 Found 36 hits of Enzyme Inhibition Constant Data
Found 36 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50261842
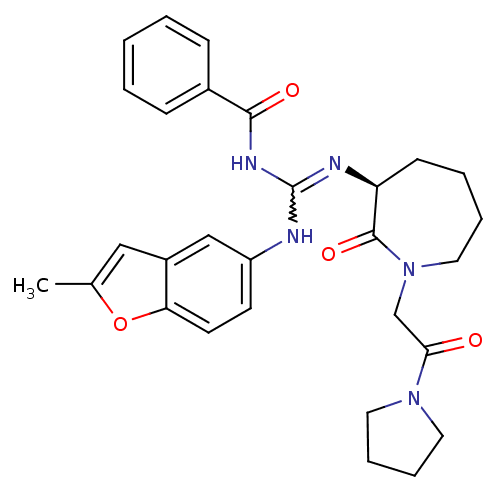
((S)-N-((2-methylbenzofuran-5-ylamino)(2-oxo-1-(2-o...)Show SMILES Cc1cc2cc(NC(NC(=O)c3ccccc3)=N[C@H]3CCCCN(CC(=O)N4CCCC4)C3=O)ccc2o1 |r,w:7.6| Show InChI InChI=1S/C29H33N5O4/c1-20-17-22-18-23(12-13-25(22)38-20)30-29(32-27(36)21-9-3-2-4-10-21)31-24-11-5-6-16-34(28(24)37)19-26(35)33-14-7-8-15-33/h2-4,9-10,12-13,17-18,24H,5-8,11,14-16,19H2,1H3,(H2,30,31,32,36)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50262351
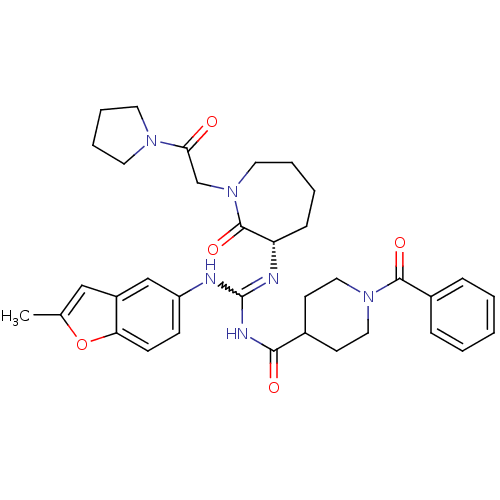
((S)-1-benzoyl-N-((2-methylbenzofuran-5-ylamino)(2-...)Show SMILES Cc1cc2cc(NC(NC(=O)C3CCN(CC3)C(=O)c3ccccc3)=N[C@H]3CCCCN(CC(=O)N4CCCC4)C3=O)ccc2o1 |r,w:7.6| Show InChI InChI=1S/C35H42N6O5/c1-24-21-27-22-28(12-13-30(27)46-24)36-35(37-29-11-5-6-18-41(34(29)45)23-31(42)39-16-7-8-17-39)38-32(43)25-14-19-40(20-15-25)33(44)26-9-3-2-4-10-26/h2-4,9-10,12-13,21-22,25,29H,5-8,11,14-20,23H2,1H3,(H2,36,37,38,43)/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50261852

((S)-N-((2-methylbenzofuran-5-ylamino)(2-oxo-1-(2-o...)Show SMILES Cc1cc2cc(NC(NC(=O)c3ccno3)=N[C@H]3CCCCN(CC(=O)N4CCCC4)C3=O)ccc2o1 |r,w:7.6| Show InChI InChI=1S/C26H30N6O5/c1-17-14-18-15-19(7-8-21(18)36-17)28-26(30-24(34)22-9-10-27-37-22)29-20-6-2-3-13-32(25(20)35)16-23(33)31-11-4-5-12-31/h7-10,14-15,20H,2-6,11-13,16H2,1H3,(H2,28,29,30,34)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50262348

((S)-2,4-dimethyl-N-((2-methylbenzofuran-5-ylamino)...)Show SMILES Cc1cc2cc(NC(NC(=O)c3sc(C)nc3C)=N[C@H]3CCCCN(CC(=O)N4CCCC4)C3=O)ccc2o1 |r,w:7.6| Show InChI InChI=1S/C28H34N6O4S/c1-17-14-20-15-21(9-10-23(20)38-17)30-28(32-26(36)25-18(2)29-19(3)39-25)31-22-8-4-5-13-34(27(22)37)16-24(35)33-11-6-7-12-33/h9-10,14-15,22H,4-8,11-13,16H2,1-3H3,(H2,30,31,32,36)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50261846

((S)-3-methoxy-N-((4-methoxyphenylamino)(2-oxo-1-(2...)Show SMILES COc1ccc(NC(NC(=O)c2cccc(OC)c2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)cc1 |r,w:7.6| Show InChI InChI=1S/C28H35N5O5/c1-37-22-13-11-21(12-14-22)29-28(31-26(35)20-8-7-9-23(18-20)38-2)30-24-10-3-4-17-33(27(24)36)19-25(34)32-15-5-6-16-32/h7-9,11-14,18,24H,3-6,10,15-17,19H2,1-2H3,(H2,29,30,31,35)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 193 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50262230

