 Found 81 hits of Enzyme Inhibition Constant Data
Found 81 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244238
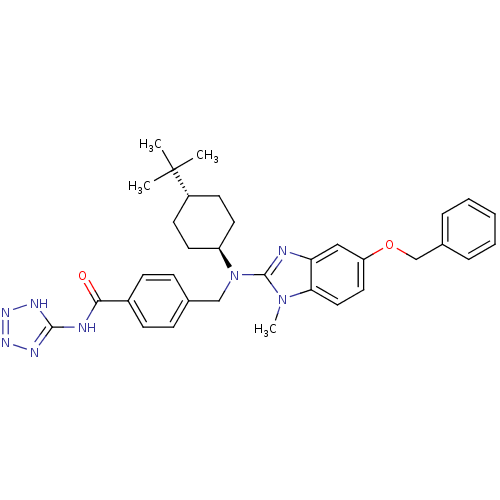
(CHEMBL499372 | trans-4-(((5-(benzyloxy)-1-methyl-1...)Show SMILES Cn1c(nc2cc(OCc3ccccc3)ccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:34.38,wD:37.45,(-7.9,-48.6,;-7.12,-47.26,;-5.7,-46.67,;-5.82,-45.14,;-7.32,-44.78,;-8.06,-43.43,;-9.59,-43.39,;-10.33,-42.04,;-9.53,-40.73,;-10.27,-39.37,;-9.47,-38.06,;-10.21,-36.71,;-11.75,-36.67,;-12.55,-37.99,;-11.81,-39.34,;-10.4,-44.71,;-9.66,-46.06,;-8.13,-46.09,;-4.37,-47.45,;-4.38,-48.99,;-3.05,-49.77,;-1.72,-49,;-.39,-49.78,;-.4,-51.32,;-1.74,-52.08,;-3.07,-51.3,;.93,-52.1,;.92,-53.64,;2.27,-51.33,;3.6,-52.11,;4.12,-53.56,;5.66,-53.52,;6.1,-52.05,;4.83,-51.17,;-3.04,-46.69,;-3.04,-45.15,;-1.69,-44.39,;-.36,-45.17,;-.38,-46.71,;-1.71,-47.47,;.98,-44.41,;2.3,-43.63,;1.75,-45.75,;.21,-43.08,)| Show InChI InChI=1S/C34H40N8O2/c1-34(2,3)26-14-16-27(17-15-26)42(21-23-10-12-25(13-11-23)31(43)36-32-37-39-40-38-32)33-35-29-20-28(18-19-30(29)41(33)4)44-22-24-8-6-5-7-9-24/h5-13,18-20,26-27H,14-17,21-22H2,1-4H3,(H2,36,37,38,39,40,43)/t26-,27- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244236
(CHEMBL500315 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(OC3CCCC3)ccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:32.36,wD:35.43,(16.18,-49.49,;16.96,-48.15,;18.38,-47.56,;18.26,-46.02,;16.76,-45.67,;16.03,-44.32,;14.49,-44.28,;13.75,-42.93,;14.55,-41.62,;16.09,-41.49,;16.44,-39.99,;15.13,-39.19,;13.96,-40.19,;13.69,-45.6,;14.43,-46.95,;15.96,-46.98,;19.71,-48.34,;19.71,-49.88,;21.04,-50.66,;22.37,-49.89,;23.69,-50.66,;23.69,-52.21,;22.34,-52.97,;21.02,-52.19,;25.02,-52.98,;25.01,-54.52,;26.36,-52.22,;27.68,-53,;28.2,-54.45,;29.74,-54.41,;30.18,-52.94,;28.91,-52.06,;21.04,-47.58,;21.05,-46.04,;22.39,-45.28,;23.72,-46.06,;23.71,-47.6,;22.37,-48.36,;25.06,-45.3,;26.39,-44.52,;25.83,-46.64,;24.3,-43.97,)| Show InChI InChI=1S/C32H42N8O2/c1-32(2,3)23-13-15-24(16-14-23)40(20-21-9-11-22(12-10-21)29(41)34-30-35-37-38-36-30)31-33-27-19-26(17-18-28(27)39(31)4)42-25-7-5-6-8-25/h9-12,17-19,23-25H,5-8,13-16,20H2,1-4H3,(H2,34,35,36,37,38,41)/t23-,24- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244265
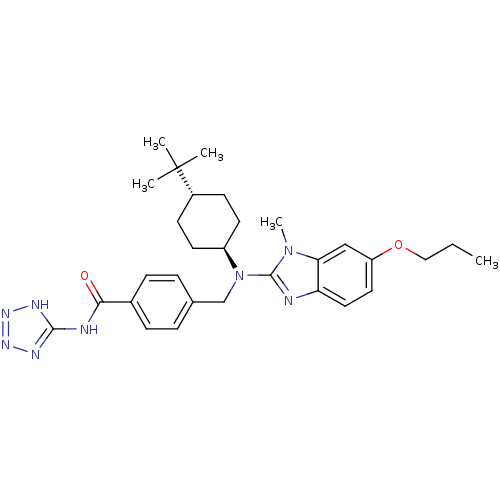
(CHEMBL480113 | trans-4-((4-tert-butylcyclohexyl)(1...)Show SMILES CCCOc1ccc2nc(N(Cc3ccc(cc3)C(=O)Nc3nnn[nH]3)[C@H]3CC[C@@H](CC3)C(C)(C)C)n(C)c2c1 |r,wU:26.27,wD:29.34,(9.13,-42.84,;9.87,-44.19,;11.41,-44.22,;12.15,-45.57,;13.69,-45.6,;14.49,-44.28,;16.03,-44.32,;16.76,-45.67,;18.26,-46.03,;18.38,-47.56,;19.71,-48.34,;19.71,-49.88,;21.04,-50.66,;22.37,-49.89,;23.7,-50.67,;23.69,-52.21,;22.34,-52.97,;21.02,-52.19,;25.02,-52.99,;25.01,-54.53,;26.36,-52.22,;27.68,-53,;28.2,-54.45,;29.74,-54.42,;30.18,-52.94,;28.91,-52.06,;21.04,-47.58,;21.05,-46.04,;22.39,-45.28,;23.72,-46.06,;23.71,-47.6,;22.37,-48.36,;25.06,-45.3,;26.39,-44.52,;25.83,-46.64,;24.3,-43.97,;16.96,-48.15,;16.19,-49.49,;15.96,-46.98,;14.43,-46.95,)| Show InChI InChI=1S/C30H40N8O2/c1-6-17-40-24-15-16-25-26(18-24)37(5)29(31-25)38(23-13-11-22(12-14-23)30(2,3)4)19-20-7-9-21(10-8-20)27(39)32-28-33-35-36-34-28/h7-10,15-16,18,22-23H,6,11-14,17,19H2,1-5H3,(H2,32,33,34,35,36,39)/t22-,23- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244199
(CHEMBL458145 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(O)ccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:27.30,wD:30.37,(-7.23,-33.1,;-6.46,-31.77,;-5.03,-31.18,;-5.16,-29.64,;-6.66,-29.29,;-7.39,-27.94,;-8.93,-27.9,;-9.67,-26.55,;-9.73,-29.22,;-8.99,-30.57,;-7.46,-30.6,;-3.7,-31.96,;-3.71,-33.5,;-2.38,-34.28,;-1.05,-33.51,;.28,-34.28,;.27,-35.83,;-1.08,-36.59,;-2.4,-35.81,;1.6,-36.6,;1.59,-38.14,;2.94,-35.84,;4.27,-36.62,;4.79,-38.07,;6.32,-38.03,;6.76,-36.56,;5.5,-35.68,;-2.37,-31.2,;-2.37,-29.66,;-1.02,-28.9,;.31,-29.68,;.29,-31.22,;-1.04,-31.98,;1.65,-28.92,;2.97,-28.14,;2.42,-30.26,;.88,-27.59,)| Show InChI InChI=1S/C27H34N8O2/c1-27(2,3)19-9-11-20(12-10-19)35(26-28-22-15-21(36)13-14-23(22)34(26)4)16-17-5-7-18(8-6-17)24(37)29-25-30-32-33-31-25/h5-8,13-15,19-20,36H,9-12,16H2,1-4H3,(H2,29,30,31,32,33,37)/t19-,20- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244235
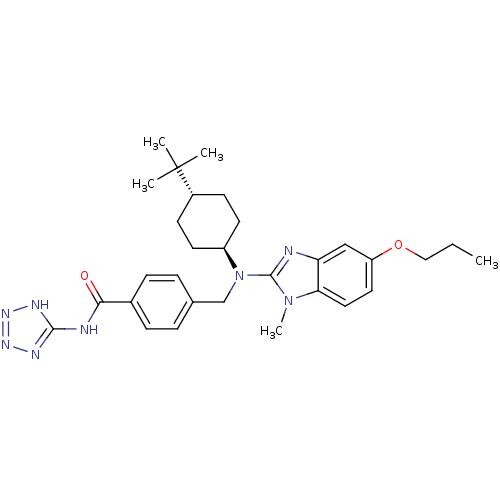
(CHEMBL480692 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES CCCOc1ccc2n(C)c(nc2c1)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:30.33,wD:33.40,(15,-40.17,;14.2,-41.49,;14.94,-42.84,;14.14,-44.16,;14.88,-45.51,;14.07,-46.83,;14.81,-48.17,;16.34,-48.21,;17.35,-49.38,;16.57,-50.71,;18.77,-48.79,;18.65,-47.25,;17.15,-46.9,;16.41,-45.55,;20.1,-49.57,;20.09,-51.11,;21.42,-51.88,;22.75,-51.12,;24.08,-51.89,;24.07,-53.43,;22.73,-54.2,;21.4,-53.42,;25.4,-54.21,;25.39,-55.75,;26.74,-53.45,;28.07,-54.23,;28.59,-55.68,;30.13,-55.64,;30.57,-54.16,;29.3,-53.29,;21.43,-48.8,;21.43,-47.27,;22.78,-46.51,;24.11,-47.29,;24.09,-48.83,;22.76,-49.58,;25.45,-46.53,;26.77,-45.75,;26.22,-47.86,;24.68,-45.19,)| Show InChI InChI=1S/C30H40N8O2/c1-6-17-40-24-15-16-26-25(18-24)31-29(37(26)5)38(23-13-11-22(12-14-23)30(2,3)4)19-20-7-9-21(10-8-20)27(39)32-28-33-35-36-34-28/h7-10,15-16,18,22-23H,6,11-14,17,19H2,1-5H3,(H2,32,33,34,35,36,39)/t22-,23- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244237
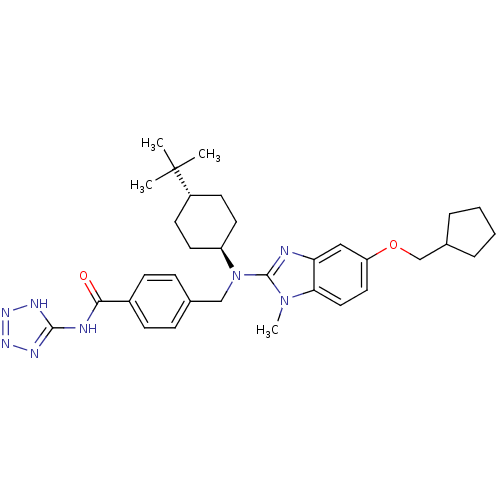
(CHEMBL448921 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(OCC3CCCC3)ccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:33.37,wD:36.44,(-7.9,-48.6,;-7.12,-47.26,;-5.7,-46.67,;-5.82,-45.13,;-7.32,-44.78,;-8.06,-43.43,;-9.59,-43.39,;-10.33,-42.04,;-9.53,-40.73,;-10.27,-39.37,;-9.61,-37.98,;-10.73,-36.92,;-12.08,-37.66,;-11.8,-39.17,;-10.4,-44.71,;-9.66,-46.06,;-8.13,-46.09,;-4.37,-47.45,;-4.38,-48.99,;-3.05,-49.77,;-1.72,-49,;-.39,-49.77,;-.4,-51.32,;-1.74,-52.08,;-3.07,-51.3,;.93,-52.09,;.92,-53.63,;2.27,-51.33,;3.6,-52.11,;4.12,-53.56,;5.66,-53.52,;6.1,-52.05,;4.83,-51.17,;-3.04,-46.69,;-3.04,-45.15,;-1.69,-44.39,;-.36,-45.17,;-.38,-46.71,;-1.71,-47.47,;.98,-44.41,;2.3,-43.63,;1.75,-45.75,;.21,-43.08,)| Show InChI InChI=1S/C33H44N8O2/c1-33(2,3)25-13-15-26(16-14-25)41(20-22-9-11-24(12-10-22)30(42)35-31-36-38-39-37-31)32-34-28-19-27(17-18-29(28)40(32)4)43-21-23-7-5-6-8-23/h9-12,17-19,23,25-26H,5-8,13-16,20-21H2,1-4H3,(H2,35,36,37,38,39,42)/t25-,26- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244234
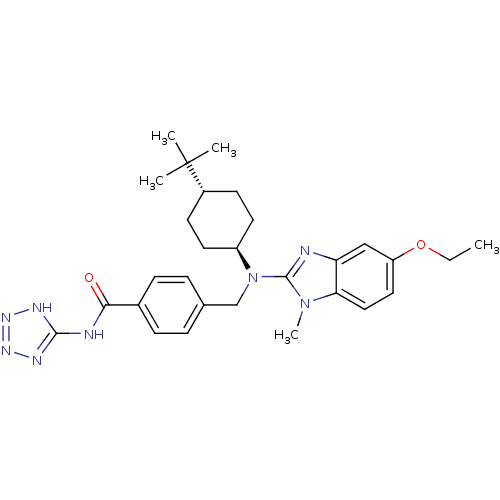
(CHEMBL518939 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES CCOc1ccc2n(C)c(nc2c1)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:29.32,wD:32.39,(-10.27,-39.37,;-9.53,-40.73,;-10.33,-42.04,;-9.59,-43.39,;-10.4,-44.71,;-9.66,-46.06,;-8.13,-46.09,;-7.12,-47.26,;-7.9,-48.6,;-5.7,-46.67,;-5.82,-45.14,;-7.32,-44.78,;-8.06,-43.43,;-4.37,-47.45,;-4.38,-48.99,;-3.05,-49.77,;-1.72,-49,;-.39,-49.78,;-.4,-51.32,;-1.74,-52.08,;-3.07,-51.3,;.93,-52.1,;.92,-53.64,;2.27,-51.33,;3.6,-52.11,;4.12,-53.56,;5.66,-53.52,;6.1,-52.05,;4.83,-51.17,;-3.04,-46.69,;-3.04,-45.15,;-1.69,-44.39,;-.36,-45.17,;-.38,-46.71,;-1.71,-47.47,;.98,-44.41,;2.3,-43.63,;1.75,-45.75,;.21,-43.08,)| Show InChI InChI=1S/C29H38N8O2/c1-6-39-23-15-16-25-24(17-23)30-28(36(25)5)37(22-13-11-21(12-14-22)29(2,3)4)18-19-7-9-20(10-8-19)26(38)31-27-32-34-35-33-27/h7-10,15-17,21-22H,6,11-14,18H2,1-5H3,(H2,31,32,33,34,35,38)/t21-,22- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244264

