 Found 70 hits of Enzyme Inhibition Constant Data
Found 70 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50372664
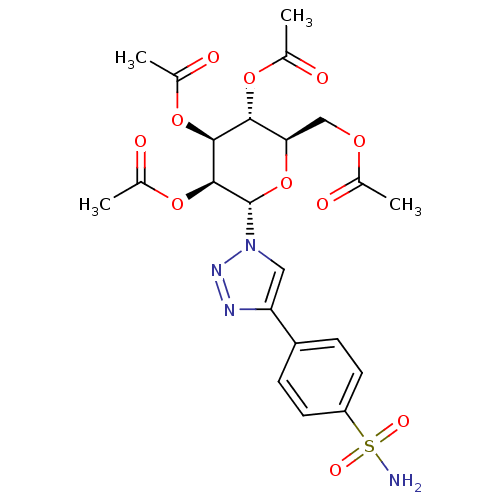
(CHEMBL273046)Show SMILES CC(=O)OC[C@H]1O[C@@H]([C@@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O)n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C22H26N4O11S/c1-11(27)33-10-18-19(34-12(2)28)20(35-13(3)29)21(36-14(4)30)22(37-18)26-9-17(24-25-26)15-5-7-16(8-6-15)38(23,31)32/h5-9,18-22H,10H2,1-4H3,(H2,23,31,32)/t18-,19-,20+,21+,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50372664

(CHEMBL273046)Show SMILES CC(=O)OC[C@H]1O[C@@H]([C@@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O)n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C22H26N4O11S/c1-11(27)33-10-18-19(34-12(2)28)20(35-13(3)29)21(36-14(4)30)22(37-18)26-9-17(24-25-26)15-5-7-16(8-6-15)38(23,31)32/h5-9,18-22H,10H2,1-4H3,(H2,23,31,32)/t18-,19-,20+,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50372658
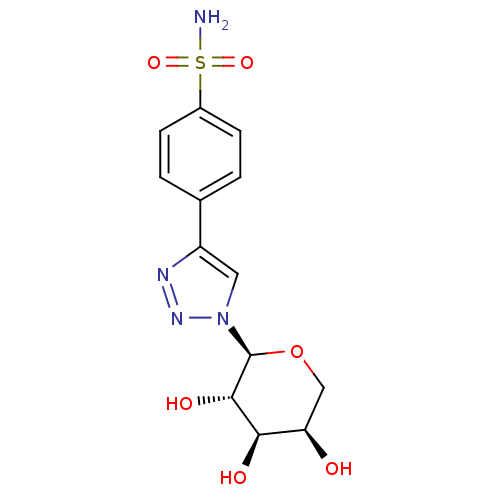
(CHEMBL409306)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@H]1OC[C@@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C13H16N4O6S/c14-24(21,22)8-3-1-7(2-4-8)9-5-17(16-15-9)13-12(20)11(19)10(18)6-23-13/h1-5,10-13,18-20H,6H2,(H2,14,21,22)/t10-,11-,12+,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50372658

(CHEMBL409306)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@H]1OC[C@@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C13H16N4O6S/c14-24(21,22)8-3-1-7(2-4-8)9-5-17(16-15-9)13-12(20)11(19)10(18)6-23-13/h1-5,10-13,18-20H,6H2,(H2,14,21,22)/t10-,11-,12+,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50372659
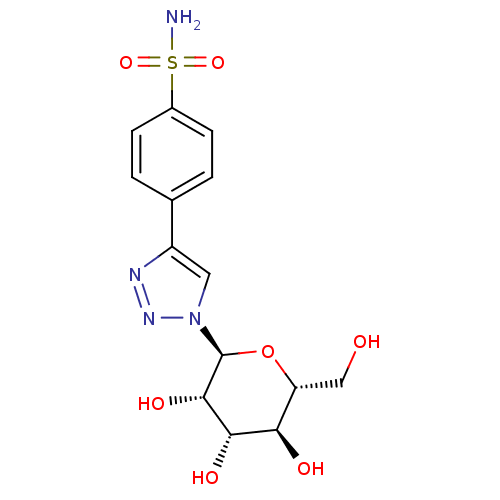
(CHEMBL259682)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]1O Show InChI InChI=1S/C14H18N4O7S/c15-26(23,24)8-3-1-7(2-4-8)9-5-18(17-16-9)14-13(22)12(21)11(20)10(6-19)25-14/h1-5,10-14,19-22H,6H2,(H2,15,23,24)/t10-,11-,12+,13+,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50372664