((S)-N-((m-toluidino)(2-oxo-1-(2-oxo-2-(pyrrolidin-...)Show SMILES Cc1cccc(NC(NC(=O)c2ccc(Cl)cc2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)c1 |r,w:7.6| Show InChI InChI=1S/C27H32ClN5O3/c1-19-7-6-8-22(17-19)29-27(31-25(35)20-10-12-21(28)13-11-20)30-23-9-2-3-16-33(26(23)36)18-24(34)32-14-4-5-15-32/h6-8,10-13,17,23H,2-5,9,14-16,18H2,1H3,(H2,29,30,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 198 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50261792
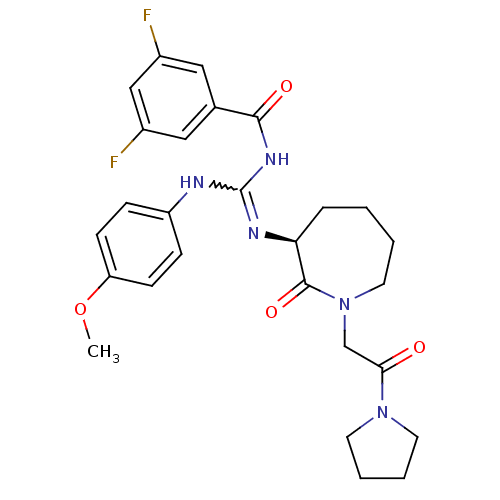
((S)-3,5-difluoro-N-((4-methoxyphenylamino)(2-oxo-1...)Show SMILES COc1ccc(NC(NC(=O)c2cc(F)cc(F)c2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)cc1 |r,w:7.6| Show InChI InChI=1S/C27H31F2N5O4/c1-38-22-9-7-21(8-10-22)30-27(32-25(36)18-14-19(28)16-20(29)15-18)31-23-6-2-3-13-34(26(23)37)17-24(35)33-11-4-5-12-33/h7-10,14-16,23H,2-6,11-13,17H2,1H3,(H2,30,31,32,36)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 208 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50262284

((S)-N-((m-toluidino)(2-oxo-1-(2-oxo-2-(pyrrolidin-...)Show SMILES Cc1cccc(NC(NC(=O)c2ccc(F)cc2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)c1 |r,w:7.6| Show InChI InChI=1S/C27H32FN5O3/c1-19-7-6-8-22(17-19)29-27(31-25(35)20-10-12-21(28)13-11-20)30-23-9-2-3-16-33(26(23)36)18-24(34)32-14-4-5-15-32/h6-8,10-13,17,23H,2-5,9,14-16,18H2,1H3,(H2,29,30,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 222 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50261847
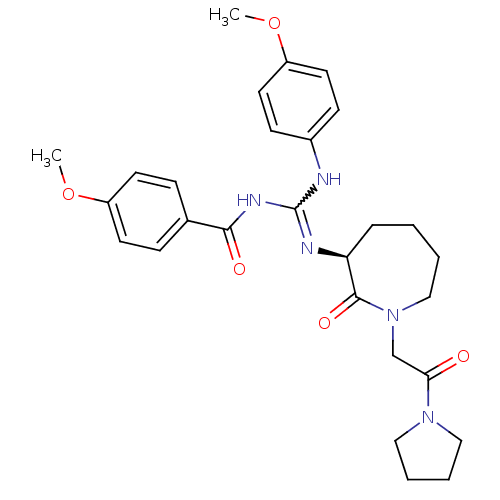
((S)-4-methoxy-N-((4-methoxyphenylamino)(2-oxo-1-(2...)Show SMILES COc1ccc(NC(NC(=O)c2ccc(OC)cc2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)cc1 |r,w:7.6| Show InChI InChI=1S/C28H35N5O5/c1-37-22-12-8-20(9-13-22)26(35)31-28(29-21-10-14-23(38-2)15-11-21)30-24-7-3-4-18-33(27(24)36)19-25(34)32-16-5-6-17-32/h8-15,24H,3-7,16-19H2,1-2H3,(H2,29,30,31,35)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 227 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50261843
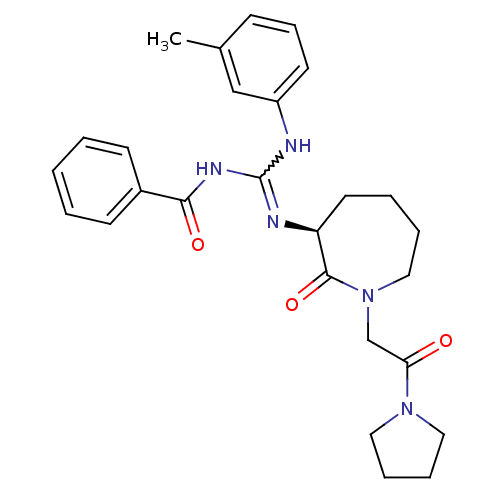
