,7,13,15,17H2,1-2H3,(H,34,35)(H,37,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228034

(CHEMBL403680 | N-{1-(5-phenethyl-1H-imidazol-2-yl)...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1ncc(CCc2ccccc2)[nH]1 |w:14.14| Show InChI InChI=1S/C29H27F3N4O5S2/c30-29(31,32)22-7-4-8-24(16-22)42(38,39)35-25(28-33-18-23(34-28)14-11-19-5-2-1-3-6-19)15-20-9-12-21(13-10-20)26-17-27(37)36-43(26,40)41/h1-10,12-13,16,18,25-26,35H,11,14-15,17H2,(H,33,34)(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228034

(CHEMBL403680 | N-{1-(5-phenethyl-1H-imidazol-2-yl)...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1ncc(CCc2ccccc2)[nH]1 |w:14.14| Show InChI InChI=1S/C29H27F3N4O5S2/c30-29(31,32)22-7-4-8-24(16-22)42(38,39)35-25(28-33-18-23(34-28)14-11-19-5-2-1-3-6-19)15-20-9-12-21(13-10-20)26-17-27(37)36-43(26,40)41/h1-10,12-13,16,18,25-26,35H,11,14-15,17H2,(H,33,34)(H,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228026

(2-[4-(2-{1-(3-fluoro-benzenesulfonylamino)-2-[3-me...)Show SMILES COC(=O)c1c(O)cccc1OCCCCc1cnc([nH]1)C(Cc1ccc(C2CC(=O)NS2(=O)=O)c(C)c1)NS(=O)(=O)c1cccc(F)c1 |w:21.41| Show InChI InChI=1S/C33H35FN4O9S2/c1-20-15-21(12-13-25(20)29-18-30(40)38-49(29,44)45)16-26(37-48(42,43)24-9-5-7-22(34)17-24)32-35-19-23(36-32)8-3-4-14-47-28-11-6-10-27(39)31(28)33(41)46-2/h5-7,9-13,15,17,19,26,29,37,39H,3-4,8,14,16,18H2,1-2H3,(H,35,36)(H,38,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228009

(2-[3-(2-{1-(3-fluoro-benzenesulfonylamino)-2-[3-me...)Show SMILES Cc1cc(CC(NS(=O)(=O)c2cccc(F)c2)c2ncc(CCCOc3cccc(O)c3C(O)=O)[nH]2)ccc1C1CC(=O)NS1(=O)=O |w:5.5| Show InChI InChI=1S/C31H31FN4O9S2/c1-18-13-19(10-11-23(18)27-16-28(38)36-47(27,43)44)14-24(35-46(41,42)22-7-2-5-20(32)15-22)30-33-17-21(34-30)6-4-12-45-26-9-3-8-25(37)29(26)31(39)40/h2-3,5,7-11,13,15,17,24,27,35,37H,4,6,12,14,16H2,1H3,(H,33,34)(H,36,38)(H,39,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228028

(3-fluoro-N-{1-(5-phenethyl-1H-imidazol-2-yl)-2-[4-...)Show SMILES Fc1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1ncc(CCc2ccccc2)[nH]1 |w:11.11| Show InChI InChI=1S/C28H27FN4O5S2/c29-22-7-4-8-24(16-22)39(35,36)32-25(28-30-18-23(31-28)14-11-19-5-2-1-3-6-19)15-20-9-12-21(13-10-20)26-17-27(34)33-40(26,37)38/h1-10,12-13,16,18,25-26,32H,11,14-15,17H2,(H,30,31)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228019

(CHEMBL403681 | N-{1-[5-(4-phenyl-butyl)-1H-imidazo...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1ncc(CCCCc2ccccc2)[nH]1 Show InChI InChI=1S/C31H31F3N4O5S2/c32-31(33,34)24-10-6-12-26(18-24)44(40,41)37-27(17-22-13-15-23(16-14-22)28-19-29(39)38-45(28,42)43)30-35-20-25(36-30)11-5-4-9-21-7-2-1-3-8-21/h1-3,6-8,10,12-16,18,20,27-28,37H,4-5,9,11,17,19H2,(H,35,36)(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228033

