

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50263215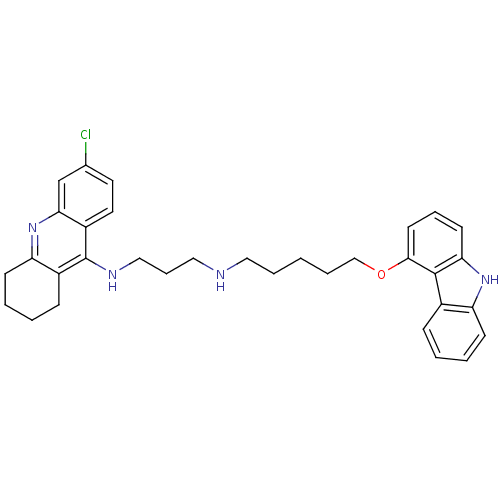 (CHEMBL473866 | N-[5-(9H-Carbazol-4-yloxy)pentyl]-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.54 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by Ellman's method | J Med Chem 51: 4381-4 (2008) Article DOI: 10.1021/jm800577j BindingDB Entry DOI: 10.7270/Q21G0M23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50263214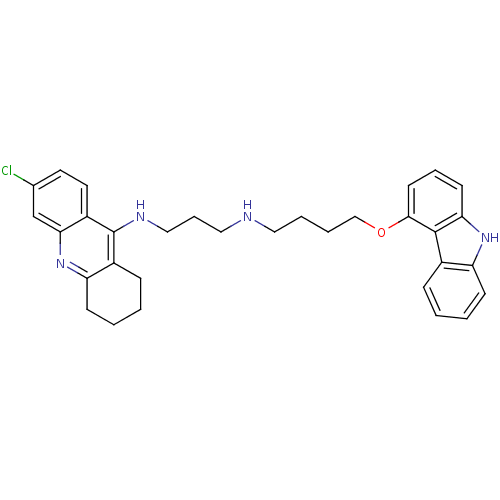 (CHEMBL478667 | N-[4-(9H-Carbazol-4-yloxy)butyl]-N'...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.65 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by Ellman's method | J Med Chem 51: 4381-4 (2008) Article DOI: 10.1021/jm800577j BindingDB Entry DOI: 10.7270/Q21G0M23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50263213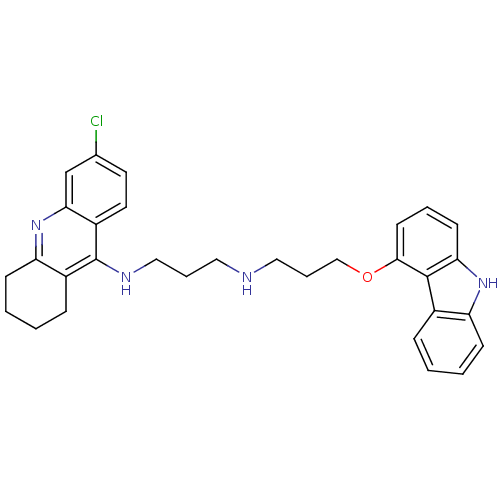 (CHEMBL478666 | N-[3-(9H-Carbazol-4-yloxy)propyl]-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.15 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by Ellman's method | J Med Chem 51: 4381-4 (2008) Article DOI: 10.1021/jm800577j BindingDB Entry DOI: 10.7270/Q21G0M23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50263262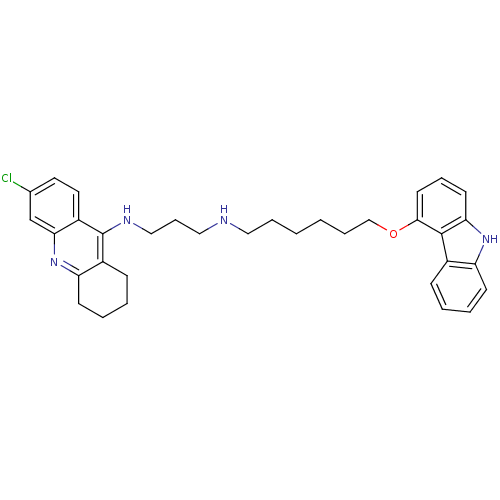 (CHEMBL474268 | N-[6-(9H-Carbazol-4-yloxy)hexyl]-N'...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.57 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by Ellman's method | J Med Chem 51: 4381-4 (2008) Article DOI: 10.1021/jm800577j BindingDB Entry DOI: 10.7270/Q21G0M23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10514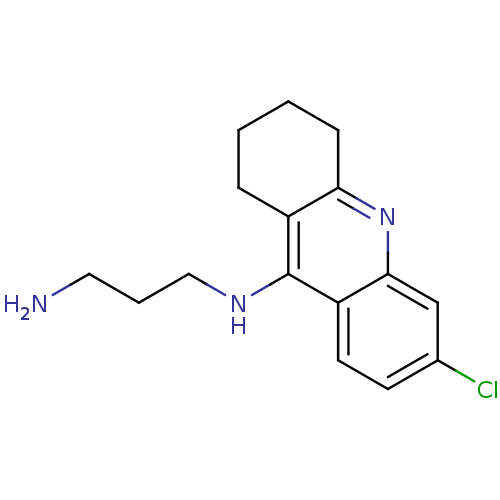 (CHEMBL191758 | N-(3-aminopropyl)-6-chloro-1,2,3,4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21.5 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by Ellman's method | J Med Chem 51: 4381-4 (2008) Article DOI: 10.1021/jm800577j BindingDB Entry DOI: 10.7270/Q21G0M23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 45.8 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BChE by Ellman's method | J Med Chem 51: 4381-4 (2008) Article DOI: 10.1021/jm800577j BindingDB Entry DOI: 10.7270/Q21G0M23 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50263262 (CHEMBL474268 | N-[6-(9H-Carbazol-4-yloxy)hexyl]-N'...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BChE by Ellman's method | J Med Chem 51: 4381-4 (2008) Article DOI: 10.1021/jm800577j BindingDB Entry DOI: 10.7270/Q21G0M23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50263215 (CHEMBL473866 | N-[5-(9H-Carbazol-4-yloxy)pentyl]-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BChE by Ellman's method | J Med Chem 51: 4381-4 (2008) Article DOI: 10.1021/jm800577j BindingDB Entry DOI: 10.7270/Q21G0M23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50263214 (CHEMBL478667 | N-[4-(9H-Carbazol-4-yloxy)butyl]-N'...