((S)-N-((m-toluidino)(2-oxo-1-(2-oxo-2-(pyrrolidin-...)Show SMILES Cc1cccc(NC(NC(=O)c2ccccc2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)c1 |r,w:7.6| Show InChI InChI=1S/C27H33N5O3/c1-20-10-9-13-22(18-20)28-27(30-25(34)21-11-3-2-4-12-21)29-23-14-5-6-17-32(26(23)35)19-24(33)31-15-7-8-16-31/h2-4,9-13,18,23H,5-8,14-17,19H2,1H3,(H2,28,29,30,34)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50261790

((S)-N-((4-methoxyphenylamino)(2-oxo-1-(2-oxo-2-(py...)Show SMILES COc1ccc(NC(NC(=O)c2ccccc2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)cc1 |r,w:7.6| Show InChI InChI=1S/C27H33N5O4/c1-36-22-14-12-21(13-15-22)28-27(30-25(34)20-9-3-2-4-10-20)29-23-11-5-6-18-32(26(23)35)19-24(33)31-16-7-8-17-31/h2-4,9-10,12-15,23H,5-8,11,16-19H2,1H3,(H2,28,29,30,34)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50262343

((S)-3-fluoro-N-((4-methoxyphenylamino)(2-oxo-1-(2-...)Show SMILES COc1ccc(NC(NC(=O)c2cccc(F)c2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)cc1 |r,w:7.6| Show InChI InChI=1S/C27H32FN5O4/c1-37-22-12-10-21(11-13-22)29-27(31-25(35)19-7-6-8-20(28)17-19)30-23-9-2-3-16-33(26(23)36)18-24(34)32-14-4-5-15-32/h6-8,10-13,17,23H,2-5,9,14-16,18H2,1H3,(H2,29,30,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 256 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50262287
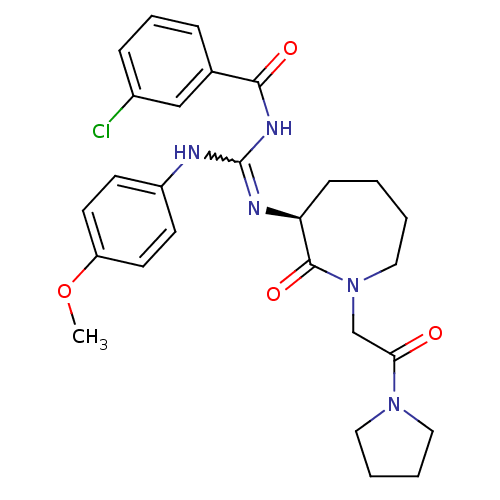
((S)-3-chloro-N-((4-methoxyphenylamino)(2-oxo-1-(2-...)Show SMILES COc1ccc(NC(NC(=O)c2cccc(Cl)c2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)cc1 |r,w:7.6| Show InChI InChI=1S/C27H32ClN5O4/c1-37-22-12-10-21(11-13-22)29-27(31-25(35)19-7-6-8-20(28)17-19)30-23-9-2-3-16-33(26(23)36)18-24(34)32-14-4-5-15-32/h6-8,10-13,17,23H,2-5,9,14-16,18H2,1H3,(H2,29,30,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 267 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50262285
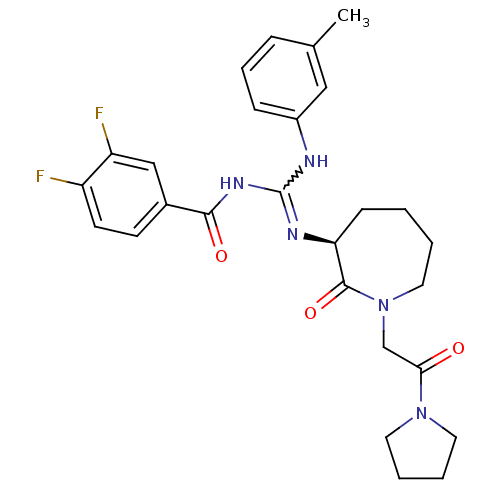
((S)-N-((m-toluidino)(2-oxo-1-(2-oxo-2-(pyrrolidin-...)Show SMILES Cc1cccc(NC(NC(=O)c2ccc(F)c(F)c2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)c1 |r,w:7.6| Show InChI InChI=1S/C27H31F2N5O3/c1-18-7-6-8-20(15-18)30-27(32-25(36)19-10-11-21(28)22(29)16-19)31-23-9-2-3-14-34(26(23)37)17-24(35)33-12-4-5-13-33/h6-8,10-11,15-16,23H,2-5,9,12-14,17H2,1H3,(H2,30,31,32,36)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 271 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50262345
