

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50067499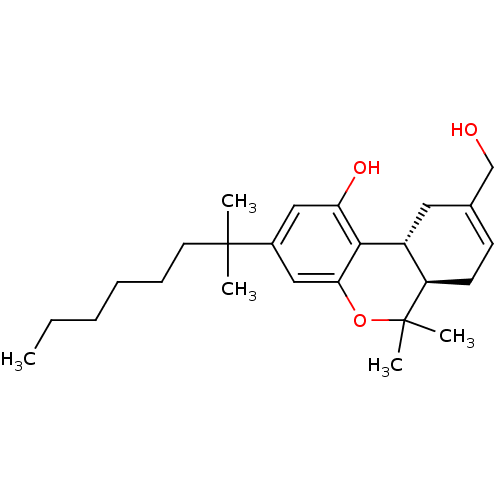 ((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266832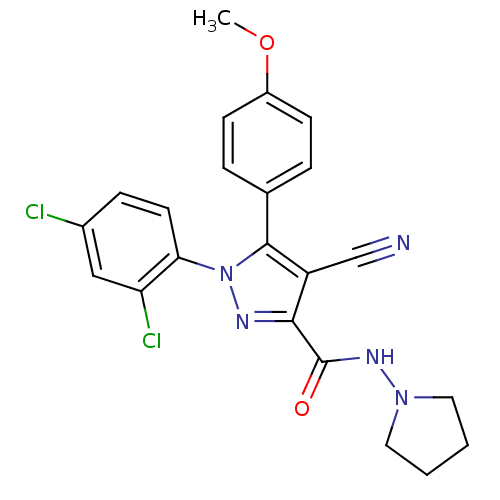 (1-(2,4-Dichlorophenyl)-4-cyano-5-(4-methoxyphenyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267374 (1-(2-bromophenyl)-4-cyano-5-(4-methoxyphenyl)-N-(p...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266833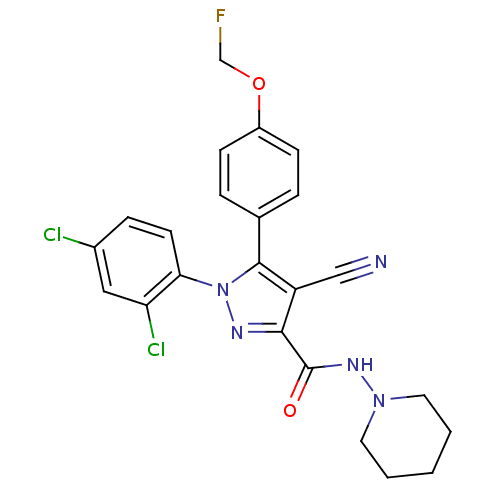 (1-(2,4-Dichlorophenyl)-4-cyano-5-(4-(fluoromethoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267373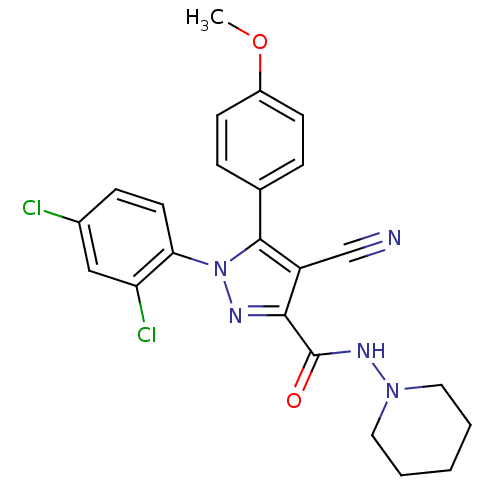 (4-cyano-1-(2,4-dichlorophenyl)-5-(4-methoxyphenyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267375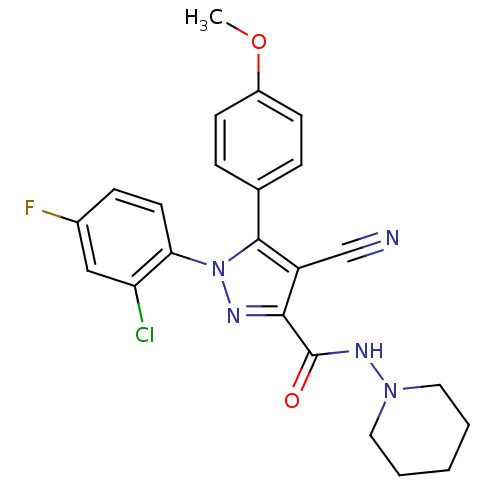 (1-(2-Chloro-4-fluorophenyl)-4-cyano-5-(4-methoxyph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266809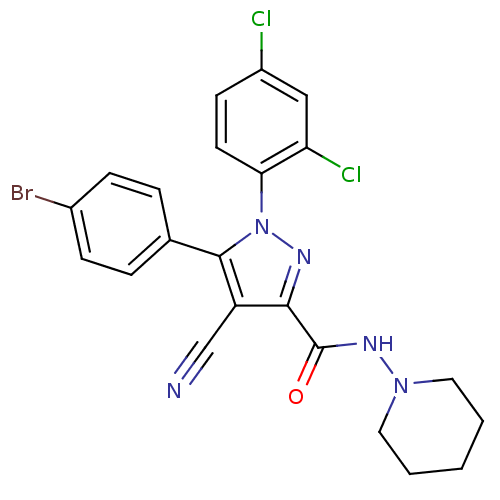 (1-(2,4-Dichlorophenyl)-4-cyano-5-(4-bromophenyl)-N...