(CHEMBL253475 | N-{1-((R)-5-benzyl-4,5-dihydro-1H-i...)Show SMILES Fc1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)C1=NC[C@@H](Cc2ccccc2)N1 |w:11.11,t:30| Show InChI InChI=1S/C27H27FN4O5S2/c28-21-7-4-8-23(15-21)38(34,35)31-24(27-29-17-22(30-27)13-18-5-2-1-3-6-18)14-19-9-11-20(12-10-19)25-16-26(33)32-39(25,36)37/h1-12,15,22,24-25,31H,13-14,16-17H2,(H,29,30)(H,32,33)/t22-,24?,25?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228027
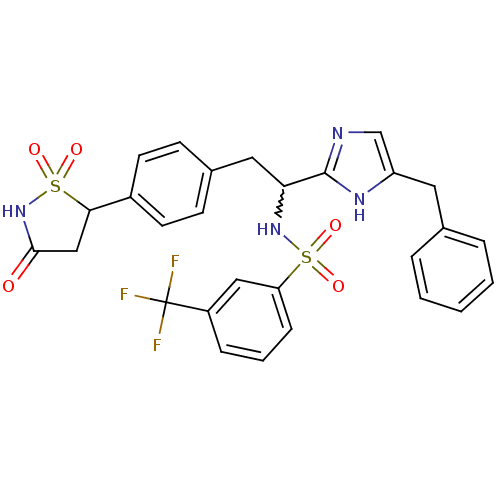
(CHEMBL252277 | N-{1-(5-benzyl-1H-imidazol-2-yl)-2-...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1ncc(Cc2ccccc2)[nH]1 |w:14.14| Show InChI InChI=1S/C28H25F3N4O5S2/c29-28(30,31)21-7-4-8-23(15-21)41(37,38)34-24(27-32-17-22(33-27)13-18-5-2-1-3-6-18)14-19-9-11-20(12-10-19)25-16-26(36)35-42(25,39)40/h1-12,15,17,24-25,34H,13-14,16H2,(H,32,33)(H,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228031

(CHEMBL254513 | N-{1-(1H-imidazol-2-yl)-2-[4-(1,1,3...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1ncc[nH]1 |w:22.23,14.14| Show InChI InChI=1S/C21H19F3N4O5S2/c22-21(23,24)15-2-1-3-16(11-15)34(30,31)27-17(20-25-8-9-26-20)10-13-4-6-14(7-5-13)18-12-19(29)28-35(18,32)33/h1-9,11,17-18,27H,10,12H2,(H,25,26)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228022

(CHEMBL404319 | N-{1-(5-benzyl-1H-imidazol-2-yl)-2-...)Show SMILES Fc1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1ncc(Cc2ccccc2)[nH]1 |w:11.11| Show InChI InChI=1S/C27H25FN4O5S2/c28-21-7-4-8-23(15-21)38(34,35)31-24(27-29-17-22(30-27)13-18-5-2-1-3-6-18)14-19-9-11-20(12-10-19)25-16-26(33)32-39(25,36)37/h1-12,15,17,24-25,31H,13-14,16H2,(H,29,30)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228029

(3-fluoro-N-{1-[5-(4-phenyl-butyl)-1H-imidazol-2-yl...)Show SMILES Fc1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1ncc(CCCCc2ccccc2)[nH]1 |w:11.11| Show InChI InChI=1S/C30H31FN4O5S2/c31-24-10-6-12-26(18-24)41(37,38)34-27(17-22-13-15-23(16-14-22)28-19-29(36)35-42(28,39)40)30-32-20-25(33-30)11-5-4-9-21-7-2-1-3-8-21/h1-3,6-8,10,12-16,18,20,27-28,34H,4-5,9,11,17,19H2,(H,32,33)(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228031

(CHEMBL254513 | N-{1-(1H-imidazol-2-yl)-2-[4-(1,1,3...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1ncc[nH]1 |w:22.23,14.14| Show InChI InChI=1S/C21H19F3N4O5S2/c22-21(23,24)15-2-1-3-16(11-15)34(30,31)27-17(20-25-8-9-26-20)10-13-4-6-14(7-5-13)18-12-19(29)28-35(18,32)33/h1-9,11,17-18,27H,10,12H2,(H,25,26)(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228022