(CHEMBL273046)Show SMILES CC(=O)OC[C@H]1O[C@@H]([C@@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O)n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C22H26N4O11S/c1-11(27)33-10-18-19(34-12(2)28)20(35-13(3)29)21(36-14(4)30)22(37-18)26-9-17(24-25-26)15-5-7-16(8-6-15)38(23,31)32/h5-9,18-22H,10H2,1-4H3,(H2,23,31,32)/t18-,19-,20+,21+,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50236140
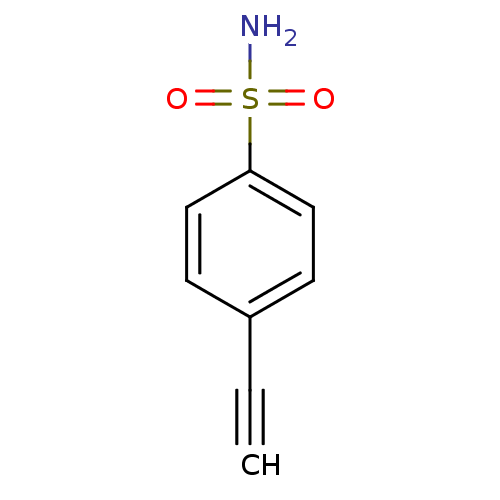
(4-ethynyl benzene sulfonamide | 4-ethynylbenzenesu...)Show InChI InChI=1S/C8H7NO2S/c1-2-7-3-5-8(6-4-7)12(9,10)11/h1,3-6H,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50372659

(CHEMBL259682)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]1O Show InChI InChI=1S/C14H18N4O7S/c15-26(23,24)8-3-1-7(2-4-8)9-5-18(17-16-9)14-13(22)12(21)11(20)10(6-19)25-14/h1-5,10-14,19-22H,6H2,(H2,15,23,24)/t10-,11-,12+,13+,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50372673

(CHEMBL411092)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C13H16N4O6S/c14-24(21,22)8-3-1-7(2-4-8)9-5-17(16-15-9)13-12(20)11(19)10(6-18)23-13/h1-5,10-13,18-20H,6H2,(H2,14,21,22)/t10-,11-,12-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50372659

(CHEMBL259682)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]1O Show InChI InChI=1S/C14H18N4O7S/c15-26(23,24)8-3-1-7(2-4-8)9-5-18(17-16-9)14-13(22)12(21)11(20)10(6-19)25-14/h1-5,10-14,19-22H,6H2,(H2,15,23,24)/t10-,11-,12+,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50236140

(4-ethynyl benzene sulfonamide | 4-ethynylbenzenesu...)Show InChI InChI=1S/C8H7NO2S/c1-2-7-3-5-8(6-4-7)12(9,10)11/h1,3-6H,(H2,9,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50372670

(CHEMBL270989)Show SMILES CC(=O)O[C@@H]1CO[C@@H]([C@@H](OC(C)=O)[C@@H]1OC(C)=O)n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H22N4O9S/c1-10(24)30-16-9-29-19(18(32-12(3)26)17(16)31-11(2)25)23-8-15(21-22-23)13-4-6-14(7-5-13)33(20,27)28/h4-8,16-19H,9H2,1-3H3,(H2,20,27,28)/t16-,17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50372660
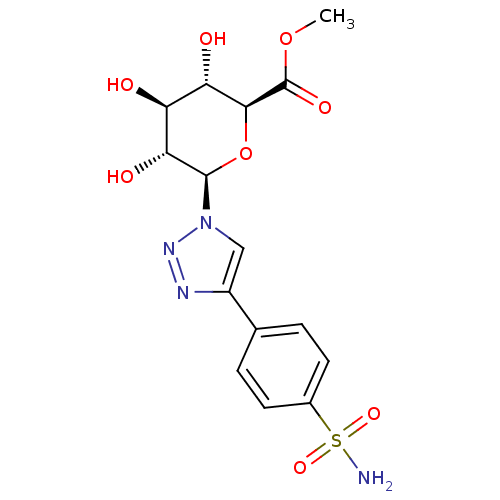
(CHEMBL270335)Show SMILES COC(=O)[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C15H18N4O8S/c1-26-15(23)13-11(21)10(20)12(22)14(27-13)19-6-9(17-18-19)7-2-4-8(5-3-7)28(16,24)25/h2-6,10-14,20-22H,1H3,(H2,16,24,25)/t10-,11-,12+,13-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50372668

