

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Homo sapiens (Human)) | BDBM50264406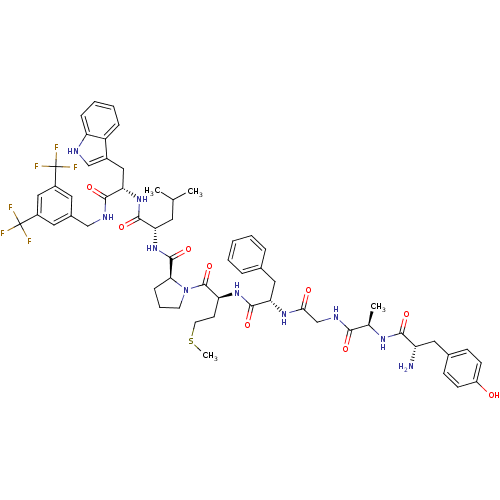 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM21021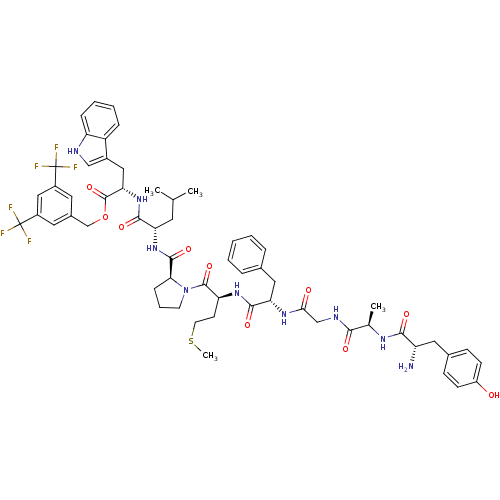 (Bifunctional Peptide Ligand, 5 (TY005) | CHEMBL389...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM21021 (Bifunctional Peptide Ligand, 5 (TY005) | CHEMBL389...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50264406 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human delta opioid receptor expressed in mouse HN9.10 cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM21016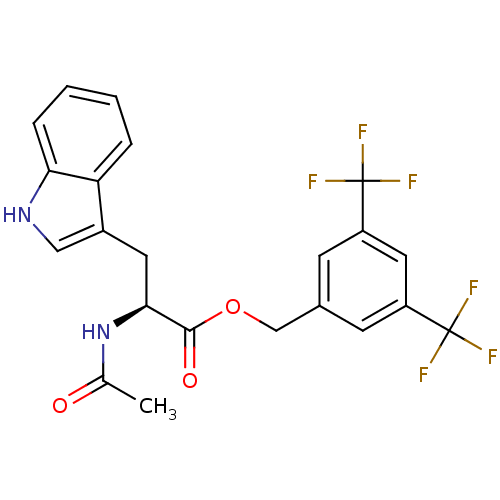 (CHEMBL22870 | L 732138 | L-732,138 | L732138 | N-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21014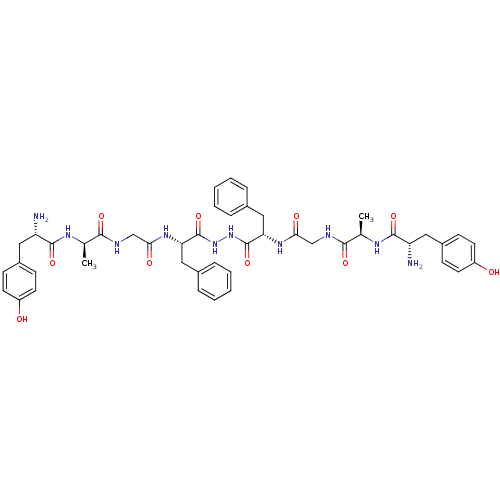 ((2S)-2-amino-N-[(1R)-1-[({[(1S)-1-{N'-[(2S)-2-{2-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in mouse HN9.10 cells by [35S]GTPgammaS binding assay | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21014 ((2S)-2-amino-N-[(1R)-1-[({[(1S)-1-{N'-[(2S)-2-{2-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human delta opioid receptor expressed in mouse HN9.10 cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50264407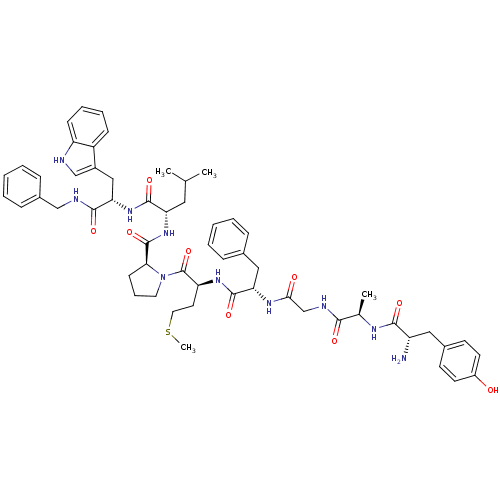 (CHEMBL448998 | Tyr-D-Ala-Gly-Phe-Met-Pro-Leu-Trp-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50264406 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM21016 (CHEMBL22870 | L 732138 | L-732,138 | L732138 | N-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50264407 (CHEMBL448998 | Tyr-D-Ala-Gly-Phe-Met-Pro-Leu-Trp-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50264406 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0126 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM21021 (Bifunctional Peptide Ligand, 5 (TY005) | CHEMBL389...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50264407 (CHEMBL448998 | Tyr-D-Ala-Gly-Phe-Met-Pro-Leu-Trp-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human delta opioid receptor expressed in mouse HN9.10 cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM21021 (Bifunctional Peptide Ligand, 5 (TY005) | CHEMBL389...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM21016 (CHEMBL22870 | L 732138 | L-732,138 | L732138 | N-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM21014 ((2S)-2-amino-N-[(1R)-1-[({[(1S)-1-{N'-[(2S)-2-{2-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor in ICR mouse vas deferens assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50264407 (CHEMBL448998 | Tyr-D-Ala-Gly-Phe-Met-Pro-Leu-Trp-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50264407 (CHEMBL448998 | Tyr-D-Ala-Gly-Phe-Met-Pro-Leu-Trp-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50264407 (CHEMBL448998 | Tyr-D-Ala-Gly-Phe-Met-Pro-Leu-Trp-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor in ICR mouse vas deferens assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21021 (Bifunctional Peptide Ligand, 5 (TY005) | CHEMBL389...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human delta opioid receptor expressed in mouse HN9.10 cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM21014 ((2S)-2-amino-N-[(1R)-1-[({[(1S)-1-{N'-[(2S)-2-{2-[...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in Hartley guinea pig ileum longitudinal muscle with myenteric plexus assessed as inhibition of electrically-s... | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50264406 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor in ICR mouse vas deferens assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM21021 (Bifunctional Peptide Ligand, 5 (TY005) | CHEMBL389...