(CHEMBL404319 | N-{1-(5-benzyl-1H-imidazol-2-yl)-2-...)Show SMILES Fc1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1ncc(Cc2ccccc2)[nH]1 |w:11.11| Show InChI InChI=1S/C27H25FN4O5S2/c28-21-7-4-8-23(15-21)38(34,35)31-24(27-29-17-22(30-27)13-18-5-2-1-3-6-18)14-19-9-11-20(12-10-19)25-16-26(33)32-39(25,36)37/h1-12,15,17,24-25,31H,13-14,16H2,(H,29,30)(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228027

(CHEMBL252277 | N-{1-(5-benzyl-1H-imidazol-2-yl)-2-...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)c1ncc(Cc2ccccc2)[nH]1 |w:14.14| Show InChI InChI=1S/C28H25F3N4O5S2/c29-28(30,31)21-7-4-8-23(15-21)41(37,38)34-24(27-32-17-22(33-27)13-18-5-2-1-3-6-18)14-19-9-11-20(12-10-19)25-16-26(36)35-42(25,39)40/h1-12,15,17,24-25,34H,13-14,16H2,(H,32,33)(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228010

(2-[3-(2-{1-(3-fluoro-benzenesulfonylamino)-2-[3-me...)Show SMILES COC(=O)c1c(O)cccc1OCCCc1cnc([nH]1)C(Cc1ccc(C2CC(=O)NS2(=O)=O)c(C)c1)NS(=O)(=O)c1cccc(F)c1 |w:20.40| Show InChI InChI=1S/C32H33FN4O9S2/c1-19-14-20(11-12-24(19)28-17-29(39)37-48(28,43)44)15-25(36-47(41,42)23-8-3-6-21(33)16-23)31-34-18-22(35-31)7-5-13-46-27-10-4-9-26(38)30(27)32(40)45-2/h3-4,6,8-12,14,16,18,25,28,36,38H,5,7,13,15,17H2,1-2H3,(H,34,35)(H,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228015
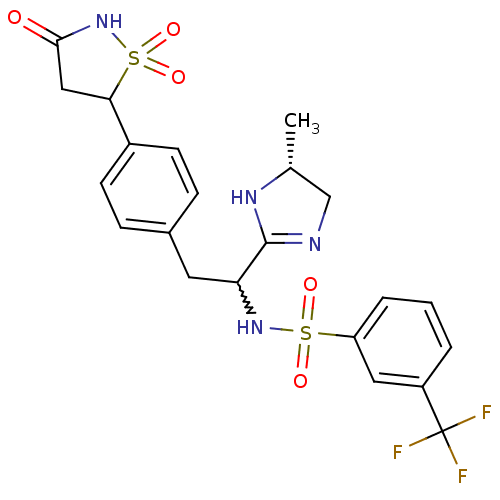
(CHEMBL253065 | N-{1-((R)-5-methyl-4,5-dihydro-1H-i...)Show SMILES C[C@@H]1CN=C(N1)C(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)NS(=O)(=O)c1cccc(c1)C(F)(F)F |w:6.24,c:3| Show InChI InChI=1S/C22H23F3N4O5S2/c1-13-12-26-21(27-13)18(28-35(31,32)17-4-2-3-16(10-17)22(23,24)25)9-14-5-7-15(8-6-14)19-11-20(30)29-36(19,33)34/h2-8,10,13,18-19,28H,9,11-12H2,1H3,(H,26,27)(H,29,30)/t13-,18?,19?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228030
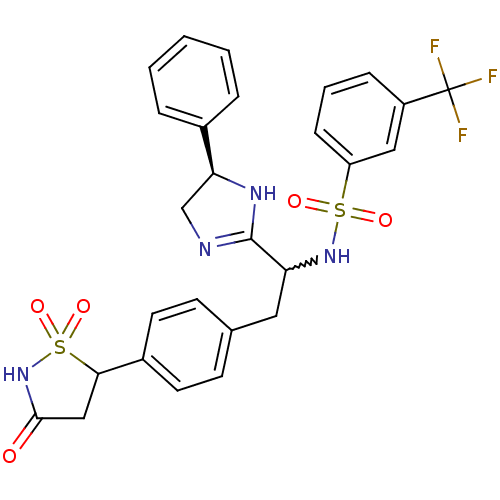
(CHEMBL403116 | N-{1-((R)-5-phenyl-4,5-dihydro-1H-i...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)C1=NC[C@H](N1)c1ccccc1 |w:14.14,t:33| Show InChI InChI=1S/C27H25F3N4O5S2/c28-27(29,30)20-7-4-8-21(14-20)40(36,37)33-22(26-31-16-23(32-26)18-5-2-1-3-6-18)13-17-9-11-19(12-10-17)24-15-25(35)34-41(24,38)39/h1-12,14,22-24,33H,13,15-16H2,(H,31,32)(H,34,35)/t22?,23-,24?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228037
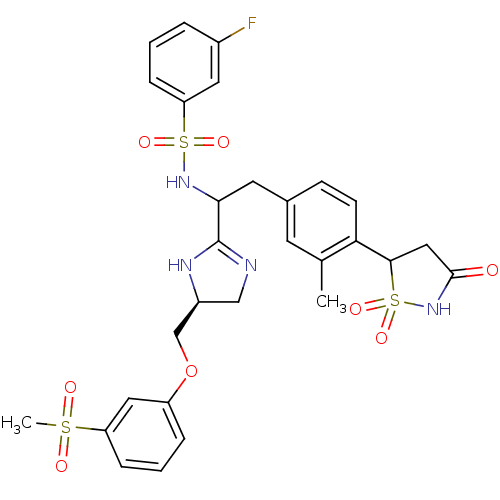
(3-fluoro-N-{1-[(S)-5-(3-methanesulfonyl-phenoxymet...)Show SMILES Cc1cc(CC(NS(=O)(=O)c2cccc(F)c2)C2=NC[C@@H](COc3cccc(c3)S(C)(=O)=O)N2)ccc1C1CC(=O)NS1(=O)=O |t:18| Show InChI InChI=1S/C29H31FN4O8S3/c1-18-11-19(9-10-25(18)27-15-28(35)34-45(27,40)41)12-26(33-44(38,39)24-8-3-5-20(30)13-24)29-31-16-21(32-29)17-42-22-6-4-7-23(14-22)43(2,36)37/h3-11,13-14,21,26-27,33H,12,15-17H2,1-2H3,(H,31,32)(H,34,35)/t21-,26?,27?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228035