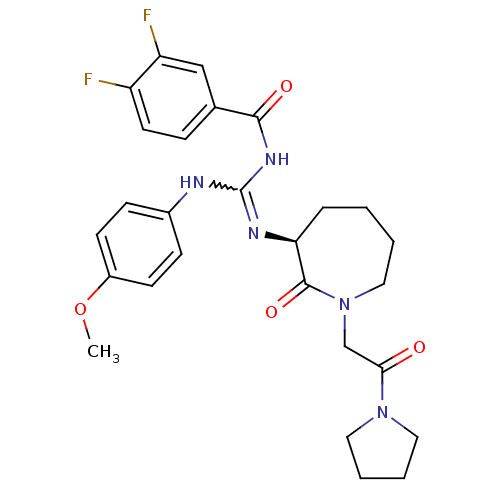
((S)-3,4-difluoro-N-((4-methoxyphenylamino)(2-oxo-1...)Show SMILES COc1ccc(NC(NC(=O)c2ccc(F)c(F)c2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)cc1 |r,w:7.6| Show InChI InChI=1S/C27H31F2N5O4/c1-38-20-10-8-19(9-11-20)30-27(32-25(36)18-7-12-21(28)22(29)16-18)31-23-6-2-3-15-34(26(23)37)17-24(35)33-13-4-5-14-33/h7-12,16,23H,2-6,13-15,17H2,1H3,(H2,30,31,32,36)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 272 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50261844

((S)-N-((m-toluidino)(2-oxo-1-(2-oxo-2-(pyrrolidin-...)Show SMILES COc1cccc(c1)C(=O)NC(Nc1cccc(C)c1)=N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:11.12| Show InChI InChI=1S/C28H35N5O4/c1-20-9-7-11-22(17-20)29-28(31-26(35)21-10-8-12-23(18-21)37-2)30-24-13-3-4-16-33(27(24)36)19-25(34)32-14-5-6-15-32/h7-12,17-18,24H,3-6,13-16,19H2,1-2H3,(H2,29,30,31,35)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 276 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50262288
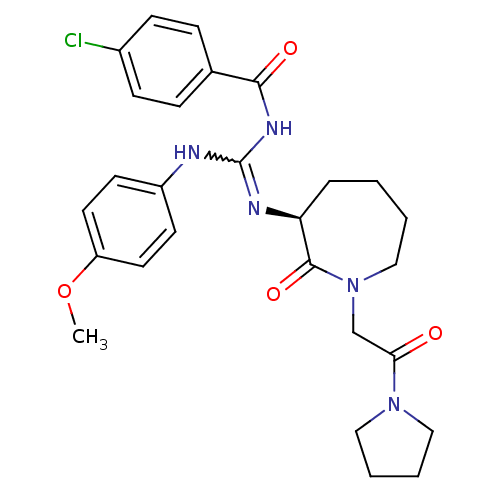
((S)-4-chloro-N-((4-methoxyphenylamino)(2-oxo-1-(2-...)Show SMILES COc1ccc(NC(NC(=O)c2ccc(Cl)cc2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)cc1 |r,w:7.6| Show InChI InChI=1S/C27H32ClN5O4/c1-37-22-13-11-21(12-14-22)29-27(31-25(35)19-7-9-20(28)10-8-19)30-23-6-2-3-17-33(26(23)36)18-24(34)32-15-4-5-16-32/h7-14,23H,2-6,15-18H2,1H3,(H2,29,30,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 294 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50262344
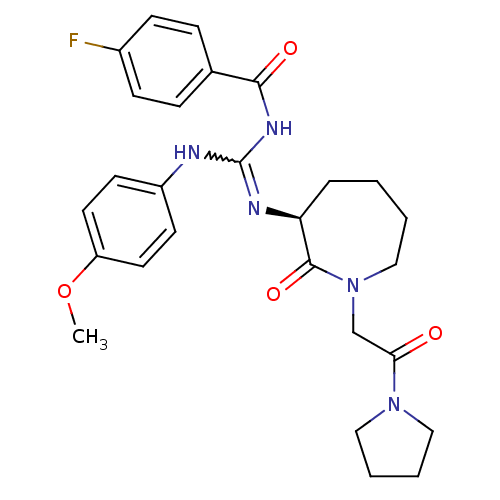
((S)-4-fluoro-N-((4-methoxyphenylamino)(2-oxo-1-(2-...)Show SMILES COc1ccc(NC(NC(=O)c2ccc(F)cc2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)cc1 |r,w:7.6| Show InChI InChI=1S/C27H32FN5O4/c1-37-22-13-11-21(12-14-22)29-27(31-25(35)19-7-9-20(28)10-8-19)30-23-6-2-3-17-33(26(23)36)18-24(34)32-15-4-5-16-32/h7-14,23H,2-6,15-18H2,1H3,(H2,29,30,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 368 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50262229

