

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264895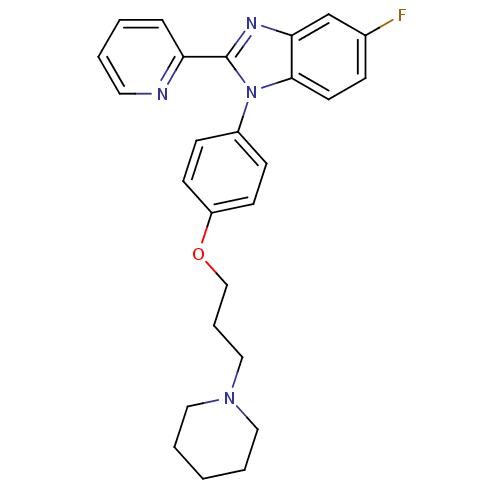 (5-fluoro-1-(4-(3-(piperidin-1-yl)propoxy)phenyl)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in HEK293 cells assessed as reversal of N-alpha-methylhistamine-induced inhibition of fo... | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264895 (5-fluoro-1-(4-(3-(piperidin-1-yl)propoxy)phenyl)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK cells by scintillation counting | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264934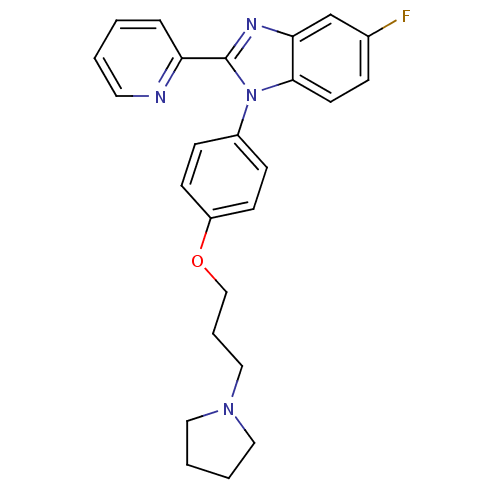 (5-fluoro-2-(pyridin-2-yl)-1-(4-(3-(pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK cells by scintillation counting | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50264895 (5-fluoro-1-(4-(3-(piperidin-1-yl)propoxy)phenyl)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity to mouse histamine H3 receptor | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50265014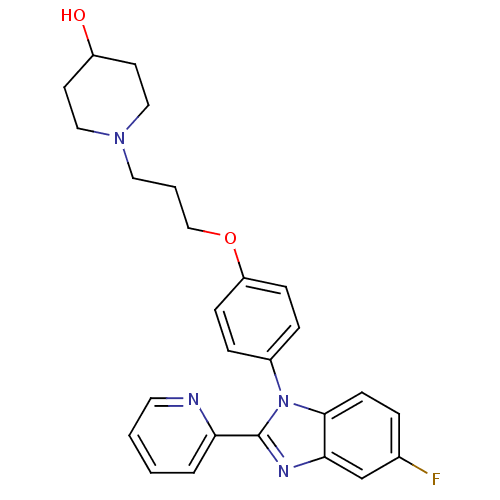 (1-(3-(4-(5-fluoro-2-(pyridin-2-yl)-1H-benzo[d]imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK cells by scintillation counting | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264932 (1-(4-(3-(4-benzylpiperidin-1-yl)propoxy)phenyl)-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK cells by scintillation counting | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50265094 (2-(ethylthio)-5-fluoro-1-(4-(3-(piperidin-1-yl)pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK cells by scintillation counting | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264897 (4-(3-(4-(5-fluoro-2-(pyridin-2-yl)-1H-benzo[d]imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK cells by scintillation counting | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264977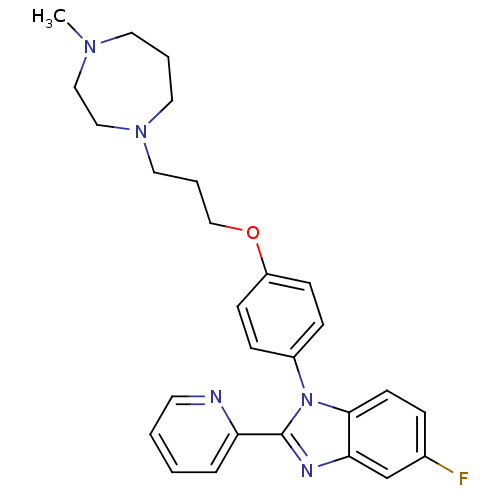 (5-fluoro-1-(4-(3-(4-methyl-1,4-diazepan-1-yl)propo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK