 Found 40 hits of Enzyme Inhibition Constant Data
Found 40 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50275723

(2,7-bis(6-(ethyl(2-methoxybenzyl)amino)hexyl)benzo...)Show SMILES CCN(CCCCCCn1c(=O)c2ccc3c4c(ccc(c24)c1=O)c(=O)n(CCCCCCN(CC)Cc1ccccc1OC)c3=O)Cc1ccccc1OC Show InChI InChI=1S/C46H56N4O6/c1-5-47(31-33-19-11-13-21-39(33)55-3)27-15-7-9-17-29-49-43(51)35-23-25-37-42-38(26-24-36(41(35)42)44(49)52)46(54)50(45(37)53)30-18-10-8-16-28-48(6-2)32-34-20-12-14-22-40(34)56-4/h11-14,19-26H,5-10,15-18,27-32H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50275719

(1,4-bis(6-(ethyl(2-methoxybenzyl)amino)hexyl)quino...)Show SMILES CCN(CCCCCCn1c2ccccc2n(CCCCCCN(CC)Cc2ccccc2OC)c(=O)c1=O)Cc1ccccc1OC Show InChI InChI=1S/C40H56N4O4/c1-5-41(31-33-21-11-15-25-37(33)47-3)27-17-7-9-19-29-43-35-23-13-14-24-36(35)44(40(46)39(43)45)30-20-10-8-18-28-42(6-2)32-34-22-12-16-26-38(34)48-4/h11-16,21-26H,5-10,17-20,27-32H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.41 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50157597
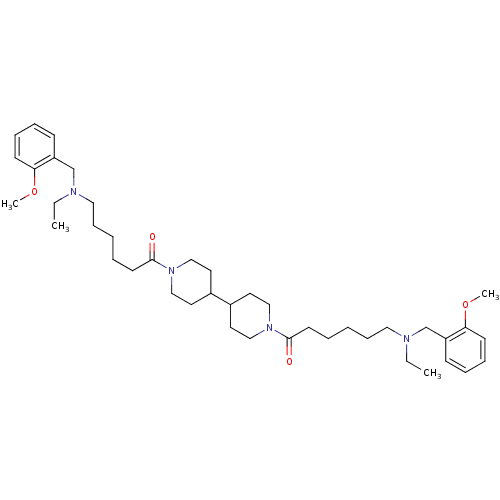
(6-[ethyl-(2-methoxy-benzyl)-amino]-1-(1'-{6-[ethyl...)Show SMILES CCN(CCCCCC(=O)N1CCC(CC1)C1CCN(CC1)C(=O)CCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC Show InChI InChI=1S/C42H66N4O4/c1-5-43(33-37-17-11-13-19-39(37)49-3)27-15-7-9-21-41(47)45-29-23-35(24-30-45)36-25-31-46(32-26-36)42(48)22-10-8-16-28-44(6-2)34-38-18-12-14-20-40(38)50-4/h11-14,17-20,35-36H,5-10,15-16,21-34H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50275716
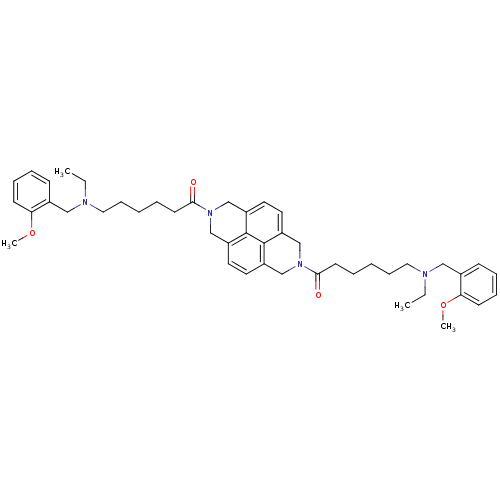