(CHEMBL471979 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES COc1ccc2nc(N(Cc3ccc(cc3)C(=O)Nc3nnn[nH]3)[C@H]3CC[C@@H](CC3)C(C)(C)C)n(C)c2c1 |r,wU:24.25,wD:27.32,(11.41,-44.22,;12.15,-45.57,;13.69,-45.6,;14.49,-44.28,;16.03,-44.32,;16.76,-45.67,;18.26,-46.03,;18.38,-47.56,;19.72,-48.34,;19.71,-49.88,;21.04,-50.66,;22.37,-49.89,;23.7,-50.67,;23.69,-52.21,;22.34,-52.97,;21.02,-52.19,;25.02,-52.99,;25.01,-54.53,;26.36,-52.23,;27.69,-53.01,;28.2,-54.45,;29.74,-54.42,;30.18,-52.94,;28.91,-52.07,;21.04,-47.58,;21.05,-46.04,;22.39,-45.28,;23.72,-46.06,;23.71,-47.61,;22.37,-48.36,;25.06,-45.31,;26.39,-44.53,;25.84,-46.64,;24.3,-43.97,;16.96,-48.16,;16.19,-49.49,;15.96,-46.98,;14.43,-46.95,)| Show InChI InChI=1S/C28H36N8O2/c1-28(2,3)20-10-12-21(13-11-20)36(27-29-23-15-14-22(38-5)16-24(23)35(27)4)17-18-6-8-19(9-7-18)25(37)30-26-31-33-34-32-26/h6-9,14-16,20-21H,10-13,17H2,1-5H3,(H2,30,31,32,33,34,37)/t20-,21- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244235

(CHEMBL480692 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES CCCOc1ccc2n(C)c(nc2c1)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:30.33,wD:33.40,(15,-40.17,;14.2,-41.49,;14.94,-42.84,;14.14,-44.16,;14.88,-45.51,;14.07,-46.83,;14.81,-48.17,;16.34,-48.21,;17.35,-49.38,;16.57,-50.71,;18.77,-48.79,;18.65,-47.25,;17.15,-46.9,;16.41,-45.55,;20.1,-49.57,;20.09,-51.11,;21.42,-51.88,;22.75,-51.12,;24.08,-51.89,;24.07,-53.43,;22.73,-54.2,;21.4,-53.42,;25.4,-54.21,;25.39,-55.75,;26.74,-53.45,;28.07,-54.23,;28.59,-55.68,;30.13,-55.64,;30.57,-54.16,;29.3,-53.29,;21.43,-48.8,;21.43,-47.27,;22.78,-46.51,;24.11,-47.29,;24.09,-48.83,;22.76,-49.58,;25.45,-46.53,;26.77,-45.75,;26.22,-47.86,;24.68,-45.19,)| Show InChI InChI=1S/C30H40N8O2/c1-6-17-40-24-15-16-26-25(18-24)31-29(37(26)5)38(23-13-11-22(12-14-23)30(2,3)4)19-20-7-9-21(10-8-20)27(39)32-28-33-35-36-34-28/h7-10,15-16,18,22-23H,6,11-14,17,19H2,1-5H3,(H2,32,33,34,35,36,39)/t22-,23- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulation |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244233
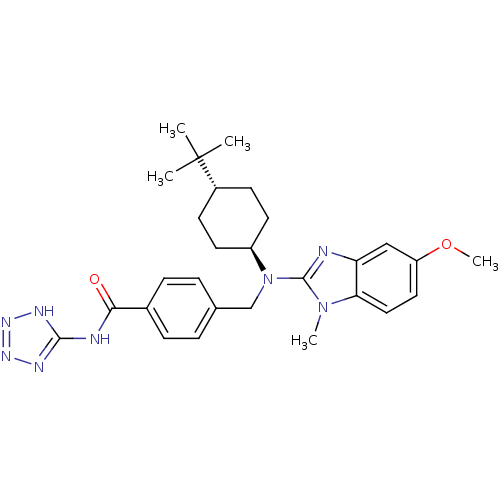
(CHEMBL513067 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES COc1ccc2n(C)c(nc2c1)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:28.31,wD:31.38,(13.19,-24.98,;12.38,-26.29,;13.12,-27.64,;12.32,-28.96,;13.06,-30.31,;14.59,-30.34,;15.59,-31.52,;14.82,-32.85,;17.02,-30.92,;16.89,-29.39,;15.39,-29.03,;14.66,-27.68,;18.35,-31.7,;18.34,-33.24,;19.67,-34.02,;21,-33.25,;22.33,-34.03,;22.32,-35.57,;20.97,-36.33,;19.65,-35.55,;23.65,-36.35,;23.64,-37.89,;24.99,-35.59,;26.32,-36.37,;26.83,-37.81,;28.37,-37.78,;28.81,-36.3,;27.55,-35.43,;19.68,-30.94,;19.68,-29.4,;21.03,-28.64,;22.36,-29.42,;22.34,-30.96,;21.01,-31.72,;23.7,-28.67,;25.02,-27.89,;24.47,-30,;22.93,-27.33,)| Show InChI InChI=1S/C28H36N8O2/c1-28(2,3)20-10-12-21(13-11-20)36(27-29-23-16-22(38-5)14-15-24(23)35(27)4)17-18-6-8-19(9-7-18)25(37)30-26-31-33-34-32-26/h6-9,14-16,20-21H,10-13,17H2,1-5H3,(H2,30,31,32,33,34,37)/t20-,21- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244122

(CHEMBL459457 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2ccccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:26.29,wD:29.36,(21.92,-38.87,;22.27,-37.38,;23.7,-36.78,;23.58,-35.25,;22.08,-34.89,;21.34,-33.54,;19.8,-33.5,;19,-34.82,;19.74,-36.17,;21.27,-36.2,;25.03,-37.56,;25.02,-39.1,;26.35,-39.88,;27.68,-39.11,;29.01,-39.89,;29,-41.43,;27.66,-42.19,;26.33,-41.41,;30.33,-42.21,;30.32,-43.75,;31.67,-41.45,;33,-42.22,;33.52,-43.67,;35.06,-43.64,;35.5,-42.16,;34.23,-41.29,;26.36,-36.8,;26.36,-35.26,;27.71,-34.5,;29.04,-35.28,;29.02,-36.82,;27.69,-37.58,;30.38,-34.53,;31.7,-33.74,;31.15,-35.86,;29.61,-33.19,)| Show InChI InChI=1S/C27H34N8O/c1-27(2,3)20-13-15-21(16-14-20)35(26-28-22-7-5-6-8-23(22)34(26)4)17-18-9-11-19(12-10-18)24(36)29-25-30-32-33-31-25/h5-12,20-21H,13-17H2,1-4H3,(H2,29,30,31,32,33,36)/t20-,21- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244300

(CHEMBL470955 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cc1cc2nc(N(Cc3ccc(cc3)C(=O)Nc3nnn[nH]3)[C@H]3CC[C@@H](CC3)C(C)(C)C)n(C)c2cc1C |r,wU:22.23,wD:25.30,(13.75,-42.93,;14.49,-44.28,;16.03,-44.32,;16.76,-45.67,;18.26,-46.03,;18.38,-47.56,;19.71,-48.34,;19.71,-49.88,;21.04,-50.66,;22.37,-49.89,;23.7,-50.67,;23.69,-52.21,;22.34,-52.97,;21.02,-52.19,;25.02,-52.99,;25.01,-54.53,;26.36,-52.23,;27.68,-53,;28.2,-54.45,;29.74,-54.42,;30.18,-52.94,;28.91,-52.07,;21.04,-47.58,;21.05,-46.04,;22.39,-45.28,;23.72,-46.06,;23.71,-47.6,;22.37,-48.36,;25.06,-45.31,;26.39,-44.52,;25.83,-46.64,;24.3,-43.97,;16.96,-48.16,;16.19,-49.49,;15.96,-46.98,;14.43,-46.95,;13.69,-45.6,;12.15,-45.57,)| Show InChI InChI=1S/C29H38N8O/c1-18-15-24-25(16-19(18)2)36(6)28(30-24)37(23-13-11-22(12-14-23)29(3,4)5)17-20-7-9-21(10-8-20)26(38)31-27-32-34-35-33-27/h7-10,15-16,22-23H,11-14,17H2,1-6H3,(H2,31,32,33,34,35,38)/t22-,23- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244266
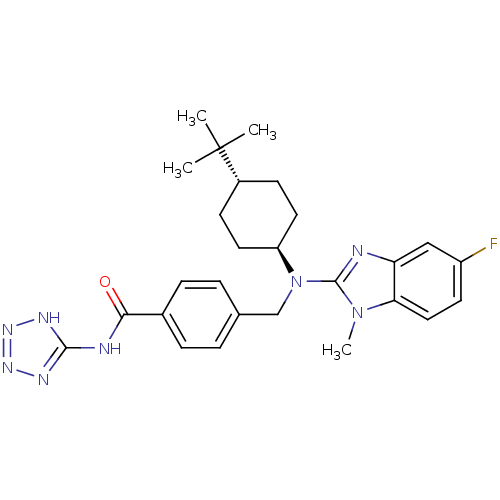
(CHEMBL472154 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(F)ccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:27.30,wD:30.37,(16.19,-49.49,;16.96,-48.16,;18.38,-47.56,;18.26,-46.03,;16.76,-45.67,;16.03,-44.32,;14.49,-44.28,;13.75,-42.93,;13.69,-45.6,;14.43,-46.95,;15.96,-46.98,;19.72,-48.34,;19.71,-49.88,;21.04,-50.66,;22.37,-49.89,;23.7,-50.67,;23.69,-52.21,;22.34,-52.97,;21.02,-52.19,;25.02,-52.99,;25.01,-54.53,;26.36,-52.23,;27.69,-53.01,;28.2,-54.45,;29.74,-54.42,;30.18,-52.94,;28.91,-52.07,;21.04,-47.58,;21.05,-46.04,;22.39,-45.28,;23.72,-46.06,;23.71,-47.61,;22.37,-48.36,;25.06,-45.31,;26.39,-44.53,;25.84,-46.64,;24.3,-43.97,)| Show InChI InChI=1S/C27H33FN8O/c1-27(2,3)19-9-12-21(13-10-19)36(26-29-22-15-20(28)11-14-23(22)35(26)4)16-17-5-7-18(8-6-17)24(37)30-25-31-33-34-32-25/h5-8,11,14-15,19,21H,9-10,12-13,16H2,1-4H3,(H2,30,31,32,33,34,37)/t19-,21- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244298
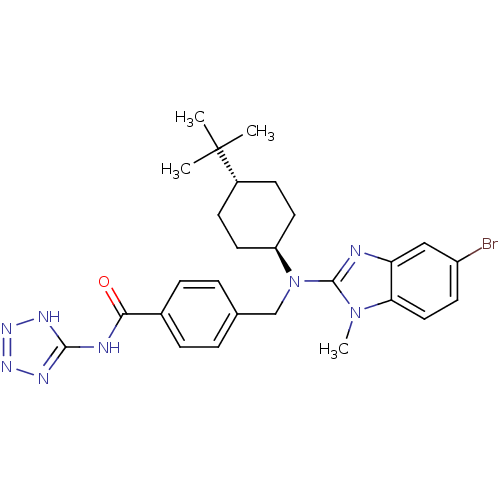
(CHEMBL472325 | trans-4-(((5-bromo-1-methyl-1H-benz...)Show SMILES Cn1c(nc2cc(Br)ccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:27.30,wD:30.37,(-7.9,-48.6,;-7.13,-47.27,;-5.7,-46.67,;-5.82,-45.14,;-7.32,-44.78,;-8.06,-43.44,;-9.59,-43.4,;-10.33,-42.04,;-10.4,-44.72,;-9.66,-46.06,;-8.13,-46.1,;-4.37,-47.45,;-4.38,-48.99,;-3.05,-49.77,;-1.72,-49,;-.39,-49.78,;-.4,-51.32,;-1.74,-52.08,;-3.07,-51.3,;.93,-52.1,;.92,-53.64,;2.27,-51.34,;3.6,-52.12,;4.12,-53.57,;5.66,-53.53,;6.1,-52.05,;4.83,-51.18,;-3.04,-46.69,;-3.04,-45.15,;-1.69,-44.39,;-.36,-45.18,;-.38,-46.72,;-1.71,-47.47,;.98,-44.42,;2.3,-43.64,;1.75,-45.75,;.21,-43.08,)| Show InChI InChI=1S/C27H33BrN8O/c1-27(2,3)19-9-12-21(13-10-19)36(26-29-22-15-20(28)11-14-23(22)35(26)4)16-17-5-7-18(8-6-17)24(37)30-25-31-33-34-32-25/h5-8,11,14-15,19,21H,9-10,12-13,16H2,1-4H3,(H2,30,31,32,33,34,37)/t19-,21- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244238

(CHEMBL499372 | trans-4-(((5-(benzyloxy)-1-methyl-1...)Show SMILES Cn1c(nc2cc(OCc3ccccc3)ccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:34.38,wD:37.45,(-7.9,-48.6,;-7.12,-47.26,;-5.7,-46.67,;-5.82,-45.14,;-7.32,-44.78,;-8.06,-43.43,;-9.59,-43.39,;-10.33,-42.04,;-9.53,-40.73,;-10.27,-39.37,;-9.47,-38.06,;-10.21,-36.71,;-11.75,-36.67,;-12.55,-37.99,;-11.81,-39.34,;-10.4,-44.71,;-9.66,-46.06,;-8.13,-46.09,;-4.37,-47.45,;-4.38,-48.99,;-3.05,-49.77,;-1.72,-49,;-.39,-49.78,;-.4,-51.32,;-1.74,-52.08,;-3.07,-51.3,;.93,-52.1,;.92,-53.64,;2.27,-51.33,;3.6,-52.11,;4.12,-53.56,;5.66,-53.52,;6.1,-52.05,;4.83,-51.17,;-3.04,-46.69,;-3.04,-45.15,;-1.69,-44.39,;-.36,-45.17,;-.38,-46.71,;-1.71,-47.47,;.98,-44.41,;2.3,-43.63,;1.75,-45.75,;.21,-43.08,)| Show InChI InChI=1S/C34H40N8O2/c1-34(2,3)26-14-16-27(17-15-26)42(21-23-10-12-25(13-11-23)31(43)36-32-37-39-40-38-32)33-35-29-20-28(18-19-30(29)41(33)4)44-22-24-8-6-5-7-9-24/h5-13,18-20,26-27H,14-17,21-22H2,1-4H3,(H2,36,37,38,39,40,43)/t26-,27- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulation |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244265

(CHEMBL480113 | trans-4-((4-tert-butylcyclohexyl)(1...)Show SMILES CCCOc1ccc2nc(N(Cc3ccc(cc3)C(=O)Nc3nnn[nH]3)[C@H]3CC[C@@H](CC3)C(C)(C)C)n(C)c2c1 |r,wU:26.27,wD:29.34,(9.13,-42.84,;9.87,-44.19,;11.41,-44.22,;12.15,-45.57,;13.69,-45.6,;14.49,-44.28,;16.03,-44.32,;16.76,-45.67,;18.26,-46.03,;18.38,-47.56,;19.71,-48.34,;19.71,-49.88,;21.04,-50.66,;22.37,-49.89,;23.7,-50.67,;23.69,-52.21,;22.34,-52.97,;21.02,-52.19,;25.02,-52.99,;25.01,-54.53,;26.36,-52.22,;27.68,-53,;28.2,-54.45,;29.74,-54.42,;30.18,-52.94,;28.91,-52.06,;21.04,-47.58,;21.05,-46.04,;22.39,-45.28,;23.72,-46.06,;23.71,-47.6,;22.37,-48.36,;25.06,-45.3,;26.39,-44.52,;25.83,-46.64,;24.3,-43.97,;16.96,-48.15,;16.19,-49.49,;15.96,-46.98,;14.43,-46.95,)| Show InChI InChI=1S/C30H40N8O2/c1-6-17-40-24-15-16-25-26(18-24)37(5)29(31-25)38(23-13-11-22(12-14-23)30(2,3)4)19-20-7-9-21(10-8-20)27(39)32-28-33-35-36-34-28/h7-10,15-16,18,22-23H,6,11-14,17,19H2,1-5H3,(H2,32,33,34,35,36,39)/t22-,23- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulation |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244198

