

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86082 (Tetraketone, 19) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86085 (Tetraketone, 22) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86070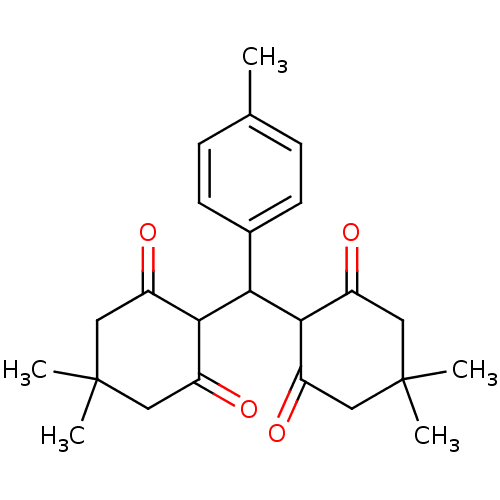 (Tetraketone, 3) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86077 (Tetraketone, 11) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86074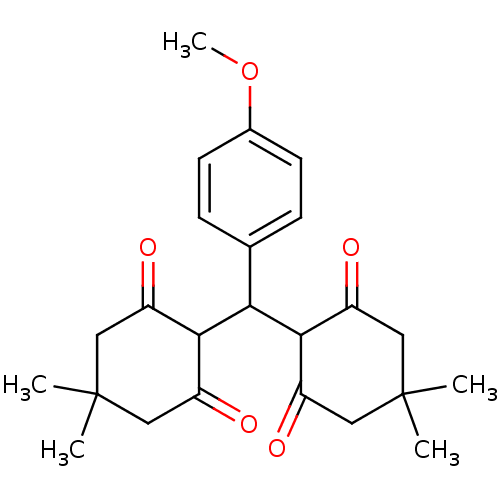 (Tetraketone, 8) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.13E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86072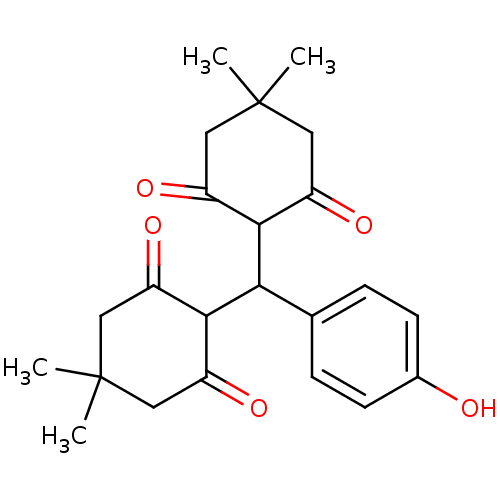 (Tetraketone, 6) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.64E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86080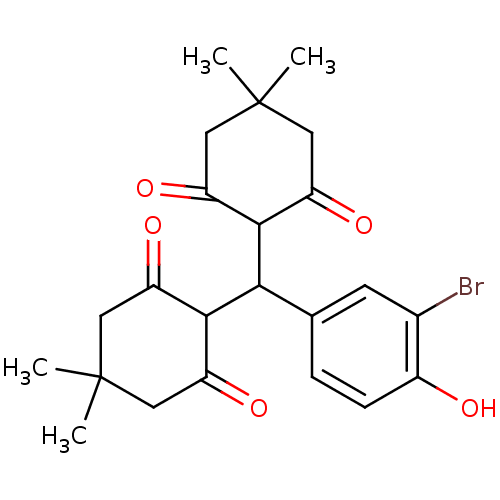 (Tetraketone, 15) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.71E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86078 (Tetraketone, 12) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.84E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86076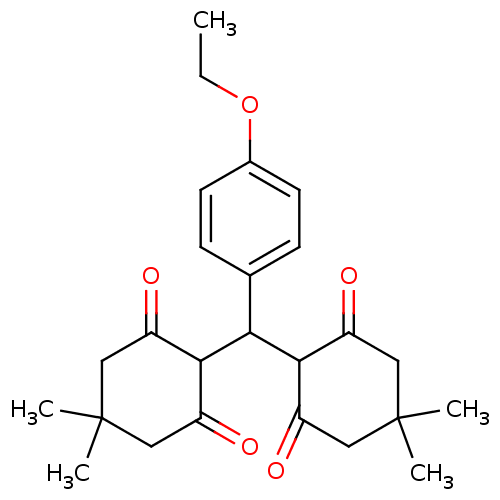 (Tetraketone, 10) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.08E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86069 (Tetraketone, 2) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86075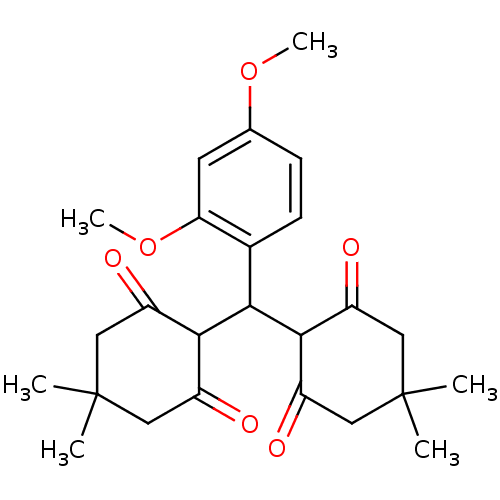 (Tetraketone, 9) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.31E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86083 (Tetraketone, 20) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.54E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86084 (Tetraketone, 21) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.15E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86079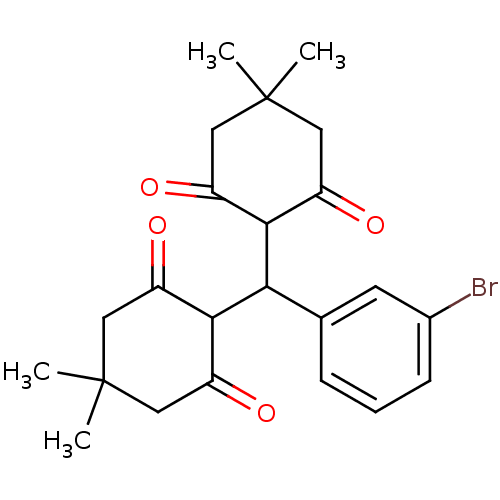 (Tetraketone, 14) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.29E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86081 (Tetraketone, 16) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.91E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86073 (Tetraketone, 7) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.96E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86071 (Tetraketone, 4) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.98E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||