(CHEMBL401833 | N-{1-(4,5-dihydro-1H-imidazol-2-yl)...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)C1=NCCN1 |w:14.14,t:33| Show InChI InChI=1S/C21H21F3N4O5S2/c22-21(23,24)15-2-1-3-16(11-15)34(30,31)27-17(20-25-8-9-26-20)10-13-4-6-14(7-5-13)18-12-19(29)28-35(18,32)33/h1-7,11,17-18,27H,8-10,12H2,(H,25,26)(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228015

(CHEMBL253065 | N-{1-((R)-5-methyl-4,5-dihydro-1H-i...)Show SMILES C[C@@H]1CN=C(N1)C(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)NS(=O)(=O)c1cccc(c1)C(F)(F)F |w:6.24,c:3| Show InChI InChI=1S/C22H23F3N4O5S2/c1-13-12-26-21(27-13)18(28-35(31,32)17-4-2-3-16(10-17)22(23,24)25)9-14-5-7-15(8-6-14)19-11-20(30)29-36(19,33)34/h2-8,10,13,18-19,28H,9,11-12H2,1H3,(H,26,27)(H,29,30)/t13-,18?,19?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228030

(CHEMBL403116 | N-{1-((R)-5-phenyl-4,5-dihydro-1H-i...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)C1=NC[C@H](N1)c1ccccc1 |w:14.14,t:33| Show InChI InChI=1S/C27H25F3N4O5S2/c28-27(29,30)20-7-4-8-21(14-20)40(36,37)33-22(26-31-16-23(32-26)18-5-2-1-3-6-18)13-17-9-11-19(12-10-17)24-15-25(35)34-41(24,38)39/h1-12,14,22-24,33H,13,15-16H2,(H,31,32)(H,34,35)/t22?,23-,24?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228016

(CHEMBL253066 | N-{1-((S)-5-methyl-4,5-dihydro-1H-i...)Show SMILES C[C@H]1CN=C(N1)C(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)NS(=O)(=O)c1cccc(c1)C(F)(F)F |w:6.24,c:3| Show InChI InChI=1S/C22H23F3N4O5S2/c1-13-12-26-21(27-13)18(28-35(31,32)17-4-2-3-16(10-17)22(23,24)25)9-14-5-7-15(8-6-14)19-11-20(30)29-36(19,33)34/h2-8,10,13,18-19,28H,9,11-12H2,1H3,(H,26,27)(H,29,30)/t13-,18?,19?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228018