(4-(((4-cyclohexylphenyl)(1-methyl-1H-benzo[d]imida...)Show SMILES Cn1c(nc2ccccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)c1ccc(cc1)C1CCCCC1 Show InChI InChI=1S/C29H30N8O/c1-36-26-10-6-5-9-25(26)30-29(36)37(24-17-15-22(16-18-24)21-7-3-2-4-8-21)19-20-11-13-23(14-12-20)27(38)31-28-32-34-35-33-28/h5-6,9-18,21H,2-4,7-8,19H2,1H3,(H2,31,32,33,34,35,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244236

(CHEMBL500315 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(OC3CCCC3)ccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:32.36,wD:35.43,(16.18,-49.49,;16.96,-48.15,;18.38,-47.56,;18.26,-46.02,;16.76,-45.67,;16.03,-44.32,;14.49,-44.28,;13.75,-42.93,;14.55,-41.62,;16.09,-41.49,;16.44,-39.99,;15.13,-39.19,;13.96,-40.19,;13.69,-45.6,;14.43,-46.95,;15.96,-46.98,;19.71,-48.34,;19.71,-49.88,;21.04,-50.66,;22.37,-49.89,;23.69,-50.66,;23.69,-52.21,;22.34,-52.97,;21.02,-52.19,;25.02,-52.98,;25.01,-54.52,;26.36,-52.22,;27.68,-53,;28.2,-54.45,;29.74,-54.41,;30.18,-52.94,;28.91,-52.06,;21.04,-47.58,;21.05,-46.04,;22.39,-45.28,;23.72,-46.06,;23.71,-47.6,;22.37,-48.36,;25.06,-45.3,;26.39,-44.52,;25.83,-46.64,;24.3,-43.97,)| Show InChI InChI=1S/C32H42N8O2/c1-32(2,3)23-13-15-24(16-14-23)40(20-21-9-11-22(12-10-21)29(41)34-30-35-37-38-36-30)31-33-27-19-26(17-18-28(27)39(31)4)42-25-7-5-6-8-25/h9-12,17-19,23-25H,5-8,13-16,20H2,1-4H3,(H2,34,35,36,37,38,41)/t23-,24- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulation |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244337

(CHEMBL511964 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(Cl)c(Cl)cc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:28.31,wD:31.38,(37.8,-49.39,;38.58,-48.06,;40,-47.47,;39.88,-45.93,;38.38,-45.58,;37.65,-44.23,;36.11,-44.19,;35.37,-42.84,;35.31,-45.51,;33.77,-45.47,;36.05,-46.85,;37.58,-46.89,;41.33,-48.24,;41.33,-49.78,;42.66,-50.56,;43.99,-49.79,;45.31,-50.57,;45.31,-52.11,;43.96,-52.87,;42.64,-52.09,;46.64,-52.89,;46.63,-54.43,;47.97,-52.13,;49.3,-52.91,;49.82,-54.36,;51.36,-54.32,;51.8,-52.84,;50.53,-51.97,;42.66,-47.48,;42.67,-45.94,;44.01,-45.19,;45.34,-45.97,;45.32,-47.51,;43.99,-48.26,;46.68,-45.21,;48,-44.43,;47.45,-46.54,;45.92,-43.87,)| Show InChI InChI=1S/C27H32Cl2N8O/c1-27(2,3)18-9-11-19(12-10-18)37(26-30-22-13-20(28)21(29)14-23(22)36(26)4)15-16-5-7-17(8-6-16)24(38)31-25-32-34-35-33-25/h5-8,13-14,18-19H,9-12,15H2,1-4H3,(H2,31,32,33,34,35,38)/t18-,19- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244080

(CHEMBL509900 | trans-4-((4-tert-butylcyclohexyl)(5...)Show SMILES Cc1ccc2nc([nH]c2c1)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:26.29,wD:29.36,(16.94,-17.66,;17.68,-19.01,;16.88,-20.33,;17.62,-21.67,;19.15,-21.71,;20.15,-22.88,;21.58,-22.29,;21.45,-20.75,;19.95,-20.4,;19.22,-19.05,;22.91,-23.07,;22.9,-24.61,;24.23,-25.38,;25.56,-24.62,;26.89,-25.39,;26.88,-26.93,;25.53,-27.7,;24.21,-26.92,;28.21,-27.71,;28.2,-29.25,;29.55,-26.95,;30.88,-27.73,;31.39,-29.18,;32.93,-29.14,;33.37,-27.67,;32.1,-26.79,;24.23,-22.3,;24.24,-20.77,;25.58,-20.01,;26.91,-20.79,;26.9,-22.33,;25.56,-23.09,;28.25,-20.03,;29.58,-19.25,;29.03,-21.36,;27.49,-18.7,)| Show InChI InChI=1S/C27H34N8O/c1-17-5-14-22-23(15-17)29-26(28-22)35(21-12-10-20(11-13-21)27(2,3)4)16-18-6-8-19(9-7-18)24(36)30-25-31-33-34-32-25/h5-9,14-15,20-21H,10-13,16H2,1-4H3,(H,28,29)(H2,30,31,32,33,34,36)/t20-,21- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244299
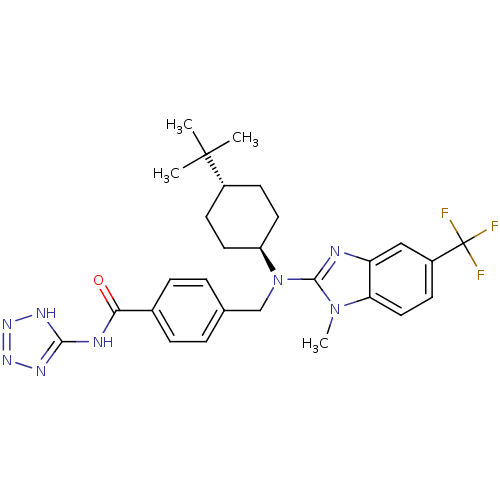
(CHEMBL480501 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(ccc12)C(F)(F)F)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:30.33,wD:33.40,(-5.67,-48.6,;-4.89,-47.26,;-3.47,-46.67,;-3.59,-45.14,;-5.09,-44.78,;-5.82,-43.43,;-7.36,-43.39,;-8.16,-44.71,;-7.42,-46.06,;-5.89,-46.09,;-8.1,-42.04,;-8.88,-40.7,;-6.76,-41.28,;-9.43,-42.81,;-2.14,-47.45,;-2.14,-48.99,;-.81,-49.77,;.52,-49,;1.84,-49.78,;1.84,-51.32,;.49,-52.08,;-.83,-51.3,;3.17,-52.1,;3.16,-53.64,;4.5,-51.33,;5.83,-52.11,;6.35,-53.56,;7.89,-53.52,;8.33,-52.05,;7.06,-51.17,;-.81,-46.69,;-.8,-45.15,;.54,-44.39,;1.87,-45.17,;1.85,-46.71,;.52,-47.47,;3.21,-44.41,;4.53,-43.63,;3.98,-45.75,;2.45,-43.08,)| Show InChI InChI=1S/C28H33F3N8O/c1-27(2,3)19-9-12-21(13-10-19)39(26-32-22-15-20(28(29,30)31)11-14-23(22)38(26)4)16-17-5-7-18(8-6-17)24(40)33-25-34-36-37-35-25/h5-8,11,14-15,19,21H,9-10,12-13,16H2,1-4H3,(H2,33,34,35,36,37,40)/t19-,21- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244079

(4-(((3-chloro-4-(4-chlorophenoxy)phenyl)(5-methyl-...)Show SMILES Cc1ccc2nc([nH]c2c1)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)c1ccc(Oc2ccc(Cl)cc2)c(Cl)c1 Show InChI InChI=1S/C29H22Cl2N8O2/c1-17-2-12-24-25(14-17)33-29(32-24)39(16-18-3-5-19(6-4-18)27(40)34-28-35-37-38-36-28)21-9-13-26(23(31)15-21)41-22-10-7-20(30)8-11-22/h2-15H,16H2,1H3,(H,32,33)(H2,34,35,36,37,38,40) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244376
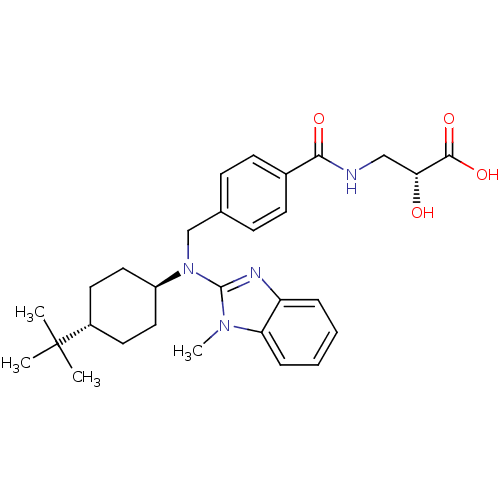
(CHEMBL488682 | trans-(R)-3-(4-(((4-tert-butylcyclo...)Show SMILES Cn1c(nc2ccccc12)N(Cc1ccc(cc1)C(=O)NC[C@@H](O)C(O)=O)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:27.29,22.25,wD:30.36,(-3.33,-14.81,;-2.56,-13.48,;-1.13,-12.88,;-1.26,-11.35,;-2.76,-10.99,;-3.49,-9.65,;-5.03,-9.61,;-5.83,-10.93,;-5.09,-12.27,;-3.56,-12.31,;.2,-13.66,;.19,-15.2,;1.52,-15.98,;2.85,-15.21,;4.18,-15.99,;4.17,-17.53,;2.82,-18.29,;1.5,-17.51,;5.5,-18.31,;5.49,-19.85,;6.84,-17.55,;8.17,-18.33,;9.51,-17.56,;9.52,-16.02,;10.83,-18.34,;12.17,-17.58,;10.82,-19.88,;1.53,-12.9,;1.53,-11.36,;2.88,-10.6,;4.2,-11.39,;4.19,-12.93,;2.85,-13.68,;5.55,-10.63,;6.87,-9.85,;6.32,-11.96,;4.78,-9.29,)| Show InChI InChI=1S/C29H38N4O4/c1-29(2,3)21-13-15-22(16-14-21)33(28-31-23-7-5-6-8-24(23)32(28)4)18-19-9-11-20(12-10-19)26(35)30-17-25(34)27(36)37/h5-12,21-22,25,34H,13-18H2,1-4H3,(H,30,35)(H,36,37)/t21-,22-,25-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244121

(CHEMBL517861 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cc1ccc2n(C)c(nc2c1)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:27.30,wD:30.37,(-7.79,-31.95,;-7.05,-33.31,;-7.85,-34.63,;-7.12,-35.97,;-5.59,-36.01,;-4.58,-37.18,;-4.94,-38.68,;-3.16,-36.58,;-3.28,-35.05,;-4.78,-34.69,;-5.51,-33.35,;-1.83,-37.36,;-1.84,-38.9,;-.51,-39.68,;.82,-38.91,;2.15,-39.69,;2.15,-41.23,;.8,-41.99,;-.53,-41.21,;3.47,-42.01,;3.46,-43.55,;4.81,-41.25,;6.14,-42.03,;6.66,-43.48,;8.2,-43.44,;8.64,-41.96,;7.37,-41.09,;-.5,-36.6,;-.5,-35.06,;.85,-34.3,;2.18,-35.09,;2.16,-36.63,;.83,-37.38,;3.52,-34.33,;4.84,-33.55,;4.29,-35.66,;2.75,-32.99,)| Show InChI InChI=1S/C28H36N8O/c1-18-6-15-24-23(16-18)29-27(35(24)5)36(22-13-11-21(12-14-22)28(2,3)4)17-19-7-9-20(10-8-19)25(37)30-26-31-33-34-32-26/h6-10,15-16,21-22H,11-14,17H2,1-5H3,(H2,30,31,32,33,34,37)/t21-,22- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244123

(CHEMBL459458 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES CCn1c(nc2ccccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:27.30,wD:30.37,(-7.03,-49.97,;-5.49,-49.92,;-4.76,-48.56,;-3.34,-47.97,;-3.46,-46.43,;-4.96,-46.08,;-5.69,-44.73,;-7.23,-44.69,;-8.03,-46.01,;-7.3,-47.35,;-5.77,-47.39,;-2.01,-48.75,;-2.02,-50.29,;-.69,-51.06,;.64,-50.3,;1.97,-51.07,;1.97,-52.61,;.62,-53.38,;-.71,-52.6,;3.29,-53.39,;3.28,-54.93,;4.63,-52.63,;5.96,-53.41,;6.48,-54.86,;8.02,-54.82,;8.46,-53.34,;7.19,-52.47,;-.68,-47.98,;-.67,-46.45,;.67,-45.69,;2,-46.47,;1.98,-48.01,;.65,-48.77,;3.34,-45.71,;4.66,-44.93,;4.11,-47.04,;2.57,-44.37,)| Show InChI InChI=1S/C28H36N8O/c1-5-35-24-9-7-6-8-23(24)29-27(35)36(22-16-14-21(15-17-22)28(2,3)4)18-19-10-12-20(13-11-19)25(37)30-26-31-33-34-32-26/h6-13,21-22H,5,14-18H2,1-4H3,(H2,30,31,32,33,34,37)/t21-,22- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244161

(CHEMBL506520 | trans-4-(((1-benzyl-1H-benzo[d]imid...)Show SMILES CC(C)(C)[C@H]1CC[C@@H](CC1)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)c1nc2ccccc2n1Cc1ccccc1 |r,wU:7.10,wD:4.3,(5.47,-12.81,;4.15,-13.59,;4.92,-14.93,;3.38,-12.26,;2.8,-14.35,;1.48,-13.57,;.13,-14.33,;.12,-15.87,;1.45,-16.65,;2.79,-15.89,;-1.2,-16.63,;-1.21,-18.17,;.12,-18.95,;1.45,-18.18,;2.78,-18.95,;2.77,-20.5,;1.42,-21.26,;.1,-20.48,;4.1,-21.28,;4.09,-22.82,;5.44,-20.51,;6.77,-21.29,;7.29,-22.74,;8.83,-22.71,;9.27,-21.23,;8,-20.35,;-2.54,-15.85,;-2.66,-14.31,;-4.16,-13.96,;-4.89,-12.61,;-6.43,-12.57,;-7.23,-13.89,;-6.49,-15.24,;-4.96,-15.27,;-3.96,-16.44,;-4.73,-17.78,;-6.27,-17.76,;-7.03,-16.43,;-8.57,-16.41,;-9.35,-17.74,;-8.58,-19.09,;-7.04,-19.09,)| Show InChI InChI=1S/C33H38N8O/c1-33(2,3)26-17-19-27(20-18-26)40(21-24-13-15-25(16-14-24)30(42)35-31-36-38-39-37-31)32-34-28-11-7-8-12-29(28)41(32)22-23-9-5-4-6-10-23/h4-16,26-27H,17-22H2,1-3H3,(H2,35,36,37,38,39,42)/t26-,27- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244300

(CHEMBL470955 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cc1cc2nc(N(Cc3ccc(cc3)C(=O)Nc3nnn[nH]3)[C@H]3CC[C@@H](CC3)C(C)(C)C)n(C)c2cc1C |r,wU:22.23,wD:25.30,(13.75,-42.93,;14.49,-44.28,;16.03,-44.32,;16.76,-45.67,;18.26,-46.03,;18.38,-47.56,;19.71,-48.34,;19.71,-49.88,;21.04,-50.66,;22.37,-49.89,;23.7,-50.67,;23.69,-52.21,;22.34,-52.97,;21.02,-52.19,;25.02,-52.99,;25.01,-54.53,;26.36,-52.23,;27.68,-53,;28.2,-54.45,;29.74,-54.42,;30.18,-52.94,;28.91,-52.07,;21.04,-47.58,;21.05,-46.04,;22.39,-45.28,;23.72,-46.06,;23.71,-47.6,;22.37,-48.36,;25.06,-45.31,;26.39,-44.52,;25.83,-46.64,;24.3,-43.97,;16.96,-48.16,;16.19,-49.49,;15.96,-46.98,;14.43,-46.95,;13.69,-45.6,;12.15,-45.57,)| Show InChI InChI=1S/C29H38N8O/c1-18-15-24-25(16-19(18)2)36(6)28(30-24)37(23-13-11-22(12-14-23)29(3,4)5)17-20-7-9-21(10-8-20)26(38)31-27-32-34-35-33-27/h7-10,15-16,22-23H,11-14,17H2,1-6H3,(H2,31,32,33,34,35,38)/t22-,23- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulation |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244237