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor in ICR mouse vas deferens assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50264406 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50264406 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50264407 (CHEMBL448998 | Tyr-D-Ala-Gly-Phe-Met-Pro-Leu-Trp-N...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in Hartley guinea pig ileum longitudinal muscle with myenteric plexus assessed as inhibition of electrically-s... | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21021 (Bifunctional Peptide Ligand, 5 (TY005) | CHEMBL389...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM21021 (Bifunctional Peptide Ligand, 5 (TY005) | CHEMBL389...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in Hartley guinea pig ileum longitudinal muscle with myenteric plexus assessed as inhibition of electrically-s... | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM21016 (CHEMBL22870 | L 732138 | L-732,138 | L732138 | N-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50264406 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in Hartley guinea pig ileum longitudinal muscle with myenteric plexus assessed as inhibition of electrically-s... | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50264407 (CHEMBL448998 | Tyr-D-Ala-Gly-Phe-Met-Pro-Leu-Trp-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50264407 (CHEMBL448998 | Tyr-D-Ala-Gly-Phe-Met-Pro-Leu-Trp-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in mouse HN9.10 cells by [35S]GTPgammaS binding assay | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21021 (Bifunctional Peptide Ligand, 5 (TY005) | CHEMBL389...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in mouse HN9.10 cells by [35S]GTPgammaS binding assay | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21021 (Bifunctional Peptide Ligand, 5 (TY005) | CHEMBL389...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in mouse HN9.10 cells by [35S]GTPgammaS binding assay | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50264406 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at human delta opioid receptor expressed in mouse HN9.10 cells by [35S]GTPgammaS binding assay | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in mouse HN9.10 cells by [35S]GTPgammaS binding assay | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in mouse HN9.10 cells by [35S]GTPgammaS binding assay | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at human delta opioid receptor expressed in mouse HN9.10 cells by [35S]GTPgammaS binding assay | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50264406 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in mouse HN9.10 cells by [35S]GTPgammaS binding assay | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50264407 (CHEMBL448998 | Tyr-D-Ala-Gly-Phe-Met-Pro-Leu-Trp-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in mouse HN9.10 cells by [35S]GTPgammaS binding assay | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50264406 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in mouse HN9.10 cells by [35S]GTPgammaS binding assay | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21014 ((2S)-2-amino-N-[(1R)-1-[({[(1S)-1-{N'-[(2S)-2-{2-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at human delta opioid receptor expressed in mouse HN9.10 cells by [35S]GTPgammaS binding assay | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50264407 (CHEMBL448998 | Tyr-D-Ala-Gly-Phe-Met-Pro-Leu-Trp-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at human delta opioid receptor expressed in mouse HN9.10 cells by [35S]GTPgammaS binding assay | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21021 (Bifunctional Peptide Ligand, 5 (TY005) | CHEMBL389...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at human delta opioid receptor expressed in mouse HN9.10 cells by [35S]GTPgammaS binding assay | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||