(1,1'-(benzo[lmn][3,8]phenanthroline-2,7(1H,3H,6H,8...)Show SMILES CCN(CCCCCC(=O)N1Cc2ccc3CN(Cc4ccc(C1)c2c34)C(=O)CCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC Show InChI InChI=1S/C46H60N4O4/c1-5-47(29-35-17-11-13-19-41(35)53-3)27-15-7-9-21-43(51)49-31-37-23-25-39-33-50(34-40-26-24-38(32-49)45(37)46(39)40)44(52)22-10-8-16-28-48(6-2)30-36-18-12-14-20-42(36)54-4/h11-14,17-20,23-26H,5-10,15-16,21-22,27-34H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.83 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50275722
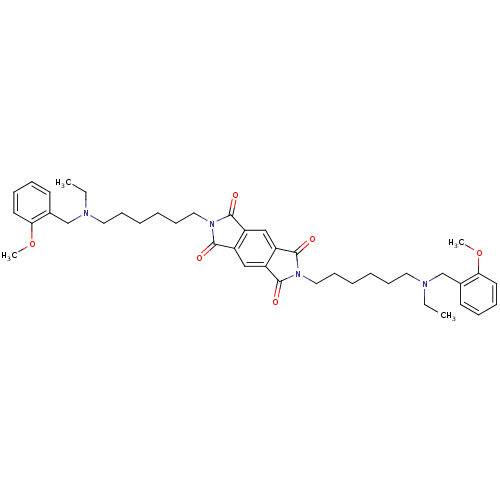
(2,6-Bis-{6-[ethyl-(2-methoxybenzyl)amino]hexyl}pyr...)Show SMILES CCN(CCCCCCn1c(=O)c2cc3c(cc2c1=O)c(=O)n(CCCCCCN(CC)Cc1ccccc1OC)c3=O)Cc1ccccc1OC Show InChI InChI=1S/C42H54N4O6/c1-5-43(29-31-19-11-13-21-37(31)51-3)23-15-7-9-17-25-45-39(47)33-27-35-36(28-34(33)40(45)48)42(50)46(41(35)49)26-18-10-8-16-24-44(6-2)30-32-20-12-14-22-38(32)52-4/h11-14,19-22,27-28H,5-10,15-18,23-26,29-30H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50275721
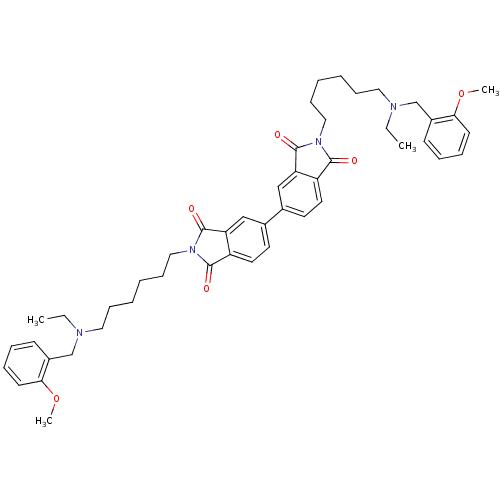
(2,2'-Bis-{6-[ethyl-(2-methoxybenzyl)amino]hexyl}-[...)Show SMILES CCN(CCCCCCN1C(=O)c2ccc(cc2C1=O)-c1ccc2C(=O)N(CCCCCCN(CC)Cc3ccccc3OC)C(=O)c2c1)Cc1ccccc1OC Show InChI InChI=1S/C48H58N4O6/c1-5-49(33-37-19-11-13-21-43(37)57-3)27-15-7-9-17-29-51-45(53)39-25-23-35(31-41(39)47(51)55)36-24-26-40-42(32-36)48(56)52(46(40)54)30-18-10-8-16-28-50(6-2)34-38-20-12-14-22-44(38)58-4/h11-14,19-26,31-32H,5-10,15-18,27-30,33-34H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50275718
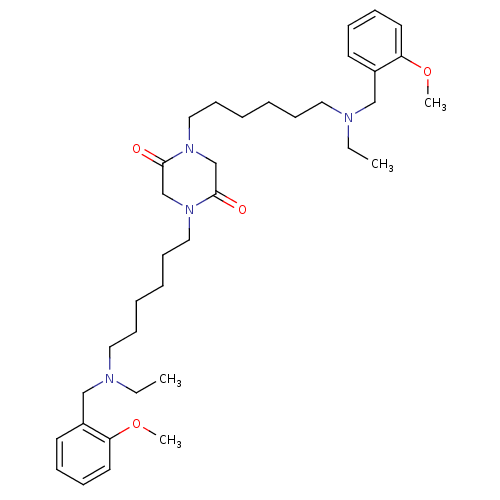