(CHEMBL448921 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(OCC3CCCC3)ccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:33.37,wD:36.44,(-7.9,-48.6,;-7.12,-47.26,;-5.7,-46.67,;-5.82,-45.13,;-7.32,-44.78,;-8.06,-43.43,;-9.59,-43.39,;-10.33,-42.04,;-9.53,-40.73,;-10.27,-39.37,;-9.61,-37.98,;-10.73,-36.92,;-12.08,-37.66,;-11.8,-39.17,;-10.4,-44.71,;-9.66,-46.06,;-8.13,-46.09,;-4.37,-47.45,;-4.38,-48.99,;-3.05,-49.77,;-1.72,-49,;-.39,-49.77,;-.4,-51.32,;-1.74,-52.08,;-3.07,-51.3,;.93,-52.09,;.92,-53.63,;2.27,-51.33,;3.6,-52.11,;4.12,-53.56,;5.66,-53.52,;6.1,-52.05,;4.83,-51.17,;-3.04,-46.69,;-3.04,-45.15,;-1.69,-44.39,;-.36,-45.17,;-.38,-46.71,;-1.71,-47.47,;.98,-44.41,;2.3,-43.63,;1.75,-45.75,;.21,-43.08,)| Show InChI InChI=1S/C33H44N8O2/c1-33(2,3)25-13-15-26(16-14-25)41(20-22-9-11-24(12-10-22)30(42)35-31-36-38-39-37-31)32-34-28-19-27(17-18-29(28)40(32)4)43-21-23-7-5-6-8-23/h9-12,17-19,23,25-26H,5-8,13-16,20-21H2,1-4H3,(H2,35,36,37,38,39,42)/t25-,26- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulation |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244234

(CHEMBL518939 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES CCOc1ccc2n(C)c(nc2c1)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:29.32,wD:32.39,(-10.27,-39.37,;-9.53,-40.73,;-10.33,-42.04,;-9.59,-43.39,;-10.4,-44.71,;-9.66,-46.06,;-8.13,-46.09,;-7.12,-47.26,;-7.9,-48.6,;-5.7,-46.67,;-5.82,-45.14,;-7.32,-44.78,;-8.06,-43.43,;-4.37,-47.45,;-4.38,-48.99,;-3.05,-49.77,;-1.72,-49,;-.39,-49.78,;-.4,-51.32,;-1.74,-52.08,;-3.07,-51.3,;.93,-52.1,;.92,-53.64,;2.27,-51.33,;3.6,-52.11,;4.12,-53.56,;5.66,-53.52,;6.1,-52.05,;4.83,-51.17,;-3.04,-46.69,;-3.04,-45.15,;-1.69,-44.39,;-.36,-45.17,;-.38,-46.71,;-1.71,-47.47,;.98,-44.41,;2.3,-43.63,;1.75,-45.75,;.21,-43.08,)| Show InChI InChI=1S/C29H38N8O2/c1-6-39-23-15-16-25-24(17-23)30-28(36(25)5)37(22-13-11-21(12-14-22)29(2,3)4)18-19-7-9-20(10-8-19)26(38)31-27-32-34-35-33-27/h7-10,15-17,21-22H,6,11-14,18H2,1-5H3,(H2,31,32,33,34,35,38)/t21-,22- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulation |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244337

(CHEMBL511964 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(Cl)c(Cl)cc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:28.31,wD:31.38,(37.8,-49.39,;38.58,-48.06,;40,-47.47,;39.88,-45.93,;38.38,-45.58,;37.65,-44.23,;36.11,-44.19,;35.37,-42.84,;35.31,-45.51,;33.77,-45.47,;36.05,-46.85,;37.58,-46.89,;41.33,-48.24,;41.33,-49.78,;42.66,-50.56,;43.99,-49.79,;45.31,-50.57,;45.31,-52.11,;43.96,-52.87,;42.64,-52.09,;46.64,-52.89,;46.63,-54.43,;47.97,-52.13,;49.3,-52.91,;49.82,-54.36,;51.36,-54.32,;51.8,-52.84,;50.53,-51.97,;42.66,-47.48,;42.67,-45.94,;44.01,-45.19,;45.34,-45.97,;45.32,-47.51,;43.99,-48.26,;46.68,-45.21,;48,-44.43,;47.45,-46.54,;45.92,-43.87,)| Show InChI InChI=1S/C27H32Cl2N8O/c1-27(2,3)18-9-11-19(12-10-18)37(26-30-22-13-20(28)21(29)14-23(22)36(26)4)15-16-5-7-17(8-6-16)24(38)31-25-32-34-35-33-25/h5-8,13-14,18-19H,9-12,15H2,1-4H3,(H2,31,32,33,34,35,38)/t18-,19- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulation |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244158
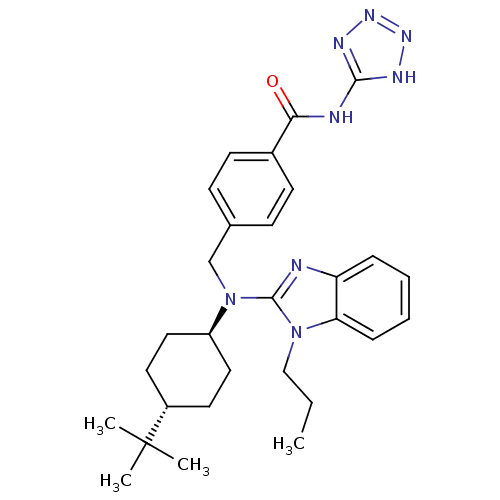
(CHEMBL456877 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES CCCn1c(nc2ccccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:28.31,wD:31.38,(19.12,-52.1,;19.9,-50.77,;21.44,-50.79,;22.22,-49.46,;23.65,-48.86,;23.52,-47.33,;22.02,-46.97,;21.29,-45.62,;19.75,-45.58,;18.95,-46.91,;19.69,-48.25,;21.22,-48.29,;24.98,-49.64,;24.97,-51.18,;26.3,-51.96,;27.63,-51.19,;28.96,-51.97,;28.95,-53.51,;27.6,-54.27,;26.28,-53.49,;30.28,-54.29,;30.27,-55.83,;31.62,-53.53,;32.95,-54.31,;33.46,-55.75,;35,-55.72,;35.44,-54.24,;34.18,-53.37,;26.31,-48.88,;26.31,-47.34,;27.66,-46.58,;28.99,-47.36,;28.97,-48.91,;27.64,-49.66,;30.33,-46.61,;31.65,-45.83,;31.1,-47.94,;29.56,-45.27,)| Show InChI InChI=1S/C29H38N8O/c1-5-18-36-25-9-7-6-8-24(25)30-28(36)37(23-16-14-22(15-17-23)29(2,3)4)19-20-10-12-21(13-11-20)26(38)31-27-32-34-35-33-27/h6-13,22-23H,5,14-19H2,1-4H3,(H2,31,32,33,34,35,38)/t22-,23- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244198

(4-(((4-cyclohexylphenyl)(1-methyl-1H-benzo[d]imida...)Show SMILES Cn1c(nc2ccccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)c1ccc(cc1)C1CCCCC1 Show InChI InChI=1S/C29H30N8O/c1-36-26-10-6-5-9-25(26)30-29(36)37(24-17-15-22(16-18-24)21-7-3-2-4-8-21)19-20-11-13-23(14-12-20)27(38)31-28-32-34-35-33-28/h5-6,9-18,21H,2-4,7-8,19H2,1H3,(H2,31,32,33,34,35,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulation |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244197
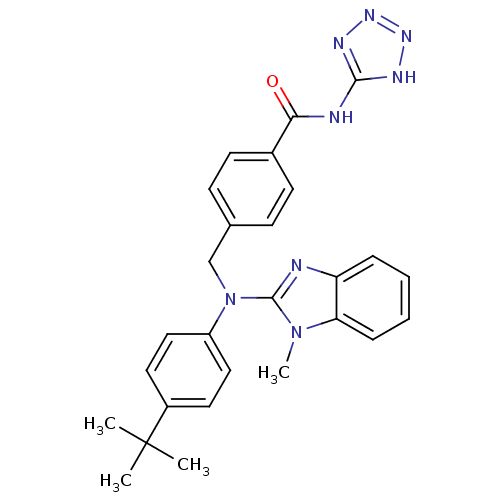
(4-(((4-tert-butylphenyl)(1-methyl-1H-benzo[d]imida...)Show SMILES Cn1c(nc2ccccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)c1ccc(cc1)C(C)(C)C Show InChI InChI=1S/C27H28N8O/c1-27(2,3)20-13-15-21(16-14-20)35(26-28-22-7-5-6-8-23(22)34(26)4)17-18-9-11-19(12-10-18)24(36)29-25-30-32-33-31-25/h5-16H,17H2,1-4H3,(H2,29,30,31,32,33,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244078

(CHEMBL502203 | trans-4-[9-tert-Butyl-2-oxo-3-(4-tr...)Show SMILES CC(C)(C)[C@H]1CC[C@]2(CC1)CCN(C(=O)N2Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)c1ccc(OC(F)(F)F)cc1 |r,wU:4.3,wD:7.16,(15.27,-10.56,;16.61,-9.8,;17.38,-11.14,;15.85,-8.46,;17.95,-9.03,;17.95,-7.49,;19.28,-6.73,;20.61,-7.5,;20.62,-9.03,;19.28,-9.8,;20.64,-5.96,;21.98,-5.2,;23.3,-5.98,;23.29,-7.52,;24.62,-8.3,;21.95,-8.27,;21.94,-9.81,;23.27,-10.59,;24.6,-9.82,;25.93,-10.6,;25.92,-12.14,;24.58,-12.91,;23.25,-12.12,;27.25,-12.92,;27.24,-14.46,;28.59,-12.16,;29.92,-12.94,;30.44,-14.39,;31.98,-14.35,;32.42,-12.87,;31.15,-12,;24.65,-5.22,;25.97,-6.01,;27.31,-5.25,;27.32,-3.71,;28.66,-2.95,;29.99,-3.73,;31.31,-4.5,;29.21,-5.06,;30.78,-2.41,;25.98,-2.93,;24.65,-3.69,)| Show InChI InChI=1S/C29H34F3N7O3/c1-27(2,3)21-12-14-28(15-13-21)16-17-38(22-8-10-23(11-9-22)42-29(30,31)32)26(41)39(28)18-19-4-6-20(7-5-19)24(40)33-25-34-36-37-35-25/h4-11,21H,12-18H2,1-3H3,(H2,33,34,35,36,37,40)/t21-,28- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244233

(CHEMBL513067 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES COc1ccc2n(C)c(nc2c1)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:28.31,wD:31.38,(13.19,-24.98,;12.38,-26.29,;13.12,-27.64,;12.32,-28.96,;13.06,-30.31,;14.59,-30.34,;15.59,-31.52,;14.82,-32.85,;17.02,-30.92,;16.89,-29.39,;15.39,-29.03,;14.66,-27.68,;18.35,-31.7,;18.34,-33.24,;19.67,-34.02,;21,-33.25,;22.33,-34.03,;22.32,-35.57,;20.97,-36.33,;19.65,-35.55,;23.65,-36.35,;23.64,-37.89,;24.99,-35.59,;26.32,-36.37,;26.83,-37.81,;28.37,-37.78,;28.81,-36.3,;27.55,-35.43,;19.68,-30.94,;19.68,-29.4,;21.03,-28.64,;22.36,-29.42,;22.34,-30.96,;21.01,-31.72,;23.7,-28.67,;25.02,-27.89,;24.47,-30,;22.93,-27.33,)| Show InChI InChI=1S/C28H36N8O2/c1-28(2,3)20-10-12-21(13-11-20)36(27-29-23-16-22(38-5)14-15-24(23)35(27)4)17-18-6-8-19(9-7-18)25(37)30-26-31-33-34-32-26/h6-9,14-16,20-21H,10-13,17H2,1-5H3,(H2,30,31,32,33,34,37)/t20-,21- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulation |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244122

(CHEMBL459457 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2ccccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:26.29,wD:29.36,(21.92,-38.87,;22.27,-37.38,;23.7,-36.78,;23.58,-35.25,;22.08,-34.89,;21.34,-33.54,;19.8,-33.5,;19,-34.82,;19.74,-36.17,;21.27,-36.2,;25.03,-37.56,;25.02,-39.1,;26.35,-39.88,;27.68,-39.11,;29.01,-39.89,;29,-41.43,;27.66,-42.19,;26.33,-41.41,;30.33,-42.21,;30.32,-43.75,;31.67,-41.45,;33,-42.22,;33.52,-43.67,;35.06,-43.64,;35.5,-42.16,;34.23,-41.29,;26.36,-36.8,;26.36,-35.26,;27.71,-34.5,;29.04,-35.28,;29.02,-36.82,;27.69,-37.58,;30.38,-34.53,;31.7,-33.74,;31.15,-35.86,;29.61,-33.19,)| Show InChI InChI=1S/C27H34N8O/c1-27(2,3)20-13-15-21(16-14-20)35(26-28-22-7-5-6-8-23(22)34(26)4)17-18-9-11-19(12-10-18)24(36)29-25-30-32-33-31-25/h5-12,20-21H,13-17H2,1-4H3,(H2,29,30,31,32,33,36)/t20-,21- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulation |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244298

(CHEMBL472325 | trans-4-(((5-bromo-1-methyl-1H-benz...)Show SMILES Cn1c(nc2cc(Br)ccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:27.30,wD:30.37,(-7.9,-48.6,;-7.13,-47.27,;-5.7,-46.67,;-5.82,-45.14,;-7.32,-44.78,;-8.06,-43.44,;-9.59,-43.4,;-10.33,-42.04,;-10.4,-44.72,;-9.66,-46.06,;-8.13,-46.1,;-4.37,-47.45,;-4.38,-48.99,;-3.05,-49.77,;-1.72,-49,;-.39,-49.78,;-.4,-51.32,;-1.74,-52.08,;-3.07,-51.3,;.93,-52.1,;.92,-53.64,;2.27,-51.34,;3.6,-52.12,;4.12,-53.57,;5.66,-53.53,;6.1,-52.05,;4.83,-51.18,;-3.04,-46.69,;-3.04,-45.15,;-1.69,-44.39,;-.36,-45.18,;-.38,-46.72,;-1.71,-47.47,;.98,-44.42,;2.3,-43.64,;1.75,-45.75,;.21,-43.08,)| Show InChI InChI=1S/C27H33BrN8O/c1-27(2,3)19-9-12-21(13-10-19)36(26-29-22-15-20(28)11-14-23(22)35(26)4)16-17-5-7-18(8-6-17)24(37)30-25-31-33-34-32-25/h5-8,11,14-15,19,21H,9-10,12-13,16H2,1-4H3,(H2,30,31,32,33,34,37)/t19-,21- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulation |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244299