(CHEMBL437092)Show SMILES CC(=O)OC[C@H]1O[C@H]([C@H](OC(C)=O)[C@@H](OC(C)=O)[C@H]1OC(C)=O)n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C22H26N4O11S/c1-11(27)33-10-18-19(34-12(2)28)20(35-13(3)29)21(36-14(4)30)22(37-18)26-9-17(24-25-26)15-5-7-16(8-6-15)38(23,31)32/h5-9,18-22H,10H2,1-4H3,(H2,23,31,32)/t18-,19+,20+,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50372662
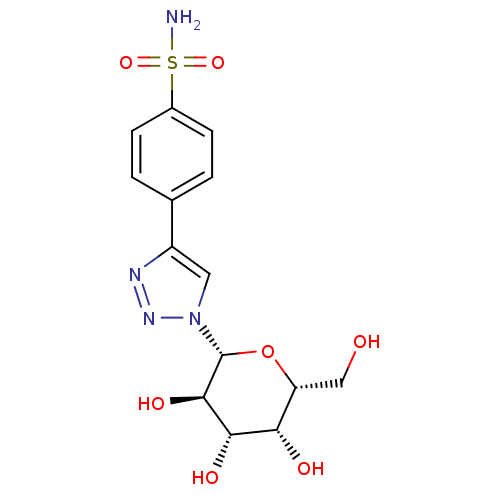
(CHEMBL272877)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C14H18N4O7S/c15-26(23,24)8-3-1-7(2-4-8)9-5-18(17-16-9)14-13(22)12(21)11(20)10(6-19)25-14/h1-5,10-14,19-22H,6H2,(H2,15,23,24)/t10-,11+,12+,13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50372672

(CHEMBL269852)Show SMILES CC(=O)OC[C@H]1O[C@H]([C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O)n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C22H26N4O11S/c1-11(27)33-10-18-19(34-12(2)28)20(35-13(3)29)21(36-14(4)30)22(37-18)26-9-17(24-25-26)15-5-7-16(8-6-15)38(23,31)32/h5-9,18-22H,10H2,1-4H3,(H2,23,31,32)/t18-,19-,20+,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50372662

(CHEMBL272877)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C14H18N4O7S/c15-26(23,24)8-3-1-7(2-4-8)9-5-18(17-16-9)14-13(22)12(21)11(20)10(6-19)25-14/h1-5,10-14,19-22H,6H2,(H2,15,23,24)/t10-,11+,12+,13-,14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50372663

(CHEMBL270990)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C14H18N4O7S/c15-26(23,24)8-3-1-7(2-4-8)9-5-18(17-16-9)14-13(22)12(21)11(20)10(6-19)25-14/h1-5,10-14,19-22H,6H2,(H2,15,23,24)/t10-,11-,12+,13-,14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50372666
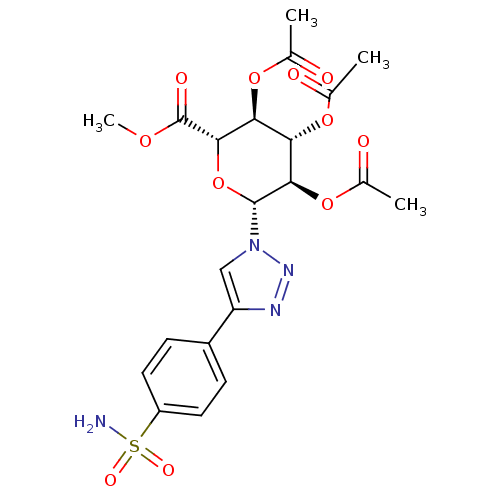
(CHEMBL412069)Show SMILES COC(=O)[C@H]1O[C@H]([C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O)n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H24N4O11S/c1-10(26)33-16-17(34-11(2)27)19(21(29)32-4)36-20(18(16)35-12(3)28)25-9-15(23-24-25)13-5-7-14(8-6-13)37(22,30)31/h5-9,16-20H,1-4H3,(H2,22,30,31)/t16-,17-,18+,19-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50372670