(1,4-Bis-{6-[ethyl-(2-methoxybenzyl)amino]hexyl}pip...)Show SMILES CCN(CCCCCCN1CC(=O)N(CCCCCCN(CC)Cc2ccccc2OC)CC1=O)Cc1ccccc1OC Show InChI InChI=1S/C36H56N4O4/c1-5-37(27-31-19-11-13-21-33(31)43-3)23-15-7-9-17-25-39-29-36(42)40(30-35(39)41)26-18-10-8-16-24-38(6-2)28-32-20-12-14-22-34(32)44-4/h11-14,19-22H,5-10,15-18,23-30H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15.9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50124570
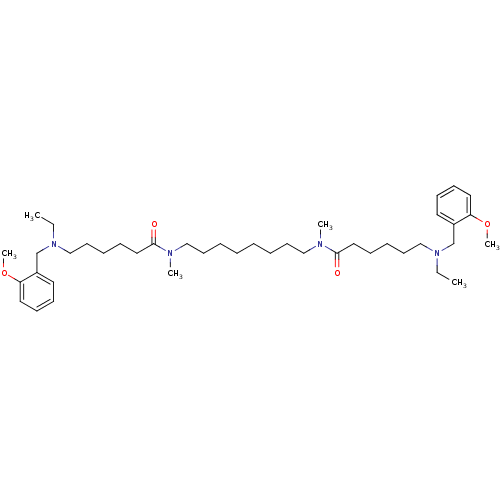
(6-[Ethyl-(2-methoxy-benzyl)-amino]-hexanoic acid [...)Show SMILES CCN(CCCCCC(=O)N(C)CCCCCCCCN(C)C(=O)CCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC Show InChI InChI=1S/C42H70N4O4/c1-7-45(35-37-25-17-19-27-39(37)49-5)33-23-13-15-29-41(47)43(3)31-21-11-9-10-12-22-32-44(4)42(48)30-16-14-24-34-46(8-2)36-38-26-18-20-28-40(38)50-6/h17-20,25-28H,7-16,21-24,29-36H2,1-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16.1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 23.1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50275712
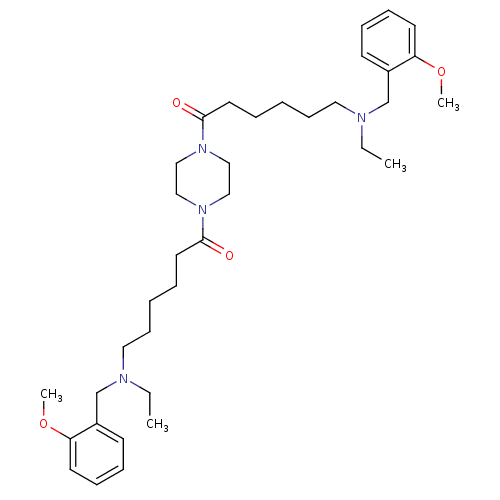
(6-[Ethyl-(2-methoxybenzyl)amino]-1-(4-{6-[ethyl-(2...)Show SMILES CCN(CCCCCC(=O)N1CCN(CC1)C(=O)CCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC Show InChI InChI=1S/C36H56N4O4/c1-5-37(29-31-17-11-13-19-33(31)43-3)23-15-7-9-21-35(41)39-25-27-40(28-26-39)36(42)22-10-8-16-24-38(6-2)30-32-18-12-14-20-34(32)44-4/h11-14,17-20H,5-10,15-16,21-30H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25.7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50275724

(2-{6-[Ethyl-(2-methoxybenzyl)amino]hexyl}benzo[de]...)Show SMILES CCN(CCCCCCN1C(=O)c2cccc3cccc(C1=O)c23)Cc1ccccc1OC Show InChI InChI=1S/C28H32N2O3/c1-3-29(20-22-12-6-7-17-25(22)33-2)18-8-4-5-9-19-30-27(31)23-15-10-13-21-14-11-16-24(26(21)23)28(30)32/h6-7,10-17H,3-5,8-9,18-20H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50275717