(CHEMBL480501 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(ccc12)C(F)(F)F)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:30.33,wD:33.40,(-5.67,-48.6,;-4.89,-47.26,;-3.47,-46.67,;-3.59,-45.14,;-5.09,-44.78,;-5.82,-43.43,;-7.36,-43.39,;-8.16,-44.71,;-7.42,-46.06,;-5.89,-46.09,;-8.1,-42.04,;-8.88,-40.7,;-6.76,-41.28,;-9.43,-42.81,;-2.14,-47.45,;-2.14,-48.99,;-.81,-49.77,;.52,-49,;1.84,-49.78,;1.84,-51.32,;.49,-52.08,;-.83,-51.3,;3.17,-52.1,;3.16,-53.64,;4.5,-51.33,;5.83,-52.11,;6.35,-53.56,;7.89,-53.52,;8.33,-52.05,;7.06,-51.17,;-.81,-46.69,;-.8,-45.15,;.54,-44.39,;1.87,-45.17,;1.85,-46.71,;.52,-47.47,;3.21,-44.41,;4.53,-43.63,;3.98,-45.75,;2.45,-43.08,)| Show InChI InChI=1S/C28H33F3N8O/c1-27(2,3)19-9-12-21(13-10-19)39(26-32-22-15-20(28(29,30)31)11-14-23(22)38(26)4)16-17-5-7-18(8-6-17)24(40)33-25-34-36-37-35-25/h5-8,11,14-15,19,21H,9-10,12-13,16H2,1-4H3,(H2,33,34,35,36,37,40)/t19-,21- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulation |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244121

(CHEMBL517861 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cc1ccc2n(C)c(nc2c1)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:27.30,wD:30.37,(-7.79,-31.95,;-7.05,-33.31,;-7.85,-34.63,;-7.12,-35.97,;-5.59,-36.01,;-4.58,-37.18,;-4.94,-38.68,;-3.16,-36.58,;-3.28,-35.05,;-4.78,-34.69,;-5.51,-33.35,;-1.83,-37.36,;-1.84,-38.9,;-.51,-39.68,;.82,-38.91,;2.15,-39.69,;2.15,-41.23,;.8,-41.99,;-.53,-41.21,;3.47,-42.01,;3.46,-43.55,;4.81,-41.25,;6.14,-42.03,;6.66,-43.48,;8.2,-43.44,;8.64,-41.96,;7.37,-41.09,;-.5,-36.6,;-.5,-35.06,;.85,-34.3,;2.18,-35.09,;2.16,-36.63,;.83,-37.38,;3.52,-34.33,;4.84,-33.55,;4.29,-35.66,;2.75,-32.99,)| Show InChI InChI=1S/C28H36N8O/c1-18-6-15-24-23(16-18)29-27(35(24)5)36(22-13-11-21(12-14-22)28(2,3)4)17-19-7-9-20(10-8-19)25(37)30-26-31-33-34-32-26/h6-10,15-16,21-22H,11-14,17H2,1-5H3,(H2,30,31,32,33,34,37)/t21-,22- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulation |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244077
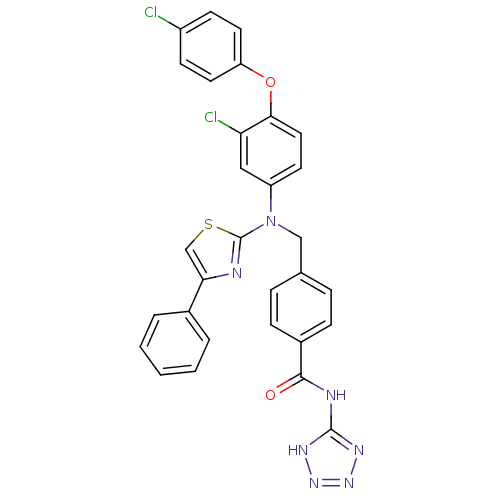
(4-(((3-chloro-4-(4-chlorophenoxy)phenyl)(4-phenylt...)Show SMILES Clc1ccc(Oc2ccc(cc2Cl)N(Cc2ccc(cc2)C(=O)Nc2nnn[nH]2)c2nc(cs2)-c2ccccc2)cc1 Show InChI InChI=1S/C30H21Cl2N7O2S/c31-22-10-13-24(14-11-22)41-27-15-12-23(16-25(27)32)39(30-33-26(18-42-30)20-4-2-1-3-5-20)17-19-6-8-21(9-7-19)28(40)34-29-35-37-38-36-29/h1-16,18H,17H2,(H2,34,35,36,37,38,40) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244338

(4-(((1-methyl-1H-benzo[d]imidazol-2-yl)(4-(trifluo...)Show SMILES Cn1c(nc2ccccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C24H19F3N8O2/c1-34-20-5-3-2-4-19(20)28-23(34)35(17-10-12-18(13-11-17)37-24(25,26)27)14-15-6-8-16(9-7-15)21(36)29-22-30-32-33-31-22/h2-13H,14H2,1H3,(H2,29,30,31,32,33,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244159

(4-(((4-tert-butylcyclohexyl)(1-cyclopentyl-1H-benz...)Show SMILES CC(C)(C)[C@H]1CC[C@@H](CC1)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)c1nc2ccccc2n1C1CCCC1 |r,wU:7.10,wD:4.3,(3.04,3.53,;1.71,2.75,;2.49,1.42,;.95,4.09,;.37,1.99,;-.95,2.78,;-2.3,2.02,;-2.31,.48,;-.98,-.3,;.36,.45,;-3.63,-.28,;-3.64,-1.82,;-2.31,-2.6,;-.98,-1.83,;.35,-2.61,;.34,-4.15,;-1.01,-4.91,;-2.33,-4.13,;1.67,-4.93,;1.66,-6.47,;3.01,-4.17,;4.34,-4.95,;4.85,-6.4,;6.39,-6.36,;6.83,-4.88,;5.56,-4.01,;-4.96,.5,;-5.09,2.03,;-6.59,2.39,;-7.32,3.74,;-8.86,3.77,;-9.66,2.45,;-8.92,1.11,;-7.39,1.07,;-6.39,-.1,;-6.87,-1.56,;-8.34,-2.03,;-8.34,-3.57,;-6.88,-4.05,;-5.97,-2.81,)| Show InChI InChI=1S/C31H40N8O/c1-31(2,3)23-16-18-24(19-17-23)38(20-21-12-14-22(15-13-21)28(40)33-29-34-36-37-35-29)30-32-26-10-6-7-11-27(26)39(30)25-8-4-5-9-25/h6-7,10-15,23-25H,4-5,8-9,16-20H2,1-3H3,(H2,33,34,35,36,37,40)/t23-,24- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244339
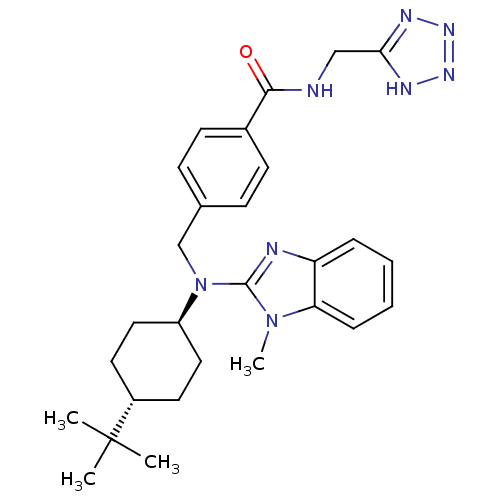
(CHEMBL469904 | trans-N-((1H-tetrazol-5-yl)methyl)-...)Show SMILES Cn1c(nc2ccccc12)N(Cc1ccc(cc1)C(=O)NCc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:27.30,wD:30.37,(-6.06,-.47,;-5.29,.86,;-3.86,1.45,;-3.99,2.99,;-5.49,3.34,;-6.22,4.69,;-7.76,4.73,;-8.56,3.41,;-7.82,2.06,;-6.29,2.03,;-2.53,.67,;-2.54,-.87,;-1.21,-1.65,;.12,-.88,;1.45,-1.65,;1.44,-3.2,;.09,-3.96,;-1.23,-3.18,;2.77,-3.97,;2.76,-5.51,;4.11,-3.21,;5.44,-3.99,;6.78,-3.23,;8.24,-3.69,;9.14,-2.44,;8.23,-1.2,;6.77,-1.68,;-1.2,1.43,;-1.2,2.97,;.15,3.73,;1.47,2.95,;1.46,1.41,;.12,.65,;2.82,3.71,;4.14,4.49,;3.59,2.37,;2.05,5.04,)| Show InChI InChI=1S/C28H36N8O/c1-28(2,3)21-13-15-22(16-14-21)36(27-30-23-7-5-6-8-24(23)35(27)4)18-19-9-11-20(12-10-19)26(37)29-17-25-31-33-34-32-25/h5-12,21-22H,13-18H2,1-4H3,(H,29,37)(H,31,32,33,34)/t21-,22- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244162
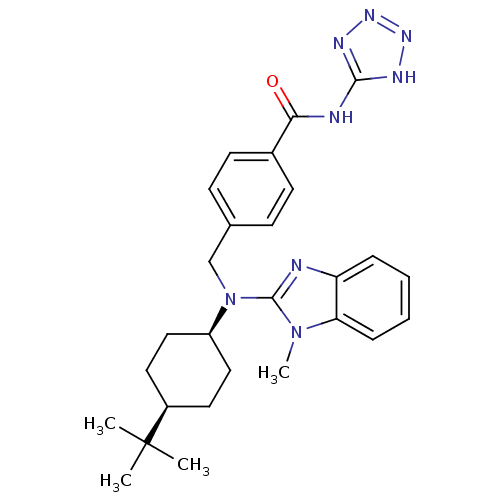
(CHEMBL515202 | cis-4-(((4-tert-butylcyclohexyl)(1-...)Show SMILES Cn1c(nc2ccccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@H](CC1)C(C)(C)C |r,wU:26.29,29.36,(-7.22,-33.1,;-6.45,-31.77,;-5.02,-31.18,;-5.15,-29.64,;-6.65,-29.29,;-7.38,-27.94,;-8.92,-27.9,;-9.72,-29.22,;-8.98,-30.56,;-7.45,-30.6,;-3.69,-31.96,;-3.7,-33.5,;-2.37,-34.27,;-1.04,-33.51,;.29,-34.28,;.28,-35.82,;-1.07,-36.59,;-2.39,-35.81,;1.61,-36.6,;1.6,-38.14,;2.95,-35.84,;4.28,-36.62,;4.79,-38.07,;6.33,-38.03,;6.77,-36.56,;5.51,-35.68,;-2.37,-31.19,;-2.36,-29.66,;-1.01,-28.9,;.31,-29.68,;.3,-31.22,;-1.04,-31.98,;1.65,-28.92,;2.98,-28.14,;2.43,-30.26,;.89,-27.59,)| Show InChI InChI=1S/C27H34N8O/c1-27(2,3)20-13-15-21(16-14-20)35(26-28-22-7-5-6-8-23(22)34(26)4)17-18-9-11-19(12-10-18)24(36)29-25-30-32-33-31-25/h5-12,20-21H,13-17H2,1-4H3,(H2,29,30,31,32,33,36)/t20-,21+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244264

(CHEMBL471979 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES COc1ccc2nc(N(Cc3ccc(cc3)C(=O)Nc3nnn[nH]3)[C@H]3CC[C@@H](CC3)C(C)(C)C)n(C)c2c1 |r,wU:24.25,wD:27.32,(11.41,-44.22,;12.15,-45.57,;13.69,-45.6,;14.49,-44.28,;16.03,-44.32,;16.76,-45.67,;18.26,-46.03,;18.38,-47.56,;19.72,-48.34,;19.71,-49.88,;21.04,-50.66,;22.37,-49.89,;23.7,-50.67,;23.69,-52.21,;22.34,-52.97,;21.02,-52.19,;25.02,-52.99,;25.01,-54.53,;26.36,-52.23,;27.69,-53.01,;28.2,-54.45,;29.74,-54.42,;30.18,-52.94,;28.91,-52.07,;21.04,-47.58,;21.05,-46.04,;22.39,-45.28,;23.72,-46.06,;23.71,-47.61,;22.37,-48.36,;25.06,-45.31,;26.39,-44.53,;25.84,-46.64,;24.3,-43.97,;16.96,-48.16,;16.19,-49.49,;15.96,-46.98,;14.43,-46.95,)| Show InChI InChI=1S/C28H36N8O2/c1-28(2,3)20-10-12-21(13-11-20)36(27-29-23-15-14-22(38-5)16-24(23)35(27)4)17-18-6-8-19(9-7-18)25(37)30-26-31-33-34-32-26/h6-9,14-16,20-21H,10-13,17H2,1-5H3,(H2,30,31,32,33,34,37)/t20-,21- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 213 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulation |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244266

(CHEMBL472154 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(F)ccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:27.30,wD:30.37,(16.19,-49.49,;16.96,-48.16,;18.38,-47.56,;18.26,-46.03,;16.76,-45.67,;16.03,-44.32,;14.49,-44.28,;13.75,-42.93,;13.69,-45.6,;14.43,-46.95,;15.96,-46.98,;19.72,-48.34,;19.71,-49.88,;21.04,-50.66,;22.37,-49.89,;23.7,-50.67,;23.69,-52.21,;22.34,-52.97,;21.02,-52.19,;25.02,-52.99,;25.01,-54.53,;26.36,-52.23,;27.69,-53.01,;28.2,-54.45,;29.74,-54.42,;30.18,-52.94,;28.91,-52.07,;21.04,-47.58,;21.05,-46.04,;22.39,-45.28,;23.72,-46.06,;23.71,-47.61,;22.37,-48.36,;25.06,-45.31,;26.39,-44.53,;25.84,-46.64,;24.3,-43.97,)| Show InChI InChI=1S/C27H33FN8O/c1-27(2,3)19-9-12-21(13-10-19)36(26-29-22-15-20(28)11-14-23(22)35(26)4)16-17-5-7-18(8-6-17)24(37)30-25-31-33-34-32-25/h5-8,11,14-15,19,21H,9-10,12-13,16H2,1-4H3,(H2,30,31,32,33,34,37)/t19-,21- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulation |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244375

(CHEMBL487828 | trans-3-(4-(((4-tert-butylcyclohexy...)Show SMILES Cn1c(nc2ccccc12)N(Cc1ccc(cc1)C(=O)NCCC(O)=O)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:26.28,wD:29.35,(17,-1.17,;17.77,.16,;19.2,.75,;19.07,2.29,;17.58,2.64,;16.84,3.99,;15.3,4.03,;14.5,2.71,;15.24,1.36,;16.77,1.33,;20.53,-.03,;20.52,-1.57,;21.85,-2.35,;23.18,-1.58,;24.51,-2.35,;24.5,-3.9,;23.15,-4.66,;21.83,-3.88,;25.83,-4.67,;25.82,-6.21,;27.17,-3.91,;28.5,-4.69,;29.84,-3.93,;31.17,-4.71,;32.5,-3.95,;31.16,-6.25,;21.86,.73,;21.86,2.27,;23.21,3.03,;24.54,2.25,;24.52,.71,;23.19,-.05,;25.88,3.01,;27.2,3.79,;26.65,1.67,;25.11,4.34,)| Show InChI InChI=1S/C29H38N4O3/c1-29(2,3)22-13-15-23(16-14-22)33(28-31-24-7-5-6-8-25(24)32(28)4)19-20-9-11-21(12-10-20)27(36)30-18-17-26(34)35/h5-12,22-23H,13-19H2,1-4H3,(H,30,36)(H,34,35)/t22-,23- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244162