(CHEMBL270989)Show SMILES CC(=O)O[C@@H]1CO[C@@H]([C@@H](OC(C)=O)[C@@H]1OC(C)=O)n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H22N4O9S/c1-10(24)30-16-9-29-19(18(32-12(3)26)17(16)31-11(2)25)23-8-15(21-22-23)13-4-6-14(7-5-13)33(20,27)28/h4-8,16-19H,9H2,1-3H3,(H2,20,27,28)/t16-,17-,18+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50372666

(CHEMBL412069)Show SMILES COC(=O)[C@H]1O[C@H]([C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O)n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H24N4O11S/c1-10(26)33-16-17(34-11(2)27)19(21(29)32-4)36-20(18(16)35-12(3)28)25-9-15(23-24-25)13-5-7-14(8-6-13)37(22,30)31/h5-9,16-20H,1-4H3,(H2,22,30,31)/t16-,17-,18+,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50372660

(CHEMBL270335)Show SMILES COC(=O)[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C15H18N4O8S/c1-26-15(23)13-11(21)10(20)12(22)14(27-13)19-6-9(17-18-19)7-2-4-8(5-3-7)28(16,24)25/h2-6,10-14,20-22H,1H3,(H2,16,24,25)/t10-,11-,12+,13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10890

(1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1cccc2c(Cl)c[nH]c12 Show InChI InChI=1S/C14H12ClN3O4S2/c15-12-8-17-14-11(12)2-1-3-13(14)18-24(21,22)10-6-4-9(5-7-10)23(16,19)20/h1-8,17-18H,(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50372658

(CHEMBL409306)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@H]1OC[C@@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C13H16N4O6S/c14-24(21,22)8-3-1-7(2-4-8)9-5-17(16-15-9)13-12(20)11(19)10(18)6-23-13/h1-5,10-13,18-20H,6H2,(H2,14,21,22)/t10-,11-,12+,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10883

(4,5-dichlorobenzene-1,3-disulfonamide | CHEMBL17 |...)Show InChI InChI=1S/C6H6Cl2N2O4S2/c7-4-1-3(15(9,11)12)2-5(6(4)8)16(10,13)14/h1-2H,(H2,9,11,12)(H2,10,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
MMDB
PDB
Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50372666

(CHEMBL412069)Show SMILES COC(=O)[C@H]1O[C@H]([C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O)n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H24N4O11S/c1-10(26)33-16-17(34-11(2)27)19(21(29)32-4)36-20(18(16)35-12(3)28)25-9-15(23-24-25)13-5-7-14(8-6-13)37(22,30)31/h5-9,16-20H,1-4H3,(H2,22,30,31)/t16-,17-,18+,19-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10883

(4,5-dichlorobenzene-1,3-disulfonamide | CHEMBL17 |...)Show InChI InChI=1S/C6H6Cl2N2O4S2/c7-4-1-3(15(9,11)12)2-5(6(4)8)16(10,13)14/h1-2H,(H2,9,11,12)(H2,10,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50372661
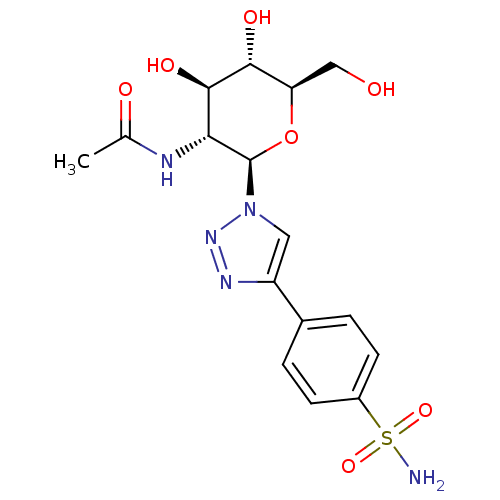
(CHEMBL270334)Show SMILES CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H21N5O7S/c1-8(23)18-13-15(25)14(24)12(7-22)28-16(13)21-6-11(19-20-21)9-2-4-10(5-3-9)29(17,26)27/h2-6,12-16,22,24-25H,7H2,1H3,(H,18,23)(H2,17,26,27)/t12-,13-,14-,15-,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50372663