cells by scintillation counting | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264974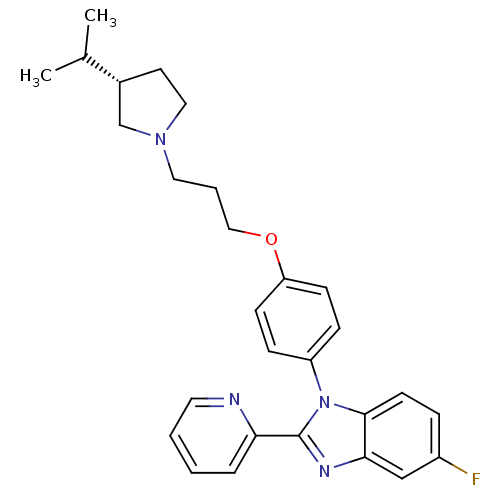 ((S)-5-fluoro-1-(4-(3-(3-isopropylpyrrolidin-1-yl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK cells by scintillation counting | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264935 (1-(4-(3-((2R,5R)-2,5-dimethylpyrrolidin-1-yl)propo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK cells by scintillation counting | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264976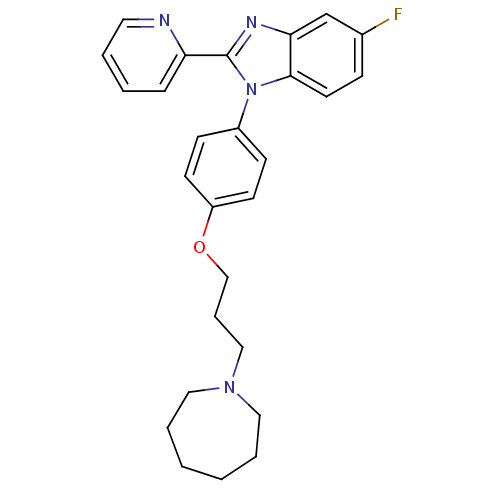 (1-(4-(3-(azepan-1-yl)propoxy)phenyl)-5-fluoro-2-(p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK cells by scintillation counting | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50265093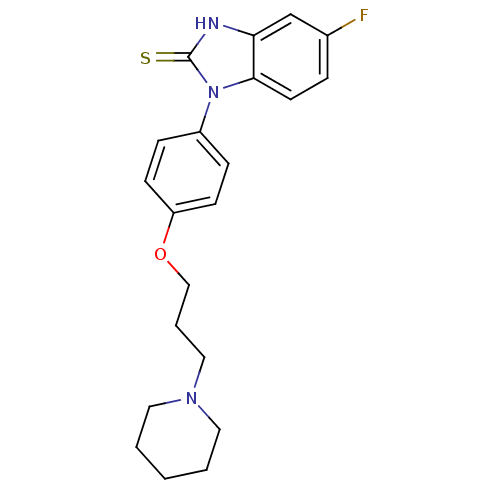 (5-fluoro-1-(4-(3-(piperidin-1-yl)propoxy)phenyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK cells by scintillation counting | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50265052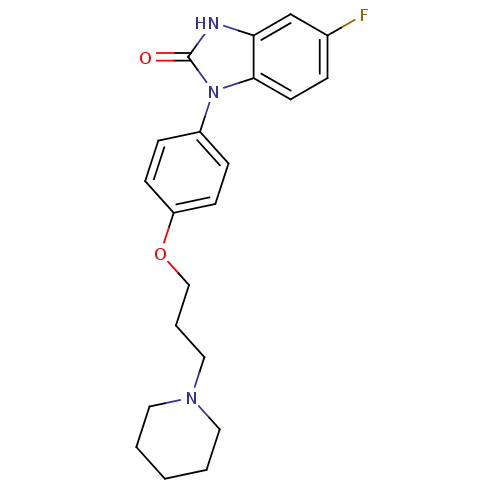 (5-fluoro-1-(4-(3-(piperidin-1-yl)propoxy)phenyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK cells by scintillation counting | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50265050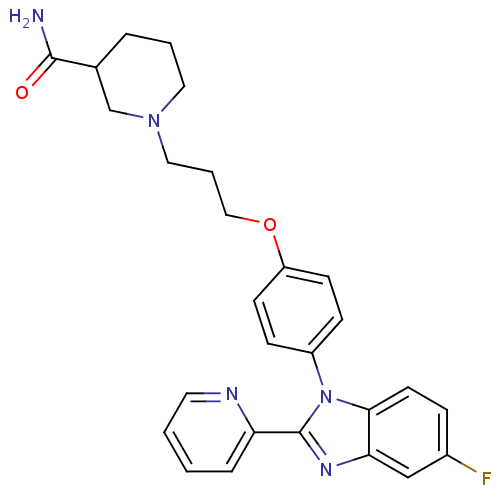 (1-(3-(4-(5-fluoro-2-(pyridin-2-yl)-1H-benzo[d]imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK cells by scintillation counting | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264896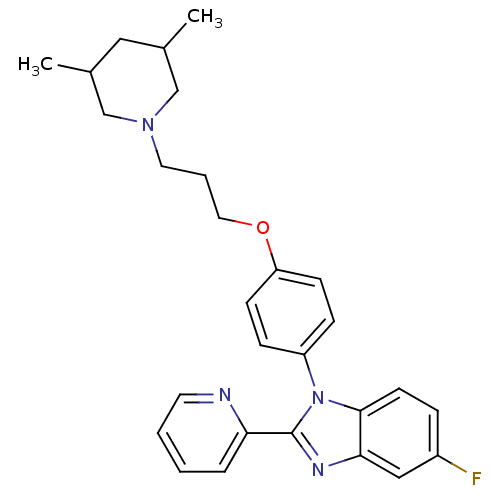 (1-(4-(3-(3,5-dimethylpiperidin-1-yl)propoxy)phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK cells by scintillation counting | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264933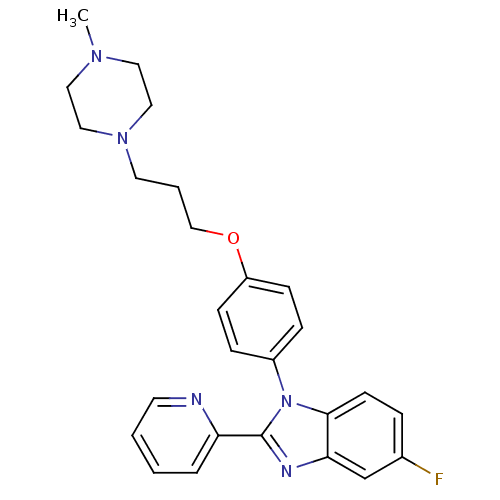 (5-fluoro-1-(4-(3-(4-methylpiperazin-1-yl)propoxy)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK cells by scintillation counting | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50265095 (2-ethoxy-5-fluoro-1-(4-(3-(piperidin-1-yl)propoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK cells by scintillation counting | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50265015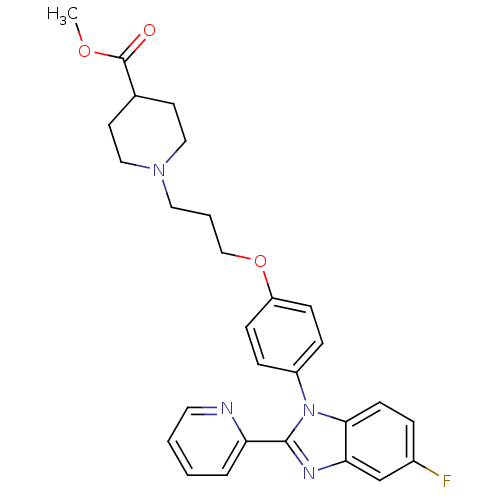 (CHEMBL497575 | methyl 1-(3-(4-(5-fluoro-2-(pyridin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK cells by scintillation counting | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50265014 (1-(3-(4-(5-fluoro-2-(pyridin-2-yl)-1H-benzo[d]imid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity to human ERG assessed as rubidium efflux at 5 ug/mL pre-equilibrated for 30 mins by DiBAC4(3)-based flame atomic absorbance spectros... | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264975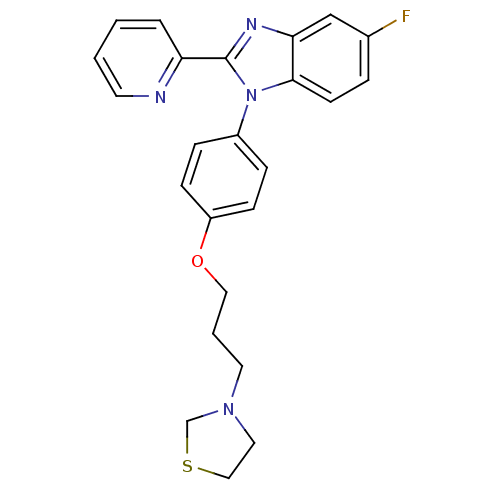 (3-(3-(4-(5-fluoro-2-(pyridin-2-yl)-1H-benzo[d]imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK cells by scintillation counting | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50265049 (1-(3-(4-(5-fluoro-2-(pyridin-2-yl)-1H-benzo[d]imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK cells by scintillation counting | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50264974 ((S)-5-fluoro-1-(4-(3-(3-isopropylpyrrolidin-1-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity to human ERG assessed as rubidium efflux at 5 ug/mL pre-equilibrated for 30 mins by DiBAC4(3)-based flame atomic absorbance spectros... | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50264975 (3-(3-(4-(5-fluoro-2-(pyridin-2-yl)-1H-benzo[d]imid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity to human ERG assessed as rubidium efflux at 5 ug/mL pre-equilibrated for 30 mins by DiBAC4(3)-based flame atomic absorbance spectros... | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50265015 (CHEMBL497575 | methyl 1-(3-(4-(5-fluoro-2-(pyridin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity to human ERG assessed as rubidium efflux at 5 ug/mL pre-equilibrated for 30 mins by DiBAC4(3)-based flame atomic absorbance spectros... | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50264897 (4-(3-(4-(5-fluoro-2-(pyridin-2-yl)-1H-benzo[d]imid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity to human ERG assessed as rubidium efflux at 5 ug/mL pre-equilibrated for 30 mins by DiBAC4(3)-based flame atomic absorbance spectros... | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50264895 (5-fluoro-1-(4-(3-(piperidin-1-yl)propoxy)phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity to human ERG assessed as rubidium efflux at 5 ug/mL pre-equilibrated for 30 mins by DiBAC4(3)-based flame atomic absorbance spectros... | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50265012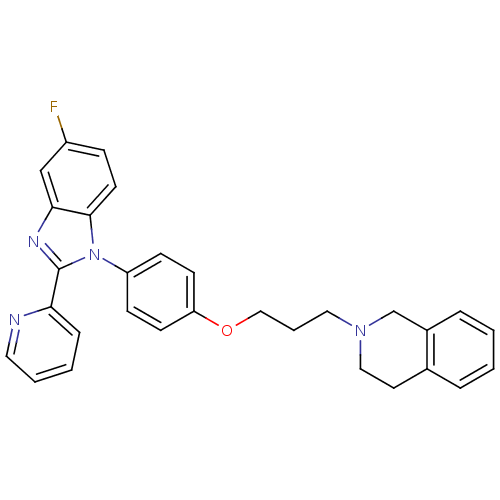 (2-(3-(4-(5-fluoro-2-(pyridin-2-yl)-1H-benzo[d]imid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity to human ERG assessed as rubidium efflux at 5 ug/mL pre-equilibrated for 30 mins by DiBAC4(3)-based flame atomic absorbance spectros... | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50265051 (3-(4-(5-fluoro-2-(pyridin-2-yl)-1H-benzo[d]imidazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK cells by scintillation counting | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50265012 (2-(3-(4-(5-fluoro-2-(pyridin-2-yl)-1H-benzo[d]imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK cells by scintillation counting | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50265013 (2-(3-(4-(5-fluoro-2-(pyridin-2-yl)-1H-benzo[d]imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 713 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in HEK cells by scintillation counting | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||