(CHEMBL515202 | cis-4-(((4-tert-butylcyclohexyl)(1-...)Show SMILES Cn1c(nc2ccccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@H](CC1)C(C)(C)C |r,wU:26.29,29.36,(-7.22,-33.1,;-6.45,-31.77,;-5.02,-31.18,;-5.15,-29.64,;-6.65,-29.29,;-7.38,-27.94,;-8.92,-27.9,;-9.72,-29.22,;-8.98,-30.56,;-7.45,-30.6,;-3.69,-31.96,;-3.7,-33.5,;-2.37,-34.27,;-1.04,-33.51,;.29,-34.28,;.28,-35.82,;-1.07,-36.59,;-2.39,-35.81,;1.61,-36.6,;1.6,-38.14,;2.95,-35.84,;4.28,-36.62,;4.79,-38.07,;6.33,-38.03,;6.77,-36.56,;5.51,-35.68,;-2.37,-31.19,;-2.36,-29.66,;-1.01,-28.9,;.31,-29.68,;.3,-31.22,;-1.04,-31.98,;1.65,-28.92,;2.98,-28.14,;2.43,-30.26,;.89,-27.59,)| Show InChI InChI=1S/C27H34N8O/c1-27(2,3)20-13-15-21(16-14-20)35(26-28-22-7-5-6-8-23(22)34(26)4)17-18-9-11-19(12-10-18)24(36)29-25-30-32-33-31-25/h5-12,20-21H,13-17H2,1-4H3,(H2,29,30,31,32,33,36)/t20-,21+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulation |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244075

(1-(3-chloro-4-(4-chlorophenoxy)phenyl)-3-(2-chloro...)Show SMILES Clc1ccc(Oc2ccc(NC(=O)NC(=O)c3ccccc3Cl)cc2Cl)cc1 Show InChI InChI=1S/C20H13Cl3N2O3/c21-12-5-8-14(9-6-12)28-18-10-7-13(11-17(18)23)24-20(27)25-19(26)15-3-1-2-4-16(15)22/h1-11H,(H2,24,25,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 482 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244197

(4-(((4-tert-butylphenyl)(1-methyl-1H-benzo[d]imida...)Show SMILES Cn1c(nc2ccccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)c1ccc(cc1)C(C)(C)C Show InChI InChI=1S/C27H28N8O/c1-27(2,3)20-13-15-21(16-14-20)35(26-28-22-7-5-6-8-23(22)34(26)4)17-18-9-11-19(12-10-18)24(36)29-25-30-32-33-31-25/h5-16H,17H2,1-4H3,(H2,29,30,31,32,33,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulation |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244160

(CHEMBL507829 | trans-4-((4-tert-butylcyclohexyl)(1...)Show SMILES CC(C)(C)[C@H]1CC[C@@H](CC1)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)c1nc2ccccc2n1-c1ccccc1 |r,wU:7.10,wD:4.3,(31.81,3.41,;30.49,2.62,;31.26,1.29,;29.72,3.96,;29.15,1.87,;27.82,2.65,;26.47,1.89,;26.47,.35,;27.8,-.43,;29.13,.33,;25.14,-.41,;25.13,-1.95,;26.46,-2.73,;27.79,-1.96,;29.12,-2.74,;29.11,-4.28,;27.77,-5.04,;26.44,-4.26,;30.44,-5.06,;30.43,-6.6,;31.78,-4.3,;33.11,-5.07,;33.63,-6.52,;35.17,-6.49,;35.61,-5.01,;34.34,-4.14,;23.81,.37,;23.69,1.9,;22.19,2.26,;21.45,3.61,;19.92,3.65,;19.11,2.33,;19.85,.98,;21.38,.95,;22.38,-.23,;21.61,-1.56,;20.07,-1.55,;19.3,-2.88,;20.06,-4.21,;21.61,-4.21,;22.38,-2.88,)| Show InChI InChI=1S/C32H36N8O/c1-32(2,3)24-17-19-25(20-18-24)39(21-22-13-15-23(16-14-22)29(41)34-30-35-37-38-36-30)31-33-27-11-7-8-12-28(27)40(31)26-9-5-4-6-10-26/h4-16,24-25H,17-21H2,1-3H3,(H2,34,35,36,37,38,41)/t24-,25- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244079

(4-(((3-chloro-4-(4-chlorophenoxy)phenyl)(5-methyl-...)Show SMILES Cc1ccc2nc([nH]c2c1)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)c1ccc(Oc2ccc(Cl)cc2)c(Cl)c1 Show InChI InChI=1S/C29H22Cl2N8O2/c1-17-2-12-24-25(14-17)33-29(32-24)39(16-18-3-5-19(6-4-18)27(40)34-28-35-37-38-36-28)21-9-13-26(23(31)15-21)41-22-10-7-20(30)8-11-22/h2-15H,16H2,1H3,(H,32,33)(H2,34,35,36,37,38,40) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulation |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Gastric inhibitory polypeptide
(Homo sapiens (Human)) | BDBM50244238

(CHEMBL499372 | trans-4-(((5-(benzyloxy)-1-methyl-1...)Show SMILES Cn1c(nc2cc(OCc3ccccc3)ccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:34.38,wD:37.45,(-7.9,-48.6,;-7.12,-47.26,;-5.7,-46.67,;-5.82,-45.14,;-7.32,-44.78,;-8.06,-43.43,;-9.59,-43.39,;-10.33,-42.04,;-9.53,-40.73,;-10.27,-39.37,;-9.47,-38.06,;-10.21,-36.71,;-11.75,-36.67,;-12.55,-37.99,;-11.81,-39.34,;-10.4,-44.71,;-9.66,-46.06,;-8.13,-46.09,;-4.37,-47.45,;-4.38,-48.99,;-3.05,-49.77,;-1.72,-49,;-.39,-49.78,;-.4,-51.32,;-1.74,-52.08,;-3.07,-51.3,;.93,-52.1,;.92,-53.64,;2.27,-51.33,;3.6,-52.11,;4.12,-53.56,;5.66,-53.52,;6.1,-52.05,;4.83,-51.17,;-3.04,-46.69,;-3.04,-45.15,;-1.69,-44.39,;-.36,-45.17,;-.38,-46.71,;-1.71,-47.47,;.98,-44.41,;2.3,-43.63,;1.75,-45.75,;.21,-43.08,)| Show InChI InChI=1S/C34H40N8O2/c1-34(2,3)26-14-16-27(17-15-26)42(21-23-10-12-25(13-11-23)31(43)36-32-37-39-40-38-32)33-35-29-20-28(18-19-30(29)41(33)4)44-22-24-8-6-5-7-9-24/h5-13,18-20,26-27H,14-17,21-22H2,1-4H3,(H2,36,37,38,39,40,43)/t26-,27- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against human GIP |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244376

(CHEMBL488682 | trans-(R)-3-(4-(((4-tert-butylcyclo...)Show SMILES Cn1c(nc2ccccc12)N(Cc1ccc(cc1)C(=O)NC[C@@H](O)C(O)=O)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:27.29,22.25,wD:30.36,(-3.33,-14.81,;-2.56,-13.48,;-1.13,-12.88,;-1.26,-11.35,;-2.76,-10.99,;-3.49,-9.65,;-5.03,-9.61,;-5.83,-10.93,;-5.09,-12.27,;-3.56,-12.31,;.2,-13.66,;.19,-15.2,;1.52,-15.98,;2.85,-15.21,;4.18,-15.99,;4.17,-17.53,;2.82,-18.29,;1.5,-17.51,;5.5,-18.31,;5.49,-19.85,;6.84,-17.55,;8.17,-18.33,;9.51,-17.56,;9.52,-16.02,;10.83,-18.34,;12.17,-17.58,;10.82,-19.88,;1.53,-12.9,;1.53,-11.36,;2.88,-10.6,;4.2,-11.39,;4.19,-12.93,;2.85,-13.68,;5.55,-10.63,;6.87,-9.85,;6.32,-11.96,;4.78,-9.29,)| Show InChI InChI=1S/C29H38N4O4/c1-29(2,3)21-13-15-22(16-14-21)33(28-31-23-7-5-6-8-24(23)32(28)4)18-19-9-11-20(12-10-19)26(35)30-17-25(34)27(36)37/h5-12,21-22,25,34H,13-18H2,1-4H3,(H,30,35)(H,36,37)/t21-,22-,25-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulation |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244163

(4-((methyl(1-methyl-1H-benzo[d]imidazol-2-yl)amino...)Show SMILES CN(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)c1nc2ccccc2n1C Show InChI InChI=1S/C18H18N8O/c1-25(18-19-14-5-3-4-6-15(14)26(18)2)11-12-7-9-13(10-8-12)16(27)20-17-21-23-24-22-17/h3-10H,11H2,1-2H3,(H2,20,21,22,23,24,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Gastric inhibitory polypeptide
(Homo sapiens (Human)) | BDBM50244237

(CHEMBL448921 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(OCC3CCCC3)ccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:33.37,wD:36.44,(-7.9,-48.6,;-7.12,-47.26,;-5.7,-46.67,;-5.82,-45.13,;-7.32,-44.78,;-8.06,-43.43,;-9.59,-43.39,;-10.33,-42.04,;-9.53,-40.73,;-10.27,-39.37,;-9.61,-37.98,;-10.73,-36.92,;-12.08,-37.66,;-11.8,-39.17,;-10.4,-44.71,;-9.66,-46.06,;-8.13,-46.09,;-4.37,-47.45,;-4.38,-48.99,;-3.05,-49.77,;-1.72,-49,;-.39,-49.77,;-.4,-51.32,;-1.74,-52.08,;-3.07,-51.3,;.93,-52.09,;.92,-53.63,;2.27,-51.33,;3.6,-52.11,;4.12,-53.56,;5.66,-53.52,;6.1,-52.05,;4.83,-51.17,;-3.04,-46.69,;-3.04,-45.15,;-1.69,-44.39,;-.36,-45.17,;-.38,-46.71,;-1.71,-47.47,;.98,-44.41,;2.3,-43.63,;1.75,-45.75,;.21,-43.08,)| Show InChI InChI=1S/C33H44N8O2/c1-33(2,3)25-13-15-26(16-14-25)41(20-22-9-11-24(12-10-22)30(42)35-31-36-38-39-37-31)32-34-28-19-27(17-18-29(28)40(32)4)43-21-23-7-5-6-8-23/h9-12,17-19,23,25-26H,5-8,13-16,20-21H2,1-4H3,(H2,35,36,37,38,39,42)/t25-,26- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against human GIP |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Gastric inhibitory polypeptide
(Homo sapiens (Human)) | BDBM50244264

(CHEMBL471979 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES COc1ccc2nc(N(Cc3ccc(cc3)C(=O)Nc3nnn[nH]3)[C@H]3CC[C@@H](CC3)C(C)(C)C)n(C)c2c1 |r,wU:24.25,wD:27.32,(11.41,-44.22,;12.15,-45.57,;13.69,-45.6,;14.49,-44.28,;16.03,-44.32,;16.76,-45.67,;18.26,-46.03,;18.38,-47.56,;19.72,-48.34,;19.71,-49.88,;21.04,-50.66,;22.37,-49.89,;23.7,-50.67,;23.69,-52.21,;22.34,-52.97,;21.02,-52.19,;25.02,-52.99,;25.01,-54.53,;26.36,-52.23,;27.69,-53.01,;28.2,-54.45,;29.74,-54.42,;30.18,-52.94,;28.91,-52.07,;21.04,-47.58,;21.05,-46.04,;22.39,-45.28,;23.72,-46.06,;23.71,-47.61,;22.37,-48.36,;25.06,-45.31,;26.39,-44.53,;25.84,-46.64,;24.3,-43.97,;16.96,-48.16,;16.19,-49.49,;15.96,-46.98,;14.43,-46.95,)| Show InChI InChI=1S/C28H36N8O2/c1-28(2,3)20-10-12-21(13-11-20)36(27-29-23-15-14-22(38-5)16-24(23)35(27)4)17-18-6-8-19(9-7-18)25(37)30-26-31-33-34-32-26/h6-9,14-16,20-21H,10-13,17H2,1-5H3,(H2,30,31,32,33,34,37)/t20-,21- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against human GIP |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Gastric inhibitory polypeptide
(Homo sapiens (Human)) | BDBM50244199

(CHEMBL458145 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(O)ccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:27.30,wD:30.37,(-7.23,-33.1,;-6.46,-31.77,;-5.03,-31.18,;-5.16,-29.64,;-6.66,-29.29,;-7.39,-27.94,;-8.93,-27.9,;-9.67,-26.55,;-9.73,-29.22,;-8.99,-30.57,;-7.46,-30.6,;-3.7,-31.96,;-3.71,-33.5,;-2.38,-34.28,;-1.05,-33.51,;.28,-34.28,;.27,-35.83,;-1.08,-36.59,;-2.4,-35.81,;1.6,-36.6,;1.59,-38.14,;2.94,-35.84,;4.27,-36.62,;4.79,-38.07,;6.32,-38.03,;6.76,-36.56,;5.5,-35.68,;-2.37,-31.2,;-2.37,-29.66,;-1.02,-28.9,;.31,-29.68,;.29,-31.22,;-1.04,-31.98,;1.65,-28.92,;2.97,-28.14,;2.42,-30.26,;.88,-27.59,)| Show InChI InChI=1S/C27H34N8O2/c1-27(2,3)19-9-11-20(12-10-19)35(26-28-22-15-21(36)13-14-23(22)34(26)4)16-17-5-7-18(8-6-17)24(37)29-25-30-32-33-31-25/h5-8,13-15,19-20,36H,9-12,16H2,1-4H3,(H2,29,30,31,32,33,37)/t19-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against human GIP |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244199

(CHEMBL458145 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(O)ccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:27.30,wD:30.37,(-7.23,-33.1,;-6.46,-31.77,;-5.03,-31.18,;-5.16,-29.64,;-6.66,-29.29,;-7.39,-27.94,;-8.93,-27.9,;-9.67,-26.55,;-9.73,-29.22,;-8.99,-30.57,;-7.46,-30.6,;-3.7,-31.96,;-3.71,-33.5,;-2.38,-34.28,;-1.05,-33.51,;.28,-34.28,;.27,-35.83,;-1.08,-36.59,;-2.4,-35.81,;1.6,-36.6,;1.59,-38.14,;2.94,-35.84,;4.27,-36.62,;4.79,-38.07,;6.32,-38.03,;6.76,-36.56,;5.5,-35.68,;-2.37,-31.2,;-2.37,-29.66,;-1.02,-28.9,;.31,-29.68,;.29,-31.22,;-1.04,-31.98,;1.65,-28.92,;2.97,-28.14,;2.42,-30.26,;.88,-27.59,)| Show InChI InChI=1S/C27H34N8O2/c1-27(2,3)19-9-11-20(12-10-19)35(26-28-22-15-21(36)13-14-23(22)34(26)4)16-17-5-7-18(8-6-17)24(37)29-25-30-32-33-31-25/h5-8,13-15,19-20,36H,9-12,16H2,1-4H3,(H2,29,30,31,32,33,37)/t19-,20- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulation |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Gastric inhibitory polypeptide
(Homo sapiens (Human)) | BDBM50244266

(CHEMBL472154 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(F)ccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:27.30,wD:30.37,(16.19,-49.49,;16.96,-48.16,;18.38,-47.56,;18.26,-46.03,;16.76,-45.67,;16.03,-44.32,;14.49,-44.28,;13.75,-42.93,;13.69,-45.6,;14.43,-46.95,;15.96,-46.98,;19.72,-48.34,;19.71,-49.88,;21.04,-50.66,;22.37,-49.89,;23.7,-50.67,;23.69,-52.21,;22.34,-52.97,;21.02,-52.19,;25.02,-52.99,;25.01,-54.53,;26.36,-52.23,;27.69,-53.01,;28.2,-54.45,;29.74,-54.42,;30.18,-52.94,;28.91,-52.07,;21.04,-47.58,;21.05,-46.04,;22.39,-45.28,;23.72,-46.06,;23.71,-47.61,;22.37,-48.36,;25.06,-45.31,;26.39,-44.53,;25.84,-46.64,;24.3,-43.97,)| Show InChI InChI=1S/C27H33FN8O/c1-27(2,3)19-9-12-21(13-10-19)36(26-29-22-15-20(28)11-14-23(22)35(26)4)16-17-5-7-18(8-6-17)24(37)30-25-31-33-34-32-25/h5-8,11,14-15,19,21H,9-10,12-13,16H2,1-4H3,(H2,30,31,32,33,34,37)/t19-,21- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against human GIP |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244080