(CHEMBL270990)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C14H18N4O7S/c15-26(23,24)8-3-1-7(2-4-8)9-5-18(17-16-9)14-13(22)12(21)11(20)10(6-19)25-14/h1-5,10-14,19-22H,6H2,(H2,15,23,24)/t10-,11-,12+,13-,14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50372665
(CHEMBL272611)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@@H]1O[C@H](COC(=O)c2ccccc2)[C@@H](OC(=O)c2ccccc2)[C@H]1OC(=O)c1ccccc1 Show InChI InChI=1S/C34H28N4O9S/c35-48(42,43)26-18-16-22(17-19-26)27-20-38(37-36-27)31-30(47-34(41)25-14-8-3-9-15-25)29(46-33(40)24-12-6-2-7-13-24)28(45-31)21-44-32(39)23-10-4-1-5-11-23/h1-20,28-31H,21H2,(H2,35,42,43)/t28-,29-,30-,31-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50372671

(CHEMBL270994)Show SMILES CC(=O)N[C@@H]1[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@@H](COC(C)=O)O[C@H]1n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C22H27N5O10S/c1-11(28)24-19-21(36-14(4)31)20(35-13(3)30)18(10-34-12(2)29)37-22(19)27-9-17(25-26-27)15-5-7-16(8-6-15)38(23,32)33/h5-9,18-22H,10H2,1-4H3,(H,24,28)(H2,23,32,33)/t18-,19-,20-,21-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50372668

(CHEMBL437092)Show SMILES CC(=O)OC[C@H]1O[C@H]([C@H](OC(C)=O)[C@@H](OC(C)=O)[C@H]1OC(C)=O)n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C22H26N4O11S/c1-11(27)33-10-18-19(34-12(2)28)20(35-13(3)29)21(36-14(4)30)22(37-18)26-9-17(24-25-26)15-5-7-16(8-6-15)38(23,31)32/h5-9,18-22H,10H2,1-4H3,(H2,23,31,32)/t18-,19+,20+,21-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50372672

(CHEMBL269852)Show SMILES CC(=O)OC[C@H]1O[C@H]([C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O)n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C22H26N4O11S/c1-11(27)33-10-18-19(34-12(2)28)20(35-13(3)29)21(36-14(4)30)22(37-18)26-9-17(24-25-26)15-5-7-16(8-6-15)38(23,31)32/h5-9,18-22H,10H2,1-4H3,(H2,23,31,32)/t18-,19-,20+,21-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50372662

(CHEMBL272877)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C14H18N4O7S/c15-26(23,24)8-3-1-7(2-4-8)9-5-18(17-16-9)14-13(22)12(21)11(20)10(6-19)25-14/h1-5,10-14,19-22H,6H2,(H2,15,23,24)/t10-,11+,12+,13-,14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50372667

(CHEMBL272900)Show SMILES CC(=O)OC[C@H]1O[C@H](O[C@@H]2[C@@H](COC(C)=O)O[C@H]([C@H](OC(C)=O)[C@H]2OC(C)=O)n2cc(nn2)-c2ccc(cc2)S(N)(=O)=O)[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O Show InChI InChI=1S/C34H42N4O19S/c1-15(39)48-13-25-28(57-34-32(54-21(7)45)30(52-19(5)43)27(50-17(3)41)26(56-34)14-49-16(2)40)29(51-18(4)42)31(53-20(6)44)33(55-25)38-12-24(36-37-38)22-8-10-23(11-9-22)58(35,46)47/h8-12,25-34H,13-14H2,1-7H3,(H2,35,46,47)/t25-,26-,27-,28-,29+,30+,31-,32-,33-,34-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50372673

(CHEMBL411092)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C13H16N4O6S/c14-24(21,22)8-3-1-7(2-4-8)9-5-17(16-15-9)13-12(20)11(19)10(6-18)23-13/h1-5,10-13,18-20H,6H2,(H2,14,21,22)/t10-,11-,12-,13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50372669

(CHEMBL407716)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@@H]1O[C@H](CO)[C@@H](O[C@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O Show InChI InChI=1S/C20H28N4O12S/c21-37(32,33)9-3-1-8(2-4-9)10-5-24(23-22-10)19-16(30)15(29)18(12(7-26)34-19)36-20-17(31)14(28)13(27)11(6-25)35-20/h1-5,11-20,25-31H,6-7H2,(H2,21,32,33)/t11-,12-,13-,14+,15-,16-,17-,18-,19-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50372670