((S)-N-((m-toluidino)(2-oxo-1-(2-oxo-2-(pyrrolidin-...)Show SMILES Cc1cccc(NC(NC(=O)c2cccc(Cl)c2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)c1 |r,w:7.6| Show InChI InChI=1S/C27H32ClN5O3/c1-19-8-6-11-22(16-19)29-27(31-25(35)20-9-7-10-21(28)17-20)30-23-12-2-3-15-33(26(23)36)18-24(34)32-13-4-5-14-32/h6-11,16-17,23H,2-5,12-15,18H2,1H3,(H2,29,30,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 371 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50262283
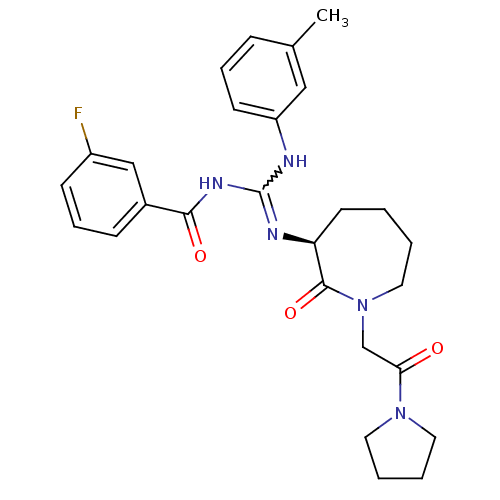
((S)-N-((m-toluidino)(2-oxo-1-(2-oxo-2-(pyrrolidin-...)Show SMILES Cc1cccc(NC(NC(=O)c2cccc(F)c2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)c1 |r,w:7.6| Show InChI InChI=1S/C27H32FN5O3/c1-19-8-6-11-22(16-19)29-27(31-25(35)20-9-7-10-21(28)17-20)30-23-12-2-3-15-33(26(23)36)18-24(34)32-13-4-5-14-32/h6-11,16-17,23H,2-5,12-15,18H2,1H3,(H2,29,30,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 373 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50261851
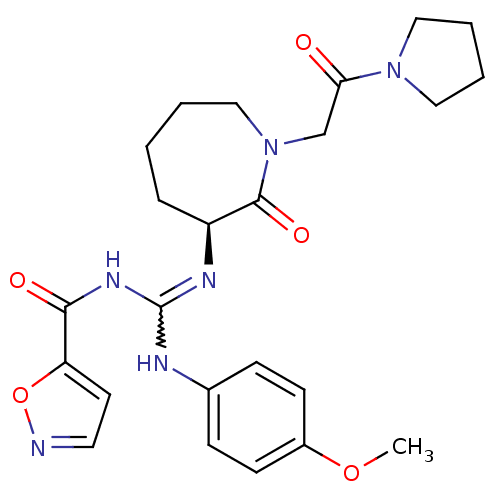
((S)-N-((4-methoxyphenylamino)(2-oxo-1-(2-oxo-2-(py...)Show SMILES COc1ccc(NC(NC(=O)c2ccno2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)cc1 |r,w:7.6| Show InChI InChI=1S/C24H30N6O5/c1-34-18-9-7-17(8-10-18)26-24(28-22(32)20-11-12-25-35-20)27-19-6-2-3-15-30(23(19)33)16-21(31)29-13-4-5-14-29/h7-12,19H,2-6,13-16H2,1H3,(H2,26,27,28,32)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50262347
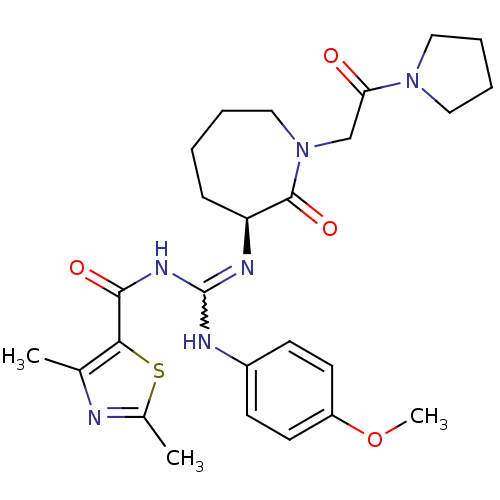
((S)-N-((4-methoxyphenylamino)(2-oxo-1-(2-oxo-2-(py...)Show SMILES COc1ccc(NC(NC(=O)c2sc(C)nc2C)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)cc1 |r,w:7.6| Show InChI InChI=1S/C26H34N6O4S/c1-17-23(37-18(2)27-17)24(34)30-26(28-19-9-11-20(36-3)12-10-19)29-21-8-4-5-15-32(25(21)35)16-22(33)31-13-6-7-14-31/h9-12,21H,4-8,13-16H2,1-3H3,(H2,28,29,30,34)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 386 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50262286
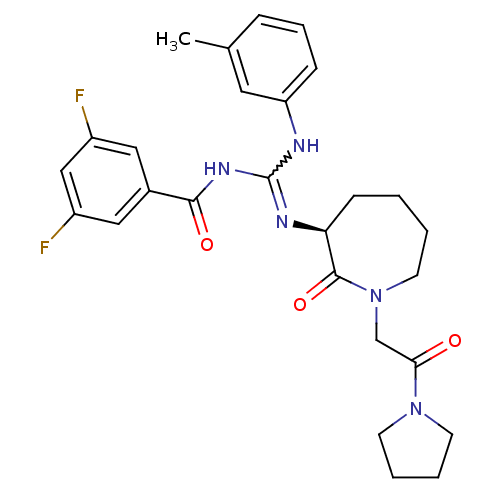
((S)-N-((m-toluidino)(2-oxo-1-(2-oxo-2-(pyrrolidin-...)Show SMILES Cc1cccc(NC(NC(=O)c2cc(F)cc(F)c2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)c1 |r,w:7.6| Show InChI InChI=1S/C27H31F2N5O3/c1-18-7-6-8-22(13-18)30-27(32-25(36)19-14-20(28)16-21(29)15-19)31-23-9-2-3-12-34(26(23)37)17-24(35)33-10-4-5-11-33/h6-8,13-16,23H,2-5,9-12,17H2,1H3,(H2,30,31,32,36)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 396 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50261788