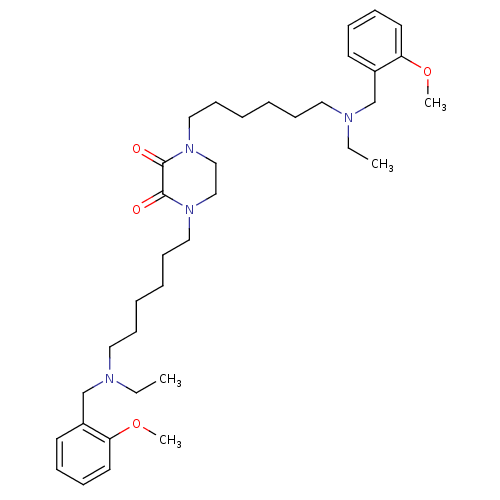
(1,4-Bis-{6-[ethyl-(2-methoxybenzyl)amino]hexyl}pip...)Show SMILES CCN(CCCCCCN1CCN(CCCCCCN(CC)Cc2ccccc2OC)C(=O)C1=O)Cc1ccccc1OC Show InChI InChI=1S/C36H56N4O4/c1-5-37(29-31-19-11-13-21-33(31)43-3)23-15-7-9-17-25-39-27-28-40(36(42)35(39)41)26-18-10-8-16-24-38(6-2)30-32-20-12-14-22-34(32)44-4/h11-14,19-22H,5-10,15-18,23-30H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68.9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50275720
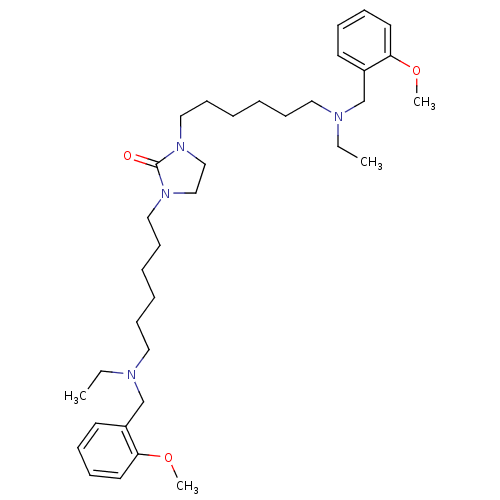
(1,3-Bis-{6-[ethyl-(2-methoxybenzyl)amino]hexyl}imi...)Show SMILES CCN(CCCCCCN1CCN(CCCCCCN(CC)Cc2ccccc2OC)C1=O)Cc1ccccc1OC Show InChI InChI=1S/C35H56N4O3/c1-5-36(29-31-19-11-13-21-33(31)41-3)23-15-7-9-17-25-38-27-28-39(35(38)40)26-18-10-8-16-24-37(6-2)30-32-20-12-14-22-34(32)42-4/h11-14,19-22H,5-10,15-18,23-30H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 72.4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50275719

(1,4-bis(6-(ethyl(2-methoxybenzyl)amino)hexyl)quino...)Show SMILES CCN(CCCCCCn1c2ccccc2n(CCCCCCN(CC)Cc2ccccc2OC)c(=O)c1=O)Cc1ccccc1OC Show InChI InChI=1S/C40H56N4O4/c1-5-41(31-33-21-11-15-25-37(33)47-3)27-17-7-9-19-29-43-35-23-13-14-24-36(35)44(40(46)39(43)45)30-20-10-8-18-28-42(6-2)32-34-22-12-16-26-38(34)48-4/h11-16,21-26H,5-10,17-20,27-32H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 95.8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50067482
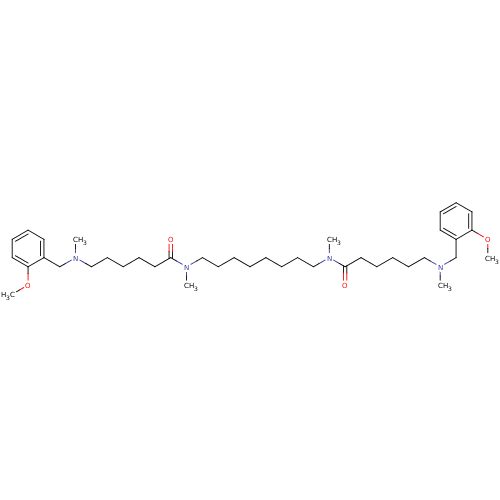
(6-[(2-Methoxy-benzyl)-methyl-amino]-hexanoic acid ...)Show SMILES COc1ccccc1CN(C)CCