

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM87059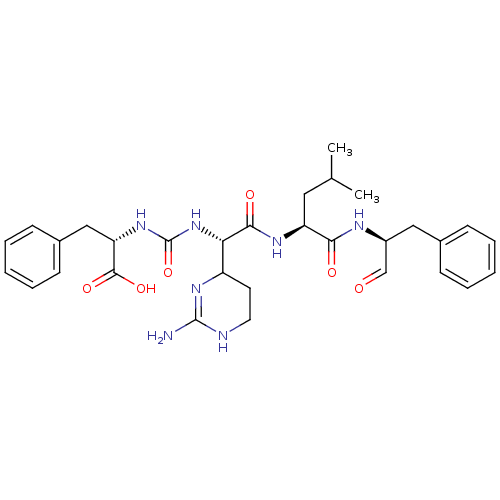 (CHEMBL247767 | Chymostatin) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.24E+3 | -29.0 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
University of Karachi | Assay Description Chymotrypsin inhibitory activity of compunds was perfomred by the method of Cannel. | J Enzyme Inhib Med Chem 23: 400-5 (2008) Article DOI: 10.1080/14756360701584653 BindingDB Entry DOI: 10.7270/Q2JS9P23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM87058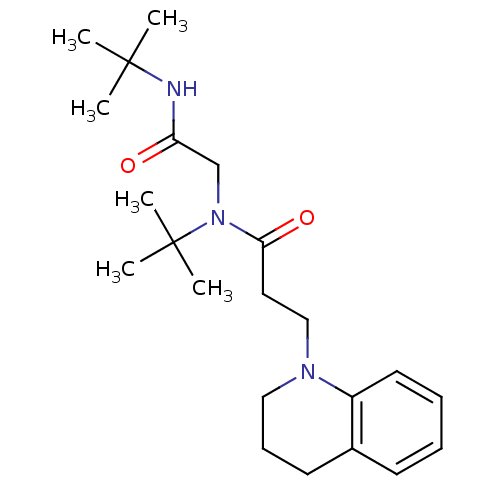 (Lignan, 3 | MLS000034632 | N-tert-Butyl-N-(tert-bu...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.18E+4 | -25.7 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
University of Karachi | Assay Description Chymotrypsin inhibitory activity of compunds was perfomred by the method of Cannel. | J Enzyme Inhib Med Chem 23: 400-5 (2008) Article DOI: 10.1080/14756360701584653 BindingDB Entry DOI: 10.7270/Q2JS9P23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM87060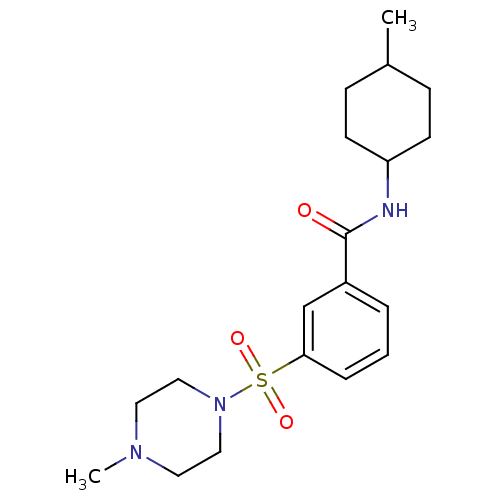 (Lignan, 4 | MLS001138823 | N-(4-methylcyclohexyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 4.71E+4 | -24.7 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
University of Karachi | Assay Description Chymotrypsin inhibitory activity of compunds was perfomred by the method of Cannel. | J Enzyme Inhib Med Chem 23: 400-5 (2008) Article DOI: 10.1080/14756360701584653 BindingDB Entry DOI: 10.7270/Q2JS9P23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||