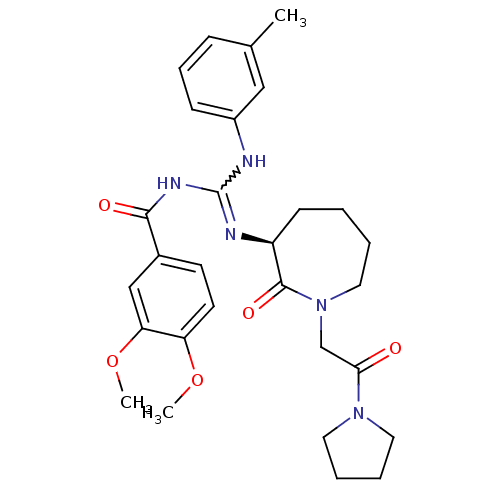
((S)-N-((m-toluidino)(2-oxo-1-(2-oxo-2-(pyrrolidin-...)Show SMILES COc1ccc(cc1OC)C(=O)NC(Nc1cccc(C)c1)=N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:13.14| Show InChI InChI=1S/C29H37N5O5/c1-20-9-8-10-22(17-20)30-29(32-27(36)21-12-13-24(38-2)25(18-21)39-3)31-23-11-4-5-16-34(28(23)37)19-26(35)33-14-6-7-15-33/h8-10,12-13,17-18,23H,4-7,11,14-16,19H2,1-3H3,(H2,30,31,32,36)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50261848
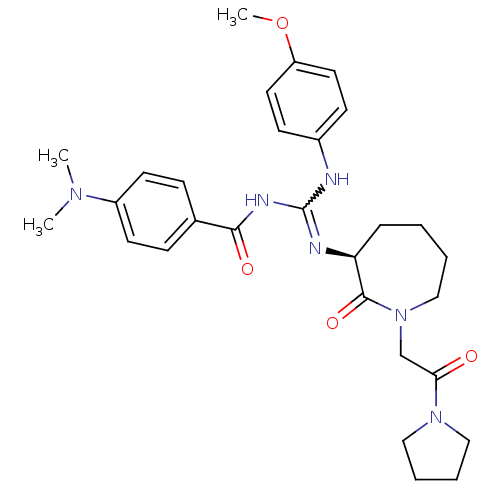
((S)-4-(dimethylamino)-N-((4-methoxyphenylamino)(2-...)Show SMILES COc1ccc(NC(NC(=O)c2ccc(cc2)N(C)C)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)cc1 |r,w:7.6| Show InChI InChI=1S/C29H38N6O4/c1-33(2)23-13-9-21(10-14-23)27(37)32-29(30-22-11-15-24(39-3)16-12-22)31-25-8-4-5-19-35(28(25)38)20-26(36)34-17-6-7-18-34/h9-16,25H,4-8,17-20H2,1-3H3,(H2,30,31,32,37)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 403 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50262346
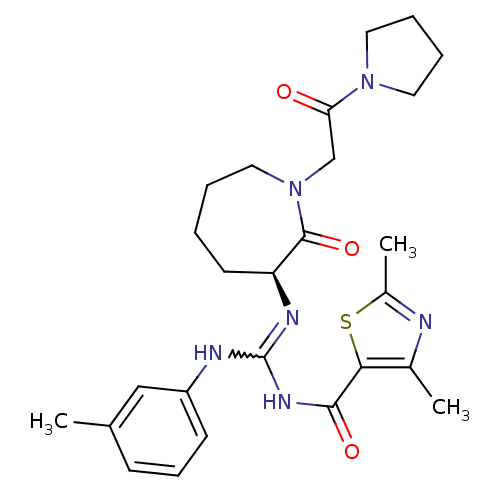
((S)-N-((m-toluidino)(2-oxo-1-(2-oxo-2-(pyrrolidin-...)Show SMILES Cc1nc(C)c(s1)C(=O)NC(Nc1cccc(C)c1)=N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C26H34N6O3S/c1-17-9-8-10-20(15-17)28-26(30-24(34)23-18(2)27-19(3)36-23)29-21-11-4-5-14-32(25(21)35)16-22(33)31-12-6-7-13-31/h8-10,15,21H,4-7,11-14,16H2,1-3H3,(H2,28,29,30,34)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 462 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50261793
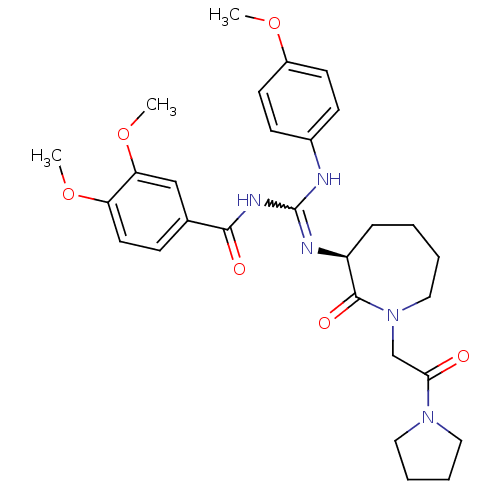
((S)-3,4-dimethoxy-N-((4-methoxyphenylamino)(2-oxo-...)Show SMILES COc1ccc(NC(NC(=O)c2ccc(OC)c(OC)c2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)cc1 |r,w:7.7| Show InChI InChI=1S/C29H37N5O6/c1-38-22-12-10-21(11-13-22)30-29(32-27(36)20-9-14-24(39-2)25(18-20)40-3)31-23-8-4-5-17-34(28(23)37)19-26(35)33-15-6-7-16-33/h9-14,18,23H,4-8,15-17,19H2,1-3H3,(H2,30,31,32,36)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 494 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50262350
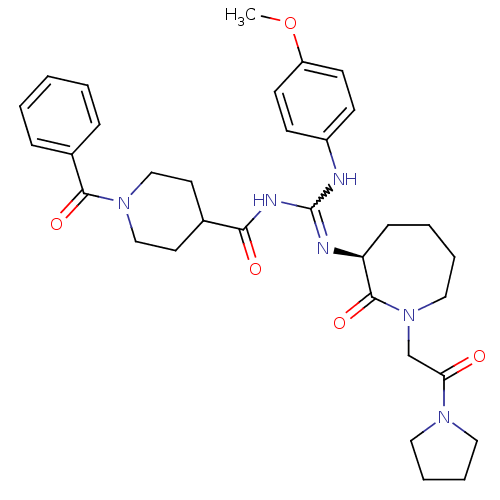