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266864 (5-(4-chlorophenyl)-4-cyano-1-(2,4-dichlorophenyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266807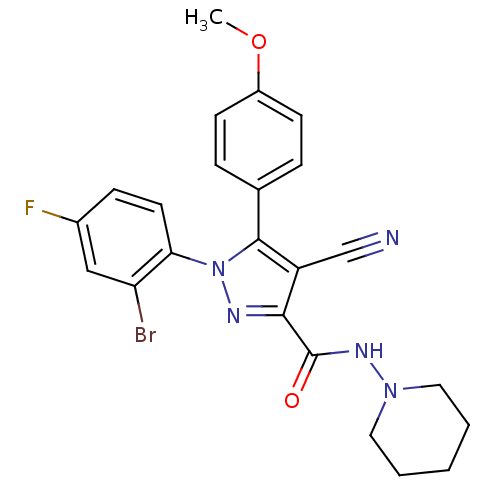 (1-(2-Bromo-4-fluorophenyl)-4-cyano-5-(4-methoxyphe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266808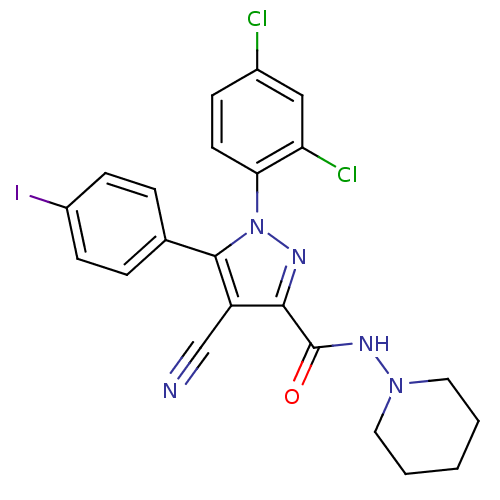 (1-(2,4-Dichlorophenyl)-4-cyano-5-(4-iodophenyl)-N-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266830 (1-(2,4-Dichlorophenyl)-4-cyano-5-(4-iodophenyl)-N-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267372 (1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 422 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266831 (1-(2,4-Dichlorophenyl)-4-cyano-5-(4-bromophenyl)-N...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266810 (1-(2-Bromophenyl)-5-(4-methoxyphenyl)-N3-(piperidi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||