(CHEMBL509900 | trans-4-((4-tert-butylcyclohexyl)(5...)Show SMILES Cc1ccc2nc([nH]c2c1)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:26.29,wD:29.36,(16.94,-17.66,;17.68,-19.01,;16.88,-20.33,;17.62,-21.67,;19.15,-21.71,;20.15,-22.88,;21.58,-22.29,;21.45,-20.75,;19.95,-20.4,;19.22,-19.05,;22.91,-23.07,;22.9,-24.61,;24.23,-25.38,;25.56,-24.62,;26.89,-25.39,;26.88,-26.93,;25.53,-27.7,;24.21,-26.92,;28.21,-27.71,;28.2,-29.25,;29.55,-26.95,;30.88,-27.73,;31.39,-29.18,;32.93,-29.14,;33.37,-27.67,;32.1,-26.79,;24.23,-22.3,;24.24,-20.77,;25.58,-20.01,;26.91,-20.79,;26.9,-22.33,;25.56,-23.09,;28.25,-20.03,;29.58,-19.25,;29.03,-21.36,;27.49,-18.7,)| Show InChI InChI=1S/C27H34N8O/c1-17-5-14-22-23(15-17)29-26(28-22)35(21-12-10-20(11-13-21)27(2,3)4)16-18-6-8-19(9-7-18)24(36)30-25-31-33-34-32-25/h5-9,14-15,20-21H,10-13,16H2,1-4H3,(H,28,29)(H2,30,31,32,33,34,36)/t20-,21- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulation |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244076
(CHEMBL452041 | N-(3-chloro-4-(4-chlorophenoxy)phen...)Show SMILES Clc1ccc(Oc2ccc(Nc3nc(cs3)-c3ccccc3)cc2Cl)cc1 Show InChI InChI=1S/C21H14Cl2N2OS/c22-15-6-9-17(10-7-15)26-20-11-8-16(12-18(20)23)24-21-25-19(13-27-21)14-4-2-1-3-5-14/h1-13H,(H,24,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244338

(4-(((1-methyl-1H-benzo[d]imidazol-2-yl)(4-(trifluo...)Show SMILES Cn1c(nc2ccccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C24H19F3N8O2/c1-34-20-5-3-2-4-19(20)28-23(34)35(17-10-12-18(13-11-17)37-24(25,26)27)14-15-6-8-16(9-7-15)21(36)29-22-30-32-33-31-22/h2-13H,14H2,1H3,(H2,29,30,31,32,33,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human GCGR expressed in CHO cells assessed as glucagon-induced cAMP accumulation |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Gastric inhibitory polypeptide
(Homo sapiens (Human)) | BDBM50244236

(CHEMBL500315 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(OC3CCCC3)ccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:32.36,wD:35.43,(16.18,-49.49,;16.96,-48.15,;18.38,-47.56,;18.26,-46.02,;16.76,-45.67,;16.03,-44.32,;14.49,-44.28,;13.75,-42.93,;14.55,-41.62,;16.09,-41.49,;16.44,-39.99,;15.13,-39.19,;13.96,-40.19,;13.69,-45.6,;14.43,-46.95,;15.96,-46.98,;19.71,-48.34,;19.71,-49.88,;21.04,-50.66,;22.37,-49.89,;23.69,-50.66,;23.69,-52.21,;22.34,-52.97,;21.02,-52.19,;25.02,-52.98,;25.01,-54.52,;26.36,-52.22,;27.68,-53,;28.2,-54.45,;29.74,-54.41,;30.18,-52.94,;28.91,-52.06,;21.04,-47.58,;21.05,-46.04,;22.39,-45.28,;23.72,-46.06,;23.71,-47.6,;22.37,-48.36,;25.06,-45.3,;26.39,-44.52,;25.83,-46.64,;24.3,-43.97,)| Show InChI InChI=1S/C32H42N8O2/c1-32(2,3)23-13-15-24(16-14-23)40(20-21-9-11-22(12-10-21)29(41)34-30-35-37-38-36-30)31-33-27-19-26(17-18-28(27)39(31)4)42-25-7-5-6-8-25/h9-12,17-19,23-25H,5-8,13-16,20H2,1-4H3,(H2,34,35,36,37,38,41)/t23-,24- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against human GIP |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Gastric inhibitory polypeptide
(Homo sapiens (Human)) | BDBM50244233

(CHEMBL513067 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES COc1ccc2n(C)c(nc2c1)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:28.31,wD:31.38,(13.19,-24.98,;12.38,-26.29,;13.12,-27.64,;12.32,-28.96,;13.06,-30.31,;14.59,-30.34,;15.59,-31.52,;14.82,-32.85,;17.02,-30.92,;16.89,-29.39,;15.39,-29.03,;14.66,-27.68,;18.35,-31.7,;18.34,-33.24,;19.67,-34.02,;21,-33.25,;22.33,-34.03,;22.32,-35.57,;20.97,-36.33,;19.65,-35.55,;23.65,-36.35,;23.64,-37.89,;24.99,-35.59,;26.32,-36.37,;26.83,-37.81,;28.37,-37.78,;28.81,-36.3,;27.55,-35.43,;19.68,-30.94,;19.68,-29.4,;21.03,-28.64,;22.36,-29.42,;22.34,-30.96,;21.01,-31.72,;23.7,-28.67,;25.02,-27.89,;24.47,-30,;22.93,-27.33,)| Show InChI InChI=1S/C28H36N8O2/c1-28(2,3)20-10-12-21(13-11-20)36(27-29-23-16-22(38-5)14-15-24(23)35(27)4)17-18-6-8-19(9-7-18)25(37)30-26-31-33-34-32-26/h6-9,14-16,20-21H,10-13,17H2,1-5H3,(H2,30,31,32,33,34,37)/t20-,21- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against human GIP |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Gastric inhibitory polypeptide
(Homo sapiens (Human)) | BDBM50244234

(CHEMBL518939 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES CCOc1ccc2n(C)c(nc2c1)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:29.32,wD:32.39,(-10.27,-39.37,;-9.53,-40.73,;-10.33,-42.04,;-9.59,-43.39,;-10.4,-44.71,;-9.66,-46.06,;-8.13,-46.09,;-7.12,-47.26,;-7.9,-48.6,;-5.7,-46.67,;-5.82,-45.14,;-7.32,-44.78,;-8.06,-43.43,;-4.37,-47.45,;-4.38,-48.99,;-3.05,-49.77,;-1.72,-49,;-.39,-49.78,;-.4,-51.32,;-1.74,-52.08,;-3.07,-51.3,;.93,-52.1,;.92,-53.64,;2.27,-51.33,;3.6,-52.11,;4.12,-53.56,;5.66,-53.52,;6.1,-52.05,;4.83,-51.17,;-3.04,-46.69,;-3.04,-45.15,;-1.69,-44.39,;-.36,-45.17,;-.38,-46.71,;-1.71,-47.47,;.98,-44.41,;2.3,-43.63,;1.75,-45.75,;.21,-43.08,)| Show InChI InChI=1S/C29H38N8O2/c1-6-39-23-15-16-25-24(17-23)30-28(36(25)5)37(22-13-11-21(12-14-22)29(2,3)4)18-19-7-9-20(10-8-19)26(38)31-27-32-34-35-33-27/h7-10,15-17,21-22H,6,11-14,18H2,1-5H3,(H2,31,32,33,34,35,38)/t21-,22- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against human GIP |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Gastric inhibitory polypeptide
(Homo sapiens (Human)) | BDBM50244337

(CHEMBL511964 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(Cl)c(Cl)cc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:28.31,wD:31.38,(37.8,-49.39,;38.58,-48.06,;40,-47.47,;39.88,-45.93,;38.38,-45.58,;37.65,-44.23,;36.11,-44.19,;35.37,-42.84,;35.31,-45.51,;33.77,-45.47,;36.05,-46.85,;37.58,-46.89,;41.33,-48.24,;41.33,-49.78,;42.66,-50.56,;43.99,-49.79,;45.31,-50.57,;45.31,-52.11,;43.96,-52.87,;42.64,-52.09,;46.64,-52.89,;46.63,-54.43,;47.97,-52.13,;49.3,-52.91,;49.82,-54.36,;51.36,-54.32,;51.8,-52.84,;50.53,-51.97,;42.66,-47.48,;42.67,-45.94,;44.01,-45.19,;45.34,-45.97,;45.32,-47.51,;43.99,-48.26,;46.68,-45.21,;48,-44.43,;47.45,-46.54,;45.92,-43.87,)| Show InChI InChI=1S/C27H32Cl2N8O/c1-27(2,3)18-9-11-19(12-10-18)37(26-30-22-13-20(28)21(29)14-23(22)36(26)4)15-16-5-7-17(8-6-16)24(38)31-25-32-34-35-33-25/h5-8,13-14,18-19H,9-12,15H2,1-4H3,(H2,31,32,33,34,35,38)/t18-,19- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against human GIP |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Gastric inhibitory polypeptide
(Homo sapiens (Human)) | BDBM50244235

(CHEMBL480692 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES CCCOc1ccc2n(C)c(nc2c1)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:30.33,wD:33.40,(15,-40.17,;14.2,-41.49,;14.94,-42.84,;14.14,-44.16,;14.88,-45.51,;14.07,-46.83,;14.81,-48.17,;16.34,-48.21,;17.35,-49.38,;16.57,-50.71,;18.77,-48.79,;18.65,-47.25,;17.15,-46.9,;16.41,-45.55,;20.1,-49.57,;20.09,-51.11,;21.42,-51.88,;22.75,-51.12,;24.08,-51.89,;24.07,-53.43,;22.73,-54.2,;21.4,-53.42,;25.4,-54.21,;25.39,-55.75,;26.74,-53.45,;28.07,-54.23,;28.59,-55.68,;30.13,-55.64,;30.57,-54.16,;29.3,-53.29,;21.43,-48.8,;21.43,-47.27,;22.78,-46.51,;24.11,-47.29,;24.09,-48.83,;22.76,-49.58,;25.45,-46.53,;26.77,-45.75,;26.22,-47.86,;24.68,-45.19,)| Show InChI InChI=1S/C30H40N8O2/c1-6-17-40-24-15-16-26-25(18-24)31-29(37(26)5)38(23-13-11-22(12-14-23)30(2,3)4)19-20-7-9-21(10-8-20)27(39)32-28-33-35-36-34-28/h7-10,15-16,18,22-23H,6,11-14,17,19H2,1-5H3,(H2,32,33,34,35,36,39)/t22-,23- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against human GIP |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Pro-glucagon
(Homo sapiens (Human)) | BDBM50244337

(CHEMBL511964 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(Cl)c(Cl)cc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:28.31,wD:31.38,(37.8,-49.39,;38.58,-48.06,;40,-47.47,;39.88,-45.93,;38.38,-45.58,;37.65,-44.23,;36.11,-44.19,;35.37,-42.84,;35.31,-45.51,;33.77,-45.47,;36.05,-46.85,;37.58,-46.89,;41.33,-48.24,;41.33,-49.78,;42.66,-50.56,;43.99,-49.79,;45.31,-50.57,;45.31,-52.11,;43.96,-52.87,;42.64,-52.09,;46.64,-52.89,;46.63,-54.43,;47.97,-52.13,;49.3,-52.91,;49.82,-54.36,;51.36,-54.32,;51.8,-52.84,;50.53,-51.97,;42.66,-47.48,;42.67,-45.94,;44.01,-45.19,;45.34,-45.97,;45.32,-47.51,;43.99,-48.26,;46.68,-45.21,;48,-44.43,;47.45,-46.54,;45.92,-43.87,)| Show InChI InChI=1S/C27H32Cl2N8O/c1-27(2,3)18-9-11-19(12-10-18)37(26-30-22-13-20(28)21(29)14-23(22)36(26)4)15-16-5-7-17(8-6-16)24(38)31-25-32-34-35-33-25/h5-8,13-14,18-19H,9-12,15H2,1-4H3,(H2,31,32,33,34,35,38)/t18-,19- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against human GLP1 |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Gastric inhibitory polypeptide
(Homo sapiens (Human)) | BDBM50244265

(CHEMBL480113 | trans-4-((4-tert-butylcyclohexyl)(1...)Show SMILES CCCOc1ccc2nc(N(Cc3ccc(cc3)C(=O)Nc3nnn[nH]3)[C@H]3CC[C@@H](CC3)C(C)(C)C)n(C)c2c1 |r,wU:26.27,wD:29.34,(9.13,-42.84,;9.87,-44.19,;11.41,-44.22,;12.15,-45.57,;13.69,-45.6,;14.49,-44.28,;16.03,-44.32,;16.76,-45.67,;18.26,-46.03,;18.38,-47.56,;19.71,-48.34,;19.71,-49.88,;21.04,-50.66,;22.37,-49.89,;23.7,-50.67,;23.69,-52.21,;22.34,-52.97,;21.02,-52.19,;25.02,-52.99,;25.01,-54.53,;26.36,-52.22,;27.68,-53,;28.2,-54.45,;29.74,-54.42,;30.18,-52.94,;28.91,-52.06,;21.04,-47.58,;21.05,-46.04,;22.39,-45.28,;23.72,-46.06,;23.71,-47.6,;22.37,-48.36,;25.06,-45.3,;26.39,-44.52,;25.83,-46.64,;24.3,-43.97,;16.96,-48.15,;16.19,-49.49,;15.96,-46.98,;14.43,-46.95,)| Show InChI InChI=1S/C30H40N8O2/c1-6-17-40-24-15-16-25-26(18-24)37(5)29(31-25)38(23-13-11-22(12-14-23)30(2,3)4)19-20-7-9-21(10-8-20)27(39)32-28-33-35-36-34-28/h7-10,15-16,18,22-23H,6,11-14,17,19H2,1-5H3,(H2,32,33,34,35,36,39)/t22-,23- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against human GIP |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Pro-glucagon
(Homo sapiens (Human)) | BDBM50244236

(CHEMBL500315 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(OC3CCCC3)ccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:32.36,wD:35.43,(16.18,-49.49,;16.96,-48.15,;18.38,-47.56,;18.26,-46.02,;16.76,-45.67,;16.03,-44.32,;14.49,-44.28,;13.75,-42.93,;14.55,-41.62,;16.09,-41.49,;16.44,-39.99,;15.13,-39.19,;13.96,-40.19,;13.69,-45.6,;14.43,-46.95,;15.96,-46.98,;19.71,-48.34,;19.71,-49.88,;21.04,-50.66,;22.37,-49.89,;23.69,-50.66,;23.69,-52.21,;22.34,-52.97,;21.02,-52.19,;25.02,-52.98,;25.01,-54.52,;26.36,-52.22,;27.68,-53,;28.2,-54.45,;29.74,-54.41,;30.18,-52.94,;28.91,-52.06,;21.04,-47.58,;21.05,-46.04,;22.39,-45.28,;23.72,-46.06,;23.71,-47.6,;22.37,-48.36,;25.06,-45.3,;26.39,-44.52,;25.83,-46.64,;24.3,-43.97,)| Show InChI InChI=1S/C32H42N8O2/c1-32(2,3)23-13-15-24(16-14-23)40(20-21-9-11-22(12-10-21)29(41)34-30-35-37-38-36-30)31-33-27-19-26(17-18-28(27)39(31)4)42-25-7-5-6-8-25/h9-12,17-19,23-25H,5-8,13-16,20H2,1-4H3,(H2,34,35,36,37,38,41)/t23-,24- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against human GLP1 |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Pro-glucagon
(Homo sapiens (Human)) | BDBM50244265

(CHEMBL480113 | trans-4-((4-tert-butylcyclohexyl)(1...)Show SMILES CCCOc1ccc2nc(N(Cc3ccc(cc3)C(=O)Nc3nnn[nH]3)[C@H]3CC[C@@H](CC3)C(C)(C)C)n(C)c2c1 |r,wU:26.27,wD:29.34,(9.13,-42.84,;9.87,-44.19,;11.41,-44.22,;12.15,-45.57,;13.69,-45.6,;14.49,-44.28,;16.03,-44.32,;16.76,-45.67,;18.26,-46.03,;18.38,-47.56,;19.71,-48.34,;19.71,-49.88,;21.04,-50.66,;22.37,-49.89,;23.7,-50.67,;23.69,-52.21,;22.34,-52.97,;21.02,-52.19,;25.02,-52.99,;25.01,-54.53,;26.36,-52.22,;27.68,-53,;28.2,-54.45,;29.74,-54.42,;30.18,-52.94,;28.91,-52.06,;21.04,-47.58,;21.05,-46.04,;22.39,-45.28,;23.72,-46.06,;23.71,-47.6,;22.37,-48.36,;25.06,-45.3,;26.39,-44.52,;25.83,-46.64,;24.3,-43.97,;16.96,-48.15,;16.19,-49.49,;15.96,-46.98,;14.43,-46.95,)| Show InChI InChI=1S/C30H40N8O2/c1-6-17-40-24-15-16-25-26(18-24)37(5)29(31-25)38(23-13-11-22(12-14-23)30(2,3)4)19-20-7-9-21(10-8-20)27(39)32-28-33-35-36-34-28/h7-10,15-16,18,22-23H,6,11-14,17,19H2,1-5H3,(H2,32,33,34,35,36,39)/t22-,23- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against human GLP1 |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Pro-glucagon
(Homo sapiens (Human)) | BDBM50244233

(CHEMBL513067 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES COc1ccc2n(C)c(nc2c1)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:28.31,wD:31.38,(13.19,-24.98,;12.38,-26.29,;13.12,-27.64,;12.32,-28.96,;13.06,-30.31,;14.59,-30.34,;15.59,-31.52,;14.82,-32.85,;17.02,-30.92,;16.89,-29.39,;15.39,-29.03,;14.66,-27.68,;18.35,-31.7,;18.34,-33.24,;19.67,-34.02,;21,-33.25,;22.33,-34.03,;22.32,-35.57,;20.97,-36.33,;19.65,-35.55,;23.65,-36.35,;23.64,-37.89,;24.99,-35.59,;26.32,-36.37,;26.83,-37.81,;28.37,-37.78,;28.81,-36.3,;27.55,-35.43,;19.68,-30.94,;19.68,-29.4,;21.03,-28.64,;22.36,-29.42,;22.34,-30.96,;21.01,-31.72,;23.7,-28.67,;25.02,-27.89,;24.47,-30,;22.93,-27.33,)| Show InChI InChI=1S/C28H36N8O2/c1-28(2,3)20-10-12-21(13-11-20)36(27-29-23-16-22(38-5)14-15-24(23)35(27)4)17-18-6-8-19(9-7-18)25(37)30-26-31-33-34-32-26/h6-9,14-16,20-21H,10-13,17H2,1-5H3,(H2,30,31,32,33,34,37)/t20-,21- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against human GLP1 |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Gastric inhibitory polypeptide
(Homo sapiens (Human)) | BDBM50244299

(CHEMBL480501 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(ccc12)C(F)(F)F)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:30.33,wD:33.40,(-5.67,-48.6,;-4.89,-47.26,;-3.47,-46.67,;-3.59,-45.14,;-5.09,-44.78,;-5.82,-43.43,;-7.36,-43.39,;-8.16,-44.71,;-7.42,-46.06,;-5.89,-46.09,;-8.1,-42.04,;-8.88,-40.7,;-6.76,-41.28,;-9.43,-42.81,;-2.14,-47.45,;-2.14,-48.99,;-.81,-49.77,;.52,-49,;1.84,-49.78,;1.84,-51.32,;.49,-52.08,;-.83,-51.3,;3.17,-52.1,;3.16,-53.64,;4.5,-51.33,;5.83,-52.11,;6.35,-53.56,;7.89,-53.52,;8.33,-52.05,;7.06,-51.17,;-.81,-46.69,;-.8,-45.15,;.54,-44.39,;1.87,-45.17,;1.85,-46.71,;.52,-47.47,;3.21,-44.41,;4.53,-43.63,;3.98,-45.75,;2.45,-43.08,)| Show InChI InChI=1S/C28H33F3N8O/c1-27(2,3)19-9-12-21(13-10-19)39(26-32-22-15-20(28(29,30)31)11-14-23(22)38(26)4)16-17-5-7-18(8-6-17)24(40)33-25-34-36-37-35-25/h5-8,11,14-15,19,21H,9-10,12-13,16H2,1-4H3,(H2,33,34,35,36,37,40)/t19-,21- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against human GIP |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Pro-glucagon
(Homo sapiens (Human)) | BDBM50244299

(CHEMBL480501 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(ccc12)C(F)(F)F)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:30.33,wD:33.40,(-5.67,-48.6,;-4.89,-47.26,;-3.47,-46.67,;-3.59,-45.14,;-5.09,-44.78,;-5.82,-43.43,;-7.36,-43.39,;-8.16,-44.71,;-7.42,-46.06,;-5.89,-46.09,;-8.1,-42.04,;-8.88,-40.7,;-6.76,-41.28,;-9.43,-42.81,;-2.14,-47.45,;-2.14,-48.99,;-.81,-49.77,;.52,-49,;1.84,-49.78,;1.84,-51.32,;.49,-52.08,;-.83,-51.3,;3.17,-52.1,;3.16,-53.64,;4.5,-51.33,;5.83,-52.11,;6.35,-53.56,;7.89,-53.52,;8.33,-52.05,;7.06,-51.17,;-.81,-46.69,;-.8,-45.15,;.54,-44.39,;1.87,-45.17,;1.85,-46.71,;.52,-47.47,;3.21,-44.41,;4.53,-43.63,;3.98,-45.75,;2.45,-43.08,)| Show InChI InChI=1S/C28H33F3N8O/c1-27(2,3)19-9-12-21(13-10-19)39(26-32-22-15-20(28(29,30)31)11-14-23(22)38(26)4)16-17-5-7-18(8-6-17)24(40)33-25-34-36-37-35-25/h5-8,11,14-15,19,21H,9-10,12-13,16H2,1-4H3,(H2,33,34,35,36,37,40)/t19-,21- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against human GLP1 |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Pro-glucagon
(Homo sapiens (Human)) | BDBM50244234

(CHEMBL518939 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES CCOc1ccc2n(C)c(nc2c1)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:29.32,wD:32.39,(-10.27,-39.37,;-9.53,-40.73,;-10.33,-42.04,;-9.59,-43.39,;-10.4,-44.71,;-9.66,-46.06,;-8.13,-46.09,;-7.12,-47.26,;-7.9,-48.6,;-5.7,-46.67,;-5.82,-45.14,;-7.32,-44.78,;-8.06,-43.43,;-4.37,-47.45,;-4.38,-48.99,;-3.05,-49.77,;-1.72,-49,;-.39,-49.78,;-.4,-51.32,;-1.74,-52.08,;-3.07,-51.3,;.93,-52.1,;.92,-53.64,;2.27,-51.33,;3.6,-52.11,;4.12,-53.56,;5.66,-53.52,;6.1,-52.05,;4.83,-51.17,;-3.04,-46.69,;-3.04,-45.15,;-1.69,-44.39,;-.36,-45.17,;-.38,-46.71,;-1.71,-47.47,;.98,-44.41,;2.3,-43.63,;1.75,-45.75,;.21,-43.08,)| Show InChI InChI=1S/C29H38N8O2/c1-6-39-23-15-16-25-24(17-23)30-28(36(25)5)37(22-13-11-21(12-14-22)29(2,3)4)18-19-7-9-20(10-8-19)26(38)31-27-32-34-35-33-27/h7-10,15-17,21-22H,6,11-14,18H2,1-5H3,(H2,31,32,33,34,35,38)/t21-,22- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against human GLP1 |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50244299

(CHEMBL480501 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(ccc12)C(F)(F)F)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:30.33,wD:33.40,(-5.67,-48.6,;-4.89,-47.26,;-3.47,-46.67,;-3.59,-45.14,;-5.09,-44.78,;-5.82,-43.43,;-7.36,-43.39,;-8.16,-44.71,;-7.42,-46.06,;-5.89,-46.09,;-8.1,-42.04,;-8.88,-40.7,;-6.76,-41.28,;-9.43,-42.81,;-2.14,-47.45,;-2.14,-48.99,;-.81,-49.77,;.52,-49,;1.84,-49.78,;1.84,-51.32,;.49,-52.08,;-.83,-51.3,;3.17,-52.1,;3.16,-53.64,;4.5,-51.33,;5.83,-52.11,;6.35,-53.56,;7.89,-53.52,;8.33,-52.05,;7.06,-51.17,;-.81,-46.69,;-.8,-45.15,;.54,-44.39,;1.87,-45.17,;1.85,-46.71,;.52,-47.47,;3.21,-44.41,;4.53,-43.63,;3.98,-45.75,;2.45,-43.08,)| Show InChI InChI=1S/C28H33F3N8O/c1-27(2,3)19-9-12-21(13-10-19)39(26-32-22-15-20(28(29,30)31)11-14-23(22)38(26)4)16-17-5-7-18(8-6-17)24(40)33-25-34-36-37-35-25/h5-8,11,14-15,19,21H,9-10,12-13,16H2,1-4H3,(H2,33,34,35,36,37,40)/t19-,21- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50244299

(CHEMBL480501 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(ccc12)C(F)(F)F)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:30.33,wD:33.40,(-5.67,-48.6,;-4.89,-47.26,;-3.47,-46.67,;-3.59,-45.14,;-5.09,-44.78,;-5.82,-43.43,;-7.36,-43.39,;-8.16,-44.71,;-7.42,-46.06,;-5.89,-46.09,;-8.1,-42.04,;-8.88,-40.7,;-6.76,-41.28,;-9.43,-42.81,;-2.14,-47.45,;-2.14,-48.99,;-.81,-49.77,;.52,-49,;1.84,-49.78,;1.84,-51.32,;.49,-52.08,;-.83,-51.3,;3.17,-52.1,;3.16,-53.64,;4.5,-51.33,;5.83,-52.11,;6.35,-53.56,;7.89,-53.52,;8.33,-52.05,;7.06,-51.17,;-.81,-46.69,;-.8,-45.15,;.54,-44.39,;1.87,-45.17,;1.85,-46.71,;.52,-47.47,;3.21,-44.41,;4.53,-43.63,;3.98,-45.75,;2.45,-43.08,)| Show InChI InChI=1S/C28H33F3N8O/c1-27(2,3)19-9-12-21(13-10-19)39(26-32-22-15-20(28(29,30)31)11-14-23(22)38(26)4)16-17-5-7-18(8-6-17)24(40)33-25-34-36-37-35-25/h5-8,11,14-15,19,21H,9-10,12-13,16H2,1-4H3,(H2,33,34,35,36,37,40)/t19-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50244299

(CHEMBL480501 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(ccc12)C(F)(F)F)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:30.33,wD:33.40,(-5.67,-48.6,;-4.89,-47.26,;-3.47,-46.67,;-3.59,-45.14,;-5.09,-44.78,;-5.82,-43.43,;-7.36,-43.39,;-8.16,-44.71,;-7.42,-46.06,;-5.89,-46.09,;-8.1,-42.04,;-8.88,-40.7,;-6.76,-41.28,;-9.43,-42.81,;-2.14,-47.45,;-2.14,-48.99,;-.81,-49.77,;.52,-49,;1.84,-49.78,;1.84,-51.32,;.49,-52.08,;-.83,-51.3,;3.17,-52.1,;3.16,-53.64,;4.5,-51.33,;5.83,-52.11,;6.35,-53.56,;7.89,-53.52,;8.33,-52.05,;7.06,-51.17,;-.81,-46.69,;-.8,-45.15,;.54,-44.39,;1.87,-45.17,;1.85,-46.71,;.52,-47.47,;3.21,-44.41,;4.53,-43.63,;3.98,-45.75,;2.45,-43.08,)| Show InChI InChI=1S/C28H33F3N8O/c1-27(2,3)19-9-12-21(13-10-19)39(26-32-22-15-20(28(29,30)31)11-14-23(22)38(26)4)16-17-5-7-18(8-6-17)24(40)33-25-34-36-37-35-25/h5-8,11,14-15,19,21H,9-10,12-13,16H2,1-4H3,(H2,33,34,35,36,37,40)/t19-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of hERG potassium channel |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Gastric inhibitory polypeptide
(Homo sapiens (Human)) | BDBM50244300

(CHEMBL470955 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cc1cc2nc(N(Cc3ccc(cc3)C(=O)Nc3nnn[nH]3)[C@H]3CC[C@@H](CC3)C(C)(C)C)n(C)c2cc1C |r,wU:22.23,wD:25.30,(13.75,-42.93,;14.49,-44.28,;16.03,-44.32,;16.76,-45.67,;18.26,-46.03,;18.38,-47.56,;19.71,-48.34,;19.71,-49.88,;21.04,-50.66,;22.37,-49.89,;23.7,-50.67,;23.69,-52.21,;22.34,-52.97,;21.02,-52.19,;25.02,-52.99,;25.01,-54.53,;26.36,-52.23,;27.68,-53,;28.2,-54.45,;29.74,-54.42,;30.18,-52.94,;28.91,-52.07,;21.04,-47.58,;21.05,-46.04,;22.39,-45.28,;23.72,-46.06,;23.71,-47.6,;22.37,-48.36,;25.06,-45.31,;26.39,-44.52,;25.83,-46.64,;24.3,-43.97,;16.96,-48.16,;16.19,-49.49,;15.96,-46.98,;14.43,-46.95,;13.69,-45.6,;12.15,-45.57,)| Show InChI InChI=1S/C29H38N8O/c1-18-15-24-25(16-19(18)2)36(6)28(30-24)37(23-13-11-22(12-14-23)29(3,4)5)17-20-7-9-21(10-8-20)26(38)31-27-32-34-35-33-27/h7-10,15-16,22-23H,11-14,17H2,1-6H3,(H2,31,32,33,34,35,38)/t22-,23- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against human GIP |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50244299

(CHEMBL480501 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(ccc12)C(F)(F)F)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:30.33,wD:33.40,(-5.67,-48.6,;-4.89,-47.26,;-3.47,-46.67,;-3.59,-45.14,;-5.09,-44.78,;-5.82,-43.43,;-7.36,-43.39,;-8.16,-44.71,;-7.42,-46.06,;-5.89,-46.09,;-8.1,-42.04,;-8.88,-40.7,;-6.76,-41.28,;-9.43,-42.81,;-2.14,-47.45,;-2.14,-48.99,;-.81,-49.77,;.52,-49,;1.84,-49.78,;1.84,-51.32,;.49,-52.08,;-.83,-51.3,;3.17,-52.1,;3.16,-53.64,;4.5,-51.33,;5.83,-52.11,;6.35,-53.56,;7.89,-53.52,;8.33,-52.05,;7.06,-51.17,;-.81,-46.69,;-.8,-45.15,;.54,-44.39,;1.87,-45.17,;1.85,-46.71,;.52,-47.47,;3.21,-44.41,;4.53,-43.63,;3.98,-45.75,;2.45,-43.08,)| Show InChI InChI=1S/C28H33F3N8O/c1-27(2,3)19-9-12-21(13-10-19)39(26-32-22-15-20(28(29,30)31)11-14-23(22)38(26)4)16-17-5-7-18(8-6-17)24(40)33-25-34-36-37-35-25/h5-8,11,14-15,19,21H,9-10,12-13,16H2,1-4H3,(H2,33,34,35,36,37,40)/t19-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
















































































