

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21464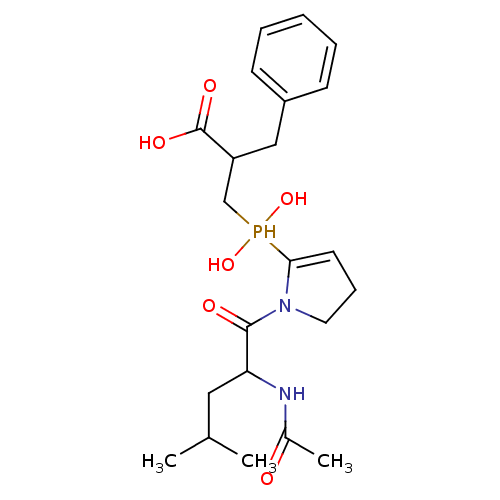 (2-benzyl-3-{[1-(2-acetamido-4-methylpentanoyl)pyrr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.130 | -56.4 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM21471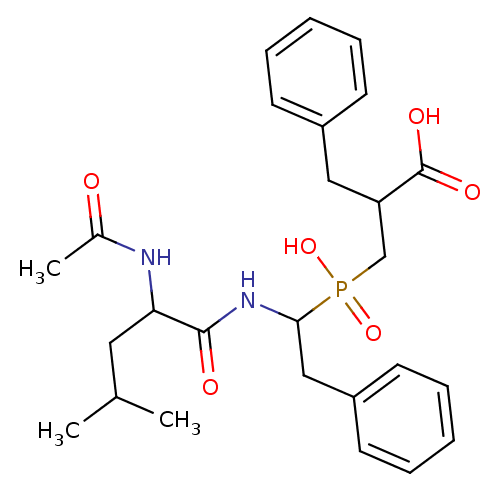 (2-benzyl-3-{[1-(2-acetamido-4-methylpentanamido)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens | Assay Description Inhibitor potencies toward bovine carboxypeptidase A (Sigma-Aldrich Co) were determined in 96-well microplates format. Activity was monitored by meas... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21474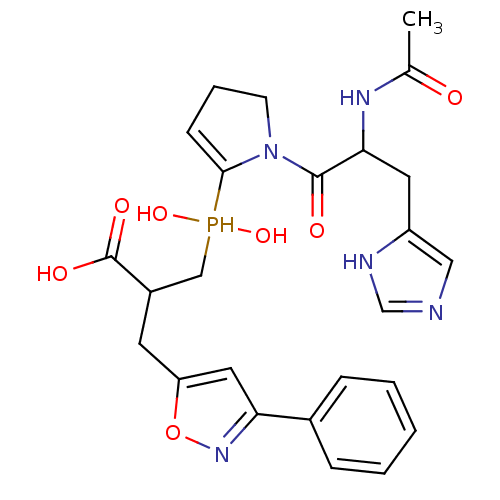 (3-({1-[2-acetamido-3-(1H-imidazol-4-yl)propanoyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.400 | -53.6 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM21464 (2-benzyl-3-{[1-(2-acetamido-4-methylpentanoyl)pyrr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens | Assay Description Inhibitor potencies toward bovine carboxypeptidase A (Sigma-Aldrich Co) were determined in 96-well microplates format. Activity was monitored by meas... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21470 (2-benzyl-3-({1-[2-acetamido-3-(1H-imidazol-4-yl)pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21473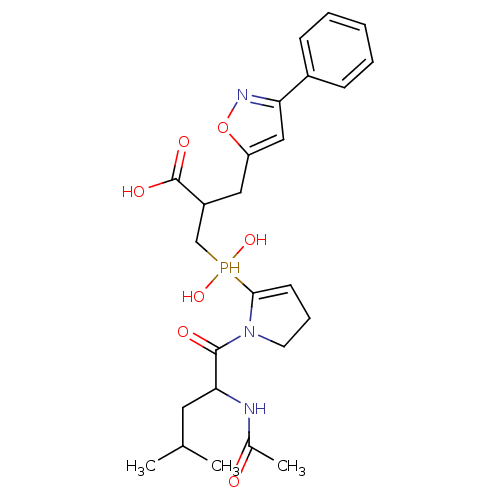 (3-{[1-(2-acetamido-4-methylpentanoyl)pyrrolidin-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.25 | -50.8 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21468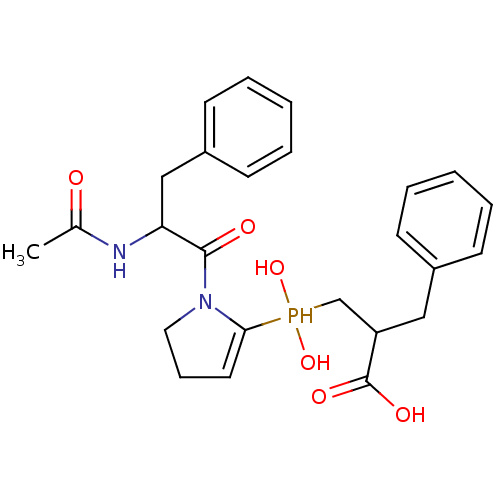 (2-benzyl-3-{[1-(2-acetamido-3-phenylpropanoyl)pyrr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.20 | -47.3 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21467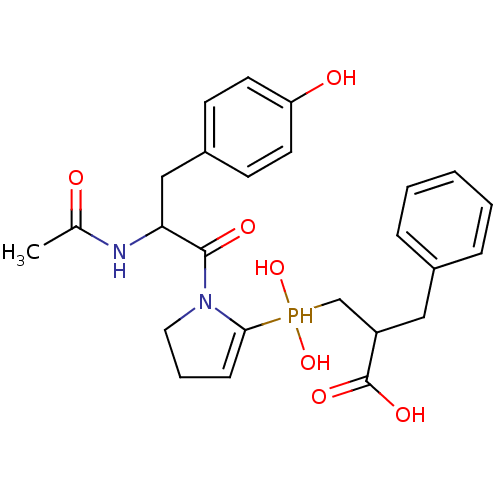 (2-benzyl-3-({1-[2-acetamido-3-(4-hydroxyphenyl)pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.20 | -47.3 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21465 (3-{[1-(6-amino-2-acetamidohexanoyl)pyrrolidin-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.5 | -46.7 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21469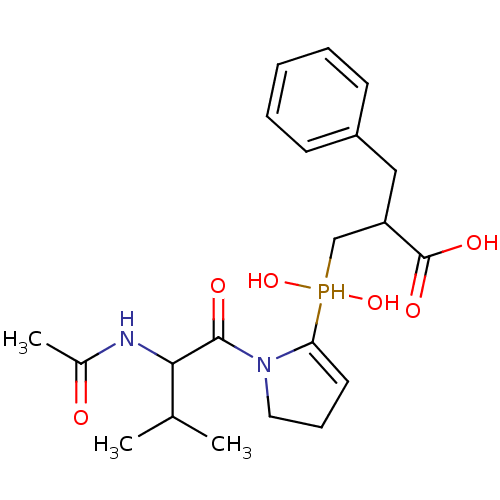 (2-benzyl-3-{[1-(2-acetamido-3-methylbutanoyl)pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.60 | -46.7 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21466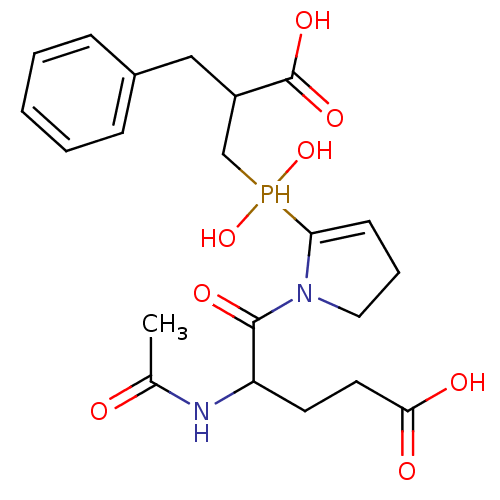 (5-{2-[(2-benzyl-2-carboxyethyl)(hydroxy)phosphoryl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | -46.5 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21463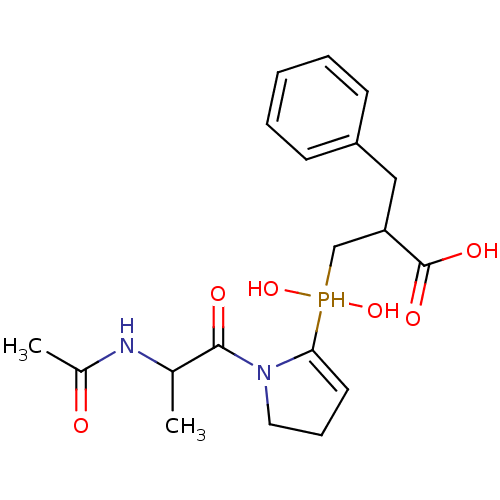 (2-benzyl-3-{[1-(2-acetamidopropanoyl)pyrrolidin-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.5 | -46.4 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM21473 (3-{[1-(2-acetamido-4-methylpentanoyl)pyrrolidin-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens | Assay Description Inhibitor potencies toward bovine carboxypeptidase A (Sigma-Aldrich Co) were determined in 96-well microplates format. Activity was monitored by meas... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM21470 (2-benzyl-3-({1-[2-acetamido-3-(1H-imidazol-4-yl)pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens | Assay Description Inhibitor potencies toward bovine carboxypeptidase A (Sigma-Aldrich Co) were determined in 96-well microplates format. Activity was monitored by meas... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM21472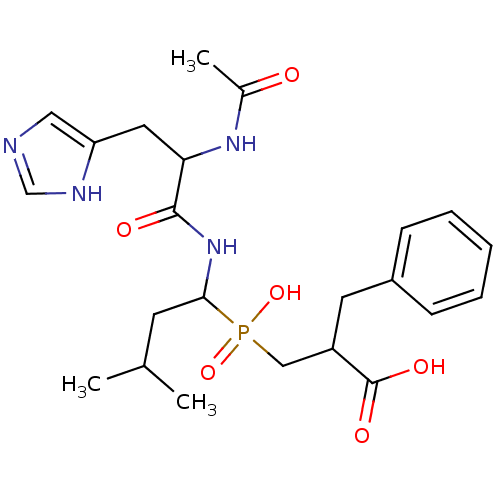 (2-benzyl-3-({1-[2-acetamido-3-(1H-imidazol-4-yl)pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens | Assay Description Inhibitor potencies toward bovine carboxypeptidase A (Sigma-Aldrich Co) were determined in 96-well microplates format. Activity was monitored by meas... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21475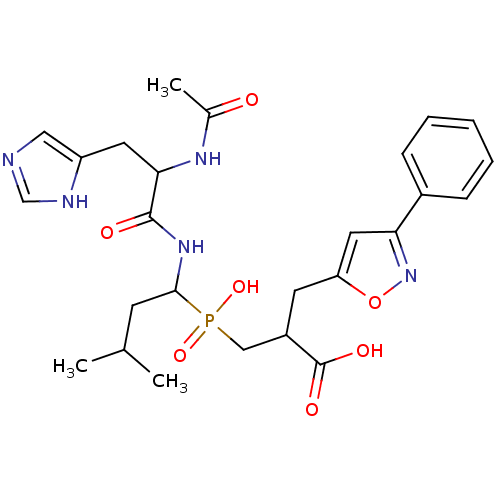 (3-({1-[2-acetamido-3-(1H-imidazol-4-yl)propanamido...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 220 | -38.0 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21454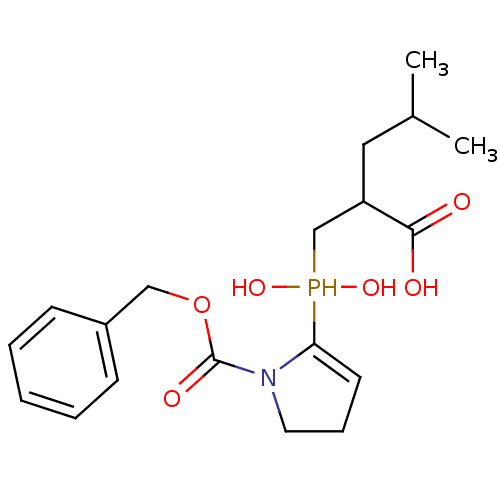 (2-[({1-[(benzyloxy)carbonyl]pyrrolidin-2-yl}(hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 300 | -37.2 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21452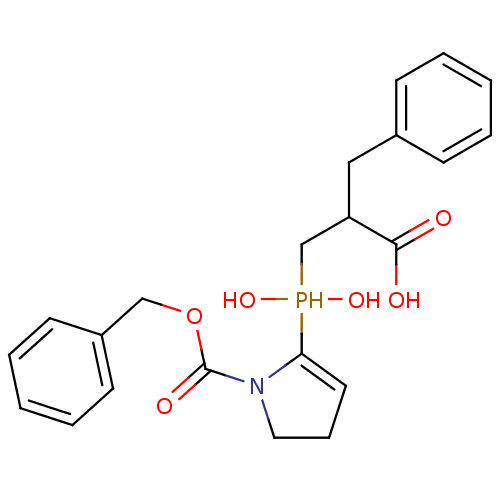 (2-benzyl-3-({1-[(benzyloxy)carbonyl]pyrrolidin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 300 | -37.2 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21472 (2-benzyl-3-({1-[2-acetamido-3-(1H-imidazol-4-yl)pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 800 | -34.8 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21471 (2-benzyl-3-{[1-(2-acetamido-4-methylpentanamido)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 920 | -34.5 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM21474 (3-({1-[2-acetamido-3-(1H-imidazol-4-yl)propanoyl]p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens | Assay Description Inhibitor potencies toward bovine carboxypeptidase A (Sigma-Aldrich Co) were determined in 96-well microplates format. Activity was monitored by meas... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21455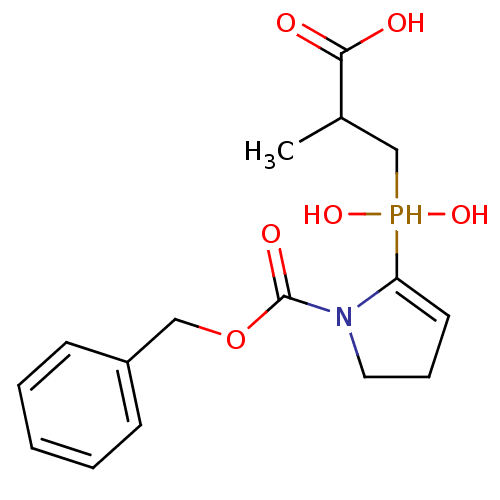 (3-({1-[(benzyloxy)carbonyl]pyrrolidin-2-yl}(hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | -31.5 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21458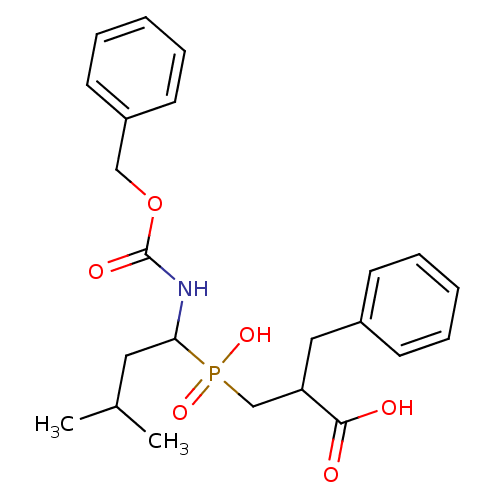 (2-benzyl-3-[(1-{[(benzyloxy)carbonyl]amino}-3-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.00E+3 | -29.1 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM21470 (2-benzyl-3-({1-[2-acetamido-3-(1H-imidazol-4-yl)pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21460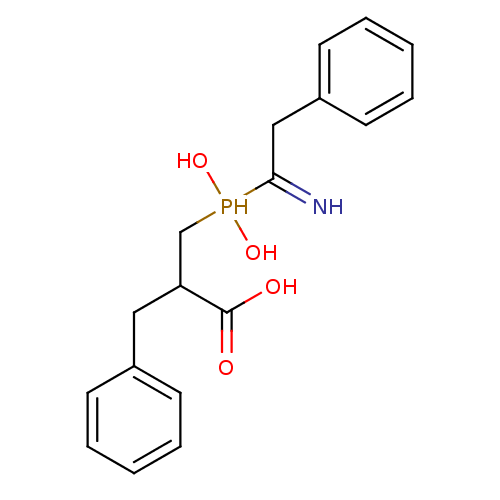 (3-[(1-amino-2-phenylethyl)(hydroxy)phosphoryl]-2-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21456 (2-benzyl-3-[(1-{[(benzyloxy)carbonyl]amino}ethyl)(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM21474 (3-({1-[2-acetamido-3-(1H-imidazol-4-yl)propanoyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM21473 (3-{[1-(2-acetamido-4-methylpentanoyl)pyrrolidin-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21457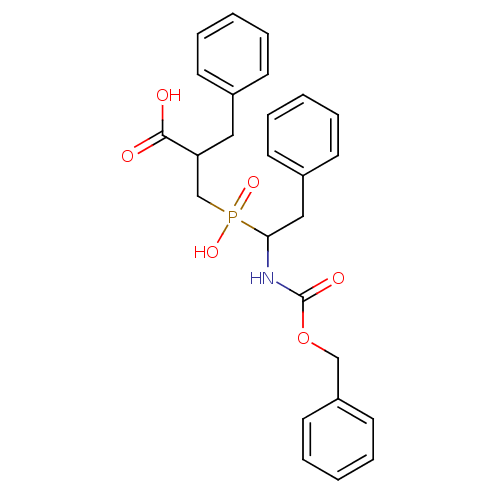 (2-benzyl-3-[(1-{[(benzyloxy)carbonyl]amino}-2-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM21464 (2-benzyl-3-{[1-(2-acetamido-4-methylpentanoyl)pyrr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21462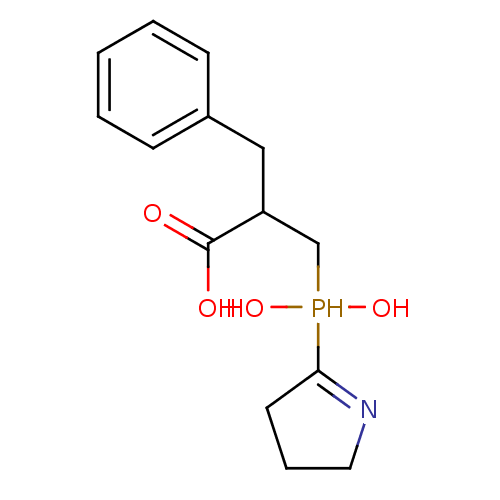 (2-benzyl-3-[hydroxy(pyrrolidin-2-yl)phosphoryl]pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21461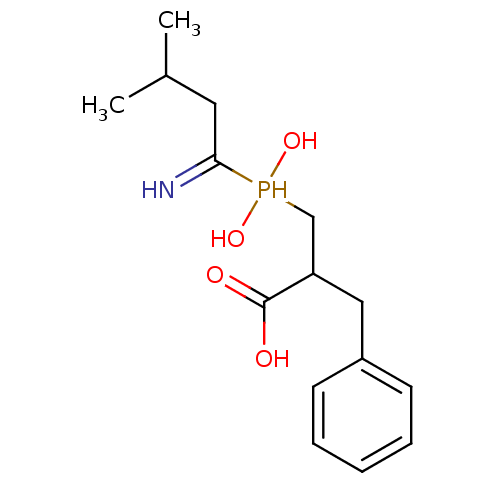 (3-[(1-amino-3-methylbutyl)(hydroxy)phosphoryl]-2-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM21475 (3-({1-[2-acetamido-3-(1H-imidazol-4-yl)propanamido...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens | Assay Description Inhibitor potencies toward bovine carboxypeptidase A (Sigma-Aldrich Co) were determined in 96-well microplates format. Activity was monitored by meas... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21459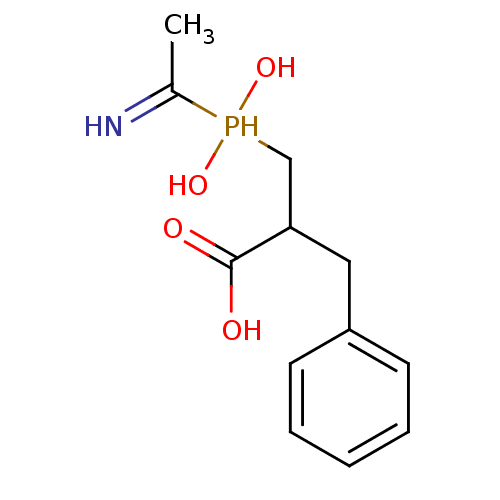 (3-[(1-aminoethyl)(hydroxy)phosphoryl]-2-benzylprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||