((S)-1-benzoyl-N-((4-methoxyphenylamino)(2-oxo-1-(2...)Show SMILES COc1ccc(NC(NC(=O)C2CCN(CC2)C(=O)c2ccccc2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)cc1 |r,w:7.6| Show InChI InChI=1S/C33H42N6O5/c1-44-27-14-12-26(13-15-27)34-33(35-28-11-5-6-20-39(32(28)43)23-29(40)37-18-7-8-19-37)36-30(41)24-16-21-38(22-17-24)31(42)25-9-3-2-4-10-25/h2-4,9-10,12-15,24,28H,5-8,11,16-23H2,1H3,(H2,34,35,36,41)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 506 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50262228
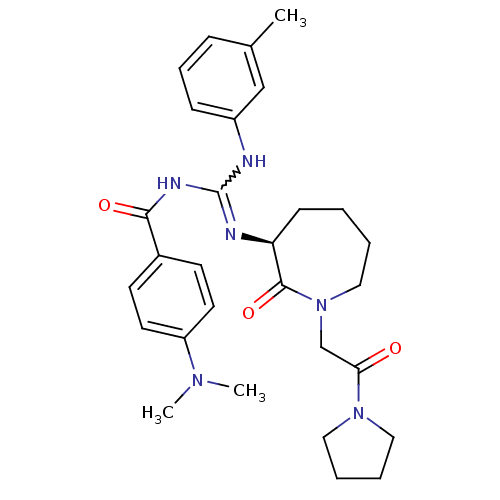
((S)-N-((m-toluidino)(2-oxo-1-(2-oxo-2-(pyrrolidin-...)Show SMILES CN(C)c1ccc(cc1)C(=O)NC(Nc1cccc(C)c1)=N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:12.13| Show InChI InChI=1S/C29H38N6O3/c1-21-9-8-10-23(19-21)30-29(32-27(37)22-12-14-24(15-13-22)33(2)3)31-25-11-4-5-18-35(28(25)38)20-26(36)34-16-6-7-17-34/h8-10,12-15,19,25H,4-7,11,16-18,20H2,1-3H3,(H2,30,31,32,37)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 517 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50262289

((S)-3,5-dichloro-N-((4-methoxyphenylamino)(2-oxo-1...)Show SMILES COc1ccc(NC(NC(=O)c2cc(Cl)cc(Cl)c2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)cc1 |r,w:7.6| Show InChI InChI=1S/C27H31Cl2N5O4/c1-38-22-9-7-21(8-10-22)30-27(32-25(36)18-14-19(28)16-20(29)15-18)31-23-6-2-3-13-34(26(23)37)17-24(35)33-11-4-5-12-33/h7-10,14-16,23H,2-6,11-13,17H2,1H3,(H2,30,31,32,36)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 546 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50261850
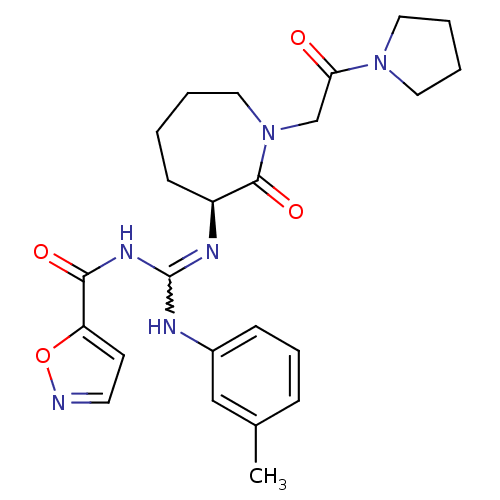
((S)-N-((m-toluidino)(2-oxo-1-(2-oxo-2-(pyrrolidin-...)Show SMILES Cc1cccc(NC(NC(=O)c2ccno2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)c1 |r,w:7.6| Show InChI InChI=1S/C24H30N6O4/c1-17-7-6-8-18(15-17)26-24(28-22(32)20-10-11-25-34-20)27-19-9-2-3-14-30(23(19)33)16-21(31)29-12-4-5-13-29/h6-8,10-11,15,19H,2-5,9,12-14,16H2,1H3,(H2,26,27,28,32)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 628 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50261845

((S)-N-((m-toluidino)(2-oxo-1-(2-oxo-2-(pyrrolidin-...)Show SMILES COc1ccc(cc1)C(=O)NC(Nc1cccc(C)c1)=N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:11.12| Show InChI InChI=1S/C28H35N5O4/c1-20-8-7-9-22(18-20)29-28(31-26(35)21-11-13-23(37-2)14-12-21)30-24-10-3-4-17-33(27(24)36)19-25(34)32-15-5-6-16-32/h7-9,11-14,18,24H,3-6,10,15-17,19H2,1-2H3,(H2,29,30,31,35)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 709 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50262231