(CHEMBL253268 | N-{1-(5,5-dimethyl-4,5-dihydro-1H-i...)Show SMILES CC1(C)CN=C(N1)C(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)NS(=O)(=O)c1cccc(c1)C(F)(F)F |w:7.25,c:4| Show InChI InChI=1S/C23H25F3N4O5S2/c1-22(2)13-27-21(28-22)18(29-36(32,33)17-5-3-4-16(11-17)23(24,25)26)10-14-6-8-15(9-7-14)19-12-20(31)30-37(19,34)35/h3-9,11,18-19,29H,10,12-13H2,1-2H3,(H,27,28)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228035

(CHEMBL401833 | N-{1-(4,5-dihydro-1H-imidazol-2-yl)...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)C1=NCCN1 |w:14.14,t:33| Show InChI InChI=1S/C21H21F3N4O5S2/c22-21(23,24)15-2-1-3-16(11-15)34(30,31)27-17(20-25-8-9-26-20)10-13-4-6-14(7-5-13)18-12-19(29)28-35(18,32)33/h1-7,11,17-18,27H,8-10,12H2,(H,25,26)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228037

(3-fluoro-N-{1-[(S)-5-(3-methanesulfonyl-phenoxymet...)Show SMILES Cc1cc(CC(NS(=O)(=O)c2cccc(F)c2)C2=NC[C@@H](COc3cccc(c3)S(C)(=O)=O)N2)ccc1C1CC(=O)NS1(=O)=O |t:18| Show InChI InChI=1S/C29H31FN4O8S3/c1-18-11-19(9-10-25(18)27-15-28(35)34-45(27,40)41)12-26(33-44(38,39)24-8-3-5-20(30)13-24)29-31-16-21(32-29)17-42-22-6-4-7-23(14-22)43(2,36)37/h3-11,13-14,21,26-27,33H,12,15-17H2,1-2H3,(H,31,32)(H,34,35)/t21-,26?,27?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228016

(CHEMBL253066 | N-{1-((S)-5-methyl-4,5-dihydro-1H-i...)Show SMILES C[C@H]1CN=C(N1)C(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)NS(=O)(=O)c1cccc(c1)C(F)(F)F |w:6.24,c:3| Show InChI InChI=1S/C22H23F3N4O5S2/c1-13-12-26-21(27-13)18(28-35(31,32)17-4-2-3-16(10-17)22(23,24)25)9-14-5-7-15(8-6-14)19-11-20(30)29-36(19,33)34/h2-8,10,13,18-19,28H,9,11-12H2,1H3,(H,26,27)(H,29,30)/t13-,18?,19?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228018

(CHEMBL253268 | N-{1-(5,5-dimethyl-4,5-dihydro-1H-i...)Show SMILES CC1(C)CN=C(N1)C(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)NS(=O)(=O)c1cccc(c1)C(F)(F)F |w:7.25,c:4| Show InChI InChI=1S/C23H25F3N4O5S2/c1-22(2)13-27-21(28-22)18(29-36(32,33)17-5-3-4-16(11-17)23(24,25)26)10-14-6-8-15(9-7-14)19-12-20(31)30-37(19,34)35/h3-9,11,18-19,29H,10,12-13H2,1-2H3,(H,27,28)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228017

(CHEMBL254301 | N-{1-((S)-5-benzyl-4,5-dihydro-1H-i...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)C1=NC[C@H](Cc2ccccc2)N1 |w:14.14,t:33| Show InChI InChI=1S/C28H27F3N4O5S2/c29-28(30,31)21-7-4-8-23(15-21)41(37,38)34-24(27-32-17-22(33-27)13-18-5-2-1-3-6-18)14-19-9-11-20(12-10-19)25-16-26(36)35-42(25,39)40/h1-12,15,22,24-25,34H,13-14,16-17H2,(H,32,33)(H,35,36)/t22-,24?,25?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228017