(CHEMBL270989)Show SMILES CC(=O)O[C@@H]1CO[C@@H]([C@@H](OC(C)=O)[C@@H]1OC(C)=O)n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H22N4O9S/c1-10(24)30-16-9-29-19(18(32-12(3)26)17(16)31-11(2)25)23-8-15(21-22-23)13-4-6-14(7-5-13)33(20,27)28/h4-8,16-19H,9H2,1-3H3,(H2,20,27,28)/t16-,17-,18+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50372667

(CHEMBL272900)Show SMILES CC(=O)OC[C@H]1O[C@H](O[C@@H]2[C@@H](COC(C)=O)O[C@H]([C@H](OC(C)=O)[C@H]2OC(C)=O)n2cc(nn2)-c2ccc(cc2)S(N)(=O)=O)[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O Show InChI InChI=1S/C34H42N4O19S/c1-15(39)48-13-25-28(57-34-32(54-21(7)45)30(52-19(5)43)27(50-17(3)41)26(56-34)14-49-16(2)40)29(51-18(4)42)31(53-20(6)44)33(55-25)38-12-24(36-37-38)22-8-10-23(11-9-22)58(35,46)47/h8-12,25-34H,13-14H2,1-7H3,(H2,35,46,47)/t25-,26-,27-,28-,29+,30+,31-,32-,33-,34-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 235 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50372663

(CHEMBL270990)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C14H18N4O7S/c15-26(23,24)8-3-1-7(2-4-8)9-5-18(17-16-9)14-13(22)12(21)11(20)10(6-19)25-14/h1-5,10-14,19-22H,6H2,(H2,15,23,24)/t10-,11-,12+,13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50372665

(CHEMBL272611)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@@H]1O[C@H](COC(=O)c2ccccc2)[C@@H](OC(=O)c2ccccc2)[C@H]1OC(=O)c1ccccc1 Show InChI InChI=1S/C34H28N4O9S/c35-48(42,43)26-18-16-22(17-19-26)27-20-38(37-36-27)31-30(47-34(41)25-14-8-3-9-15-25)29(46-33(40)24-12-6-2-7-13-24)28(45-31)21-44-32(39)23-10-4-1-5-11-23/h1-20,28-31H,21H2,(H2,35,42,43)/t28-,29-,30-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 427 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50372669

(CHEMBL407716)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@@H]1O[C@H](CO)[C@@H](O[C@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O Show InChI InChI=1S/C20H28N4O12S/c21-37(32,33)9-3-1-8(2-4-9)10-5-24(23-22-10)19-16(30)15(29)18(12(7-26)34-19)36-20-17(31)14(28)13(27)11(6-25)35-20/h1-5,11-20,25-31H,6-7H2,(H2,21,32,33)/t11-,12-,13-,14+,15-,16-,17-,18-,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 432 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50372671

(CHEMBL270994)Show SMILES CC(=O)N[C@@H]1[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@@H](COC(C)=O)O[C@H]1n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C22H27N5O10S/c1-11(28)24-19-21(36-14(4)31)20(35-13(3)30)18(10-34-12(2)29)37-22(19)27-9-17(25-26-27)15-5-7-16(8-6-15)38(23,32)33/h5-9,18-22H,10H2,1-4H3,(H,24,28)(H2,23,32,33)/t18-,19-,20-,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50372661

(CHEMBL270334)Show SMILES CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H21N5O7S/c1-8(23)18-13-15(25)14(24)12(7-22)28-16(13)21-6-11(19-20-21)9-2-4-10(5-3-9)29(17,26)27/h2-6,12-16,22,24-25H,7H2,1H3,(H,18,23)(H2,17,26,27)/t12-,13-,14-,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50372660

(CHEMBL270335)Show SMILES COC(=O)[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C15H18N4O8S/c1-26-15(23)13-11(21)10(20)12(22)14(27-13)19-6-9(17-18-19)7-2-4-8(5-3-7)28(16,24)25/h2-6,10-14,20-22H,1H3,(H2,16,24,25)/t10-,11-,12+,13-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50236140

(4-ethynyl benzene sulfonamide | 4-ethynylbenzenesu...)Show InChI InChI=1S/C8H7NO2S/c1-2-7-3-5-8(6-4-7)12(9,10)11/h1,3-6H,(H2,9,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10883

(4,5-dichlorobenzene-1,3-disulfonamide | CHEMBL17 |...)Show InChI InChI=1S/C6H6Cl2N2O4S2/c7-4-1-3(15(9,11)12)2-5(6(4)8)16(10,13)14/h1-2H,(H2,9,11,12)(H2,10,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50372667