((S)-N-((m-toluidino)(2-oxo-1-(2-oxo-2-(pyrrolidin-...)Show SMILES Cc1cccc(NC(NC(=O)c2cc(Cl)cc(Cl)c2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)c1 |r,w:7.6| Show InChI InChI=1S/C27H31Cl2N5O3/c1-18-7-6-8-22(13-18)30-27(32-25(36)19-14-20(28)16-21(29)15-19)31-23-9-2-3-12-34(26(23)37)17-24(35)33-10-4-5-11-33/h6-8,13-16,23H,2-5,9-12,17H2,1H3,(H2,30,31,32,36)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 835 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50261849

((S)-3,5-dimethoxy-N-((4-methoxyphenylamino)(2-oxo-...)Show SMILES COc1ccc(NC(NC(=O)c2cc(OC)cc(OC)c2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)cc1 |r,w:7.7| Show InChI InChI=1S/C29H37N5O6/c1-38-22-11-9-21(10-12-22)30-29(32-27(36)20-16-23(39-2)18-24(17-20)40-3)31-25-8-4-5-15-34(28(25)37)19-26(35)33-13-6-7-14-33/h9-12,16-18,25H,4-8,13-15,19H2,1-3H3,(H2,30,31,32,36)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 872 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50262349
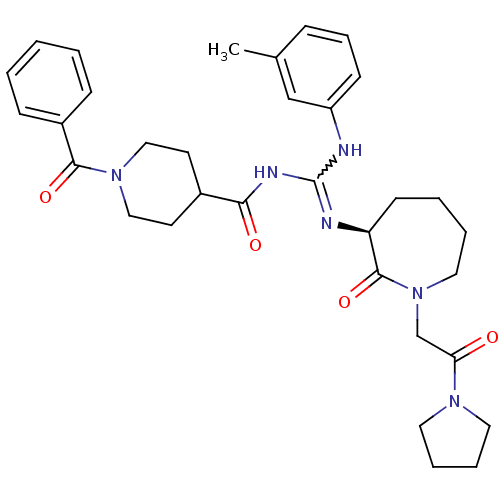
((S)-N-((m-toluidino)(2-oxo-1-(2-oxo-2-(pyrrolidin-...)Show SMILES Cc1cccc(NC(NC(=O)C2CCN(CC2)C(=O)c2ccccc2)=N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)c1 |r,w:7.6| Show InChI InChI=1S/C33H42N6O4/c1-24-10-9-13-27(22-24)34-33(35-28-14-5-6-19-39(32(28)43)23-29(40)37-17-7-8-18-37)36-30(41)25-15-20-38(21-16-25)31(42)26-11-3-2-4-12-26/h2-4,9-13,22,25,28H,5-8,14-21,23H2,1H3,(H2,34,35,36,41)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50261789

((S)-N-((m-toluidino)(2-oxo-1-(2-oxo-2-(pyrrolidin-...)Show SMILES COc1cc(OC)cc(c1)C(=O)NC(Nc1cccc(C)c1)=N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:13.14| Show InChI InChI=1S/C29H37N5O5/c1-20-9-8-10-22(15-20)30-29(32-27(36)21-16-23(38-2)18-24(17-21)39-3)31-25-11-4-5-14-34(28(25)37)19-26(35)33-12-6-7-13-33/h8-10,15-18,25H,4-7,11-14,19H2,1-3H3,(H2,30,31,32,36)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4696-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.004
BindingDB Entry DOI: 10.7270/Q2QV3MBT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data