

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50232355 ((3aR,6aR)-2-(cyclopropylmethyl)-6-(4-(2-(pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50243638 (1-((3aR,6aR)-6-(4-(2-((R)-2-methylpyrrolidin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50243123 (CHEMBL488464 | cyclopentyl((3aR,6aR)-6-(4-(2-((R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50243122 (2-methyl-1-((3aR,6aR)-6-(4-(2-((R)-2-methylpyrroli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50243674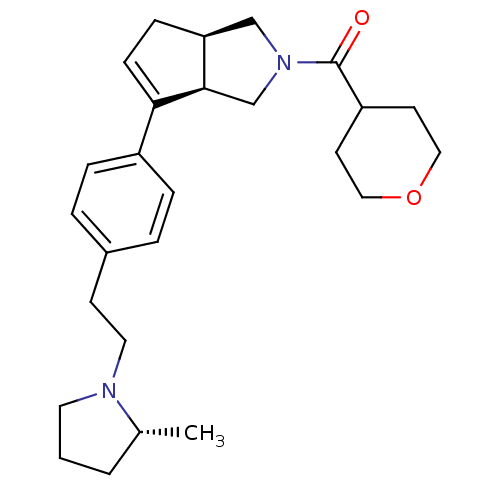 (((3aR,6aR)-6-(4-(2-((R)-2-methylpyrrolidin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50232355 ((3aR,6aR)-2-(cyclopropylmethyl)-6-(4-(2-(pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in CHOK1 cells by [3H]R(-)-alpha-methylhistamine displacement assay | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50232355 ((3aR,6aR)-2-(cyclopropylmethyl)-6-(4-(2-(pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Inverse agonist activity at human H3 receptor expressed in CHOK1 cells by GTPgammaS binding assay | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50243639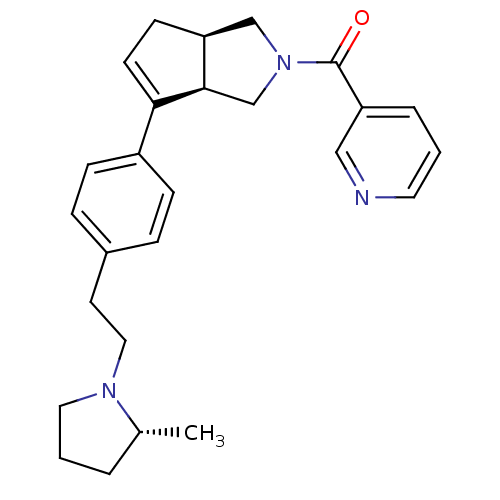 (((3aR,6aR)-6-(4-(2-((R)-2-methylpyrrolidin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50243674 (((3aR,6aR)-6-(4-(2-((R)-2-methylpyrrolidin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in CHOK1 cells by [3H]R(-)-alpha-methylhistamine displacement assay | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50243175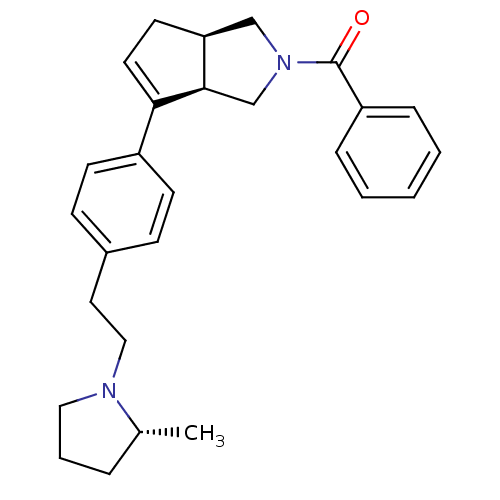 (((3aR,6aR)-6-(4-(2-((R)-2-methylpyrrolidin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50243120 (CHEMBL520719 | cyclopentyl((3aR,6aR)-6-(4-(2-(pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50243674 (((3aR,6aR)-6-(4-(2-((R)-2-methylpyrrolidin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Inverse