(CHEMBL272900)Show SMILES CC(=O)OC[C@H]1O[C@H](O[C@@H]2[C@@H](COC(C)=O)O[C@H]([C@H](OC(C)=O)[C@H]2OC(C)=O)n2cc(nn2)-c2ccc(cc2)S(N)(=O)=O)[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O Show InChI InChI=1S/C34H42N4O19S/c1-15(39)48-13-25-28(57-34-32(54-21(7)45)30(52-19(5)43)27(50-17(3)41)26(56-34)14-49-16(2)40)29(51-18(4)42)31(53-20(6)44)33(55-25)38-12-24(36-37-38)22-8-10-23(11-9-22)58(35,46)47/h8-12,25-34H,13-14H2,1-7H3,(H2,35,46,47)/t25-,26-,27-,28-,29+,30+,31-,32-,33-,34-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50372668

(CHEMBL437092)Show SMILES CC(=O)OC[C@H]1O[C@H]([C@H](OC(C)=O)[C@@H](OC(C)=O)[C@H]1OC(C)=O)n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C22H26N4O11S/c1-11(27)33-10-18-19(34-12(2)28)20(35-13(3)29)21(36-14(4)30)22(37-18)26-9-17(24-25-26)15-5-7-16(8-6-15)38(23,31)32/h5-9,18-22H,10H2,1-4H3,(H2,23,31,32)/t18-,19+,20+,21-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50372673

(CHEMBL411092)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C13H16N4O6S/c14-24(21,22)8-3-1-7(2-4-8)9-5-17(16-15-9)13-12(20)11(19)10(6-18)23-13/h1-5,10-13,18-20H,6H2,(H2,14,21,22)/t10-,11-,12-,13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50372661

(CHEMBL270334)Show SMILES CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H21N5O7S/c1-8(23)18-13-15(25)14(24)12(7-22)28-16(13)21-6-11(19-20-21)9-2-4-10(5-3-9)29(17,26)27/h2-6,12-16,22,24-25H,7H2,1H3,(H,18,23)(H2,17,26,27)/t12-,13-,14-,15-,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50372672

(CHEMBL269852)Show SMILES CC(=O)OC[C@H]1O[C@H]([C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O)n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C22H26N4O11S/c1-11(27)33-10-18-19(34-12(2)28)20(35-13(3)29)21(36-14(4)30)22(37-18)26-9-17(24-25-26)15-5-7-16(8-6-15)38(23,31)32/h5-9,18-22H,10H2,1-4H3,(H2,23,31,32)/t18-,19-,20+,21-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50372669

(CHEMBL407716)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@@H]1O[C@H](CO)[C@@H](O[C@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O Show InChI InChI=1S/C20H28N4O12S/c21-37(32,33)9-3-1-8(2-4-9)10-5-24(23-22-10)19-16(30)15(29)18(12(7-26)34-19)36-20-17(31)14(28)13(27)11(6-25)35-20/h1-5,11-20,25-31H,6-7H2,(H2,21,32,33)/t11-,12-,13-,14+,15-,16-,17-,18-,19-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50372671

(CHEMBL270994)Show SMILES CC(=O)N[C@@H]1[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@@H](COC(C)=O)O[C@H]1n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C22H27N5O10S/c1-11(28)24-19-21(36-14(4)31)20(35-13(3)30)18(10-34-12(2)29)37-22(19)27-9-17(25-26-27)15-5-7-16(8-6-15)38(23,32)33/h5-9,18-22H,10H2,1-4H3,(H,24,28)(H2,23,32,33)/t18-,19-,20-,21-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50372665

(CHEMBL272611)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@@H]1O[C@H](COC(=O)c2ccccc2)[C@@H](OC(=O)c2ccccc2)[C@H]1OC(=O)c1ccccc1 Show InChI InChI=1S/C34H28N4O9S/c35-48(42,43)26-18-16-22(17-19-26)27-20-38(37-36-27)31-30(47-34(41)25-14-8-3-9-15-25)29(46-33(40)24-12-6-2-7-13-24)28(45-31)21-44-32(39)23-10-4-1-5-11-23/h1-20,28-31H,21H2,(H2,35,42,43)/t28-,29-,30-,31-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data