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 211 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BChE by Ellman's method | J Med Chem 51: 4381-4 (2008) Article DOI: 10.1021/jm800577j BindingDB Entry DOI: 10.7270/Q21G0M23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50263213 (CHEMBL478666 | N-[3-(9H-Carbazol-4-yloxy)propyl]-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 296 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BChE by Ellman's method | J Med Chem 51: 4381-4 (2008) Article DOI: 10.1021/jm800577j BindingDB Entry DOI: 10.7270/Q21G0M23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 424 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by Ellman's method | J Med Chem 51: 4381-4 (2008) Article DOI: 10.1021/jm800577j BindingDB Entry DOI: 10.7270/Q21G0M23 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50263213 (CHEMBL478666 | N-[3-(9H-Carbazol-4-yloxy)propyl]-N...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna Curated by ChEMBL | Assay Description Inhibition of NMDA NR1/NR2B receptor (unknown origin) expressed in xenopus oocytes assessed as inhibition of NMDA and glycine-induced current respons... | J Med Chem 51: 4381-4 (2008) Article DOI: 10.1021/jm800577j BindingDB Entry DOI: 10.7270/Q21G0M23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10514 (CHEMBL191758 | N-(3-aminopropyl)-6-chloro-1,2,3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BChE by Ellman's method | J Med Chem 51: 4381-4 (2008) Article DOI: 10.1021/jm800577j BindingDB Entry DOI: 10.7270/Q21G0M23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50062599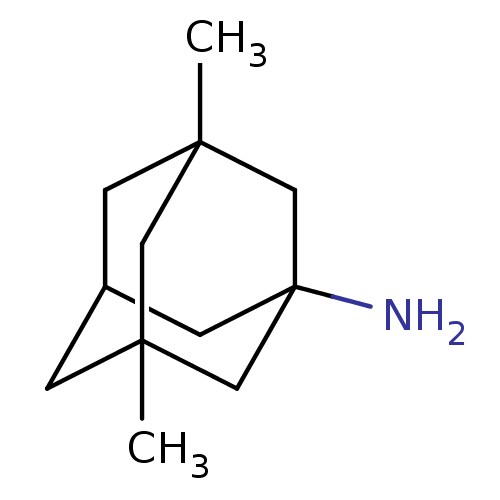 (3,5-Dimethyl-adamantan-1-ylamine | CHEMBL807 | EN3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 9.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna Curated by ChEMBL | Assay Description Inhibition of NMDA NR1/NR2B receptor (unknown origin) expressed in xenopus oocytes assessed as inhibition of NMDA and glycine-induced current respons... | J Med Chem 51: 4381-4 (2008) Article DOI: 10.1021/jm800577j BindingDB Entry DOI: 10.7270/Q21G0M23 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50263262 (CHEMBL474268 | N-[6-(9H-Carbazol-4-yloxy)hexyl]-N'...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna Curated by ChEMBL | Assay Description Inhibition of NMDA NR1/NR2B receptor (unknown origin) expressed in xenopus oocytes assessed as inhibition of NMDA and glycine-induced current respons... | J Med Chem 51: 4381-4 (2008) Article DOI: 10.1021/jm800577j BindingDB Entry DOI: 10.7270/Q21G0M23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50263215 (CHEMBL473866 | N-[5-(9H-Carbazol-4-yloxy)pentyl]-N...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna Curated by ChEMBL | Assay Description Inhibition of NMDA NR1/NR2B receptor (unknown origin) expressed in xenopus oocytes assessed as inhibition of NMDA and glycine-induced current respons... | J Med Chem 51: 4381-4 (2008) Article DOI: 10.1021/jm800577j BindingDB Entry DOI: 10.7270/Q21G0M23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50263214 (CHEMBL478667 | N-[4-(9H-Carbazol-4-yloxy)butyl]-N'...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UniVersity of Bologna Curated by ChEMBL | Assay Description Inhibition of NMDA NR1/NR2B receptor (unknown origin) expressed in xenopus oocytes assessed as inhibition of NMDA and glycine-induced current respons... | J Med Chem 51: 4381-4 (2008) Article DOI: 10.1021/jm800577j BindingDB Entry DOI: 10.7270/Q21G0M23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||