agonist activity at human H3 receptor expressed in CHOK1 cells by GTPgammaS binding assay | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50243676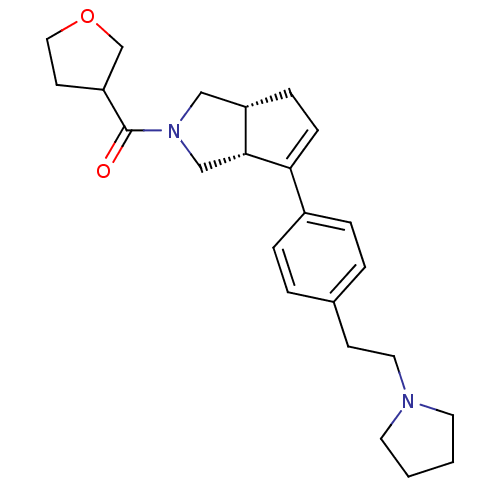 (((3aR,6aR)-6-(4-(2-(pyrrolidin-1-yl)ethyl)phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50243119 (2-methyl-1-((3aR,6aR)-6-(4-(2-(pyrrolidin-1-yl)eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50243121 (2-hydroxy-1-((3aR,6aR)-6-(4-(2-(pyrrolidin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50243072 (CHEMBL470286 | pyridin-3-yl((3aR,6aR)-6-(4-(2-(pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50243073 (2-phenyl-1-((3aR,6aR)-6-(4-(2-(pyrrolidin-1-yl)eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50243070 ((3aR,6aR)-2-(benzylsulfonyl)-6-(4-(2-(pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50242917 ((3aR,6aR)-2-(isopropylsulfonyl)-6-(4-(2-(pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50242845 (CHEMBL512484 | morpholino((3aR,6aR)-6-(4-(2-(pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50242844 ((3aR,6aR)-N,N-dimethyl-6-(4-(2-(pyrrolidin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50242843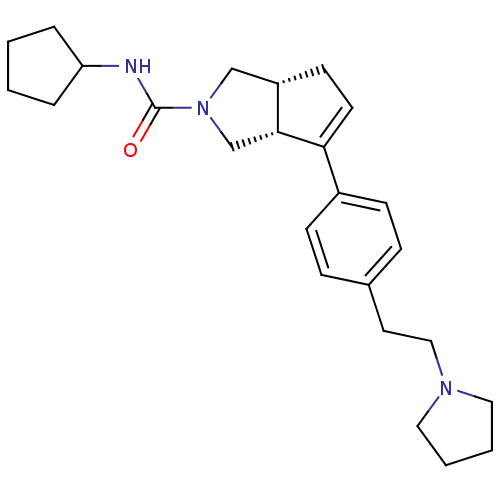 ((3aR,6aR)-N-cyclopentyl-6-(4-(2-(pyrrolidin-1-yl)e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50243071 ((3aR,6aR)-2-(phenylsulfonyl)-6-(4-(2-(pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50242842 ((3aR,6aR)-N-isopropyl-6-(4-(2-(pyrrolidin-1-yl)eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50243675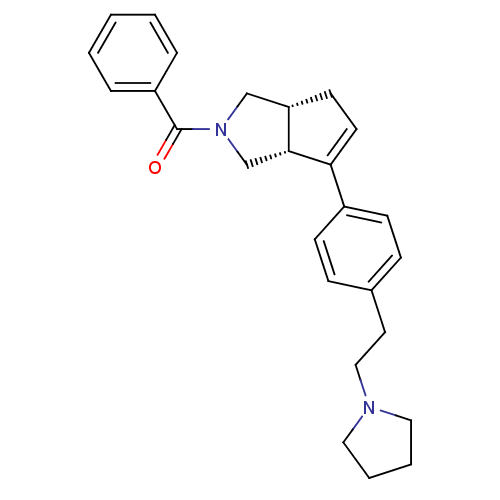 (CHEMBL512707 | phenyl((3aR,6aR)-6-(4-(2-(pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50242846 ((3aR,6aR)-2-(methylsulfonyl)-6-(4-(2-(pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||