(CHEMBL254301 | N-{1-((S)-5-benzyl-4,5-dihydro-1H-i...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)C1=NC[C@H](Cc2ccccc2)N1 |w:14.14,t:33| Show InChI InChI=1S/C28H27F3N4O5S2/c29-28(30,31)21-7-4-8-23(15-21)41(37,38)34-24(27-32-17-22(33-27)13-18-5-2-1-3-6-18)14-19-9-11-20(12-10-19)25-16-26(36)35-42(25,39)40/h1-12,15,22,24-25,34H,13-14,16-17H2,(H,32,33)(H,35,36)/t22-,24?,25?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228023
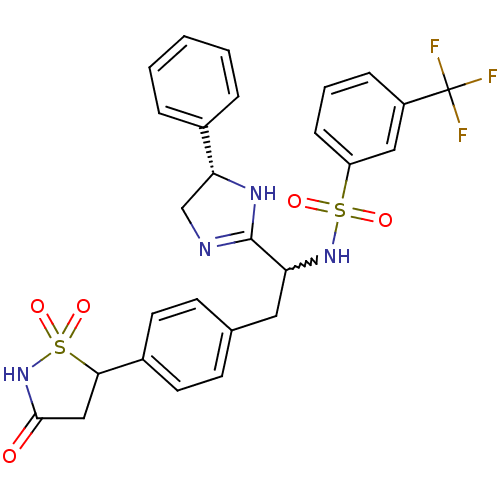
(CHEMBL403117 | N-{1-((S)-5-phenyl-4,5-dihydro-1H-i...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)C1=NC[C@@H](N1)c1ccccc1 |w:14.14,t:33| Show InChI InChI=1S/C27H25F3N4O5S2/c28-27(29,30)20-7-4-8-21(14-20)40(36,37)33-22(26-31-16-23(32-26)18-5-2-1-3-6-18)13-17-9-11-19(12-10-17)24-15-25(35)34-41(24,38)39/h1-12,14,22-24,33H,13,15-16H2,(H,31,32)(H,34,35)/t22?,23-,24?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228023

(CHEMBL403117 | N-{1-((S)-5-phenyl-4,5-dihydro-1H-i...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)NC(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)C1=NC[C@@H](N1)c1ccccc1 |w:14.14,t:33| Show InChI InChI=1S/C27H25F3N4O5S2/c28-27(29,30)20-7-4-8-21(14-20)40(36,37)33-22(26-31-16-23(32-26)18-5-2-1-3-6-18)13-17-9-11-19(12-10-17)24-15-25(35)34-41(24,38)39/h1-12,14,22-24,33H,13,15-16H2,(H,31,32)(H,34,35)/t22?,23-,24?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50228011
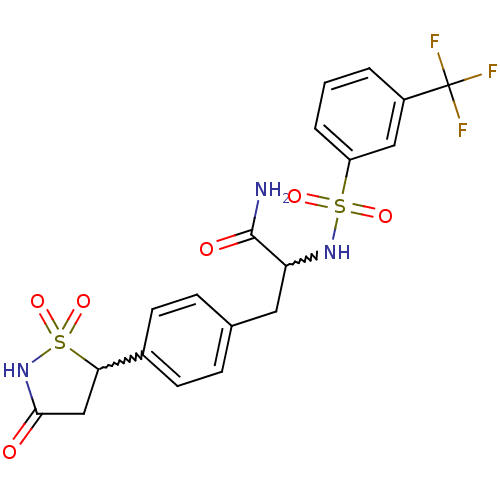
(2-(3-trifluoromethyl-benzenesulfonylamino)-3-[4-(1...)Show SMILES NC(=O)C(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)NS(=O)(=O)c1cccc(c1)C(F)(F)F |w:11.11,3.20| Show InChI InChI=1S/C19H18F3N3O6S2/c20-19(21,22)13-2-1-3-14(9-13)32(28,29)24-15(18(23)27)8-11-4-6-12(7-5-11)16-10-17(26)25-33(16,30)31/h1-7,9,15-16,24H,8,10H2,(H2,23,27)(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50228011

(2-(3-trifluoromethyl-benzenesulfonylamino)-3-[4-(1...)Show SMILES NC(=O)C(Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)NS(=O)(=O)c1cccc(c1)C(F)(F)F |w:11.11,3.20| Show InChI InChI=1S/C19H18F3N3O6S2/c20-19(21,22)13-2-1-3-14(9-13)32(28,29)24-15(18(23)27)8-11-4-6-12(7-5-11)16-10-17(26)25-33(16,30)31/h1-7,9,15-16,24H,8,10H2,(H2,23,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
Bioorg Med Chem Lett 18: 66-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.012
BindingDB Entry DOI: 10.7270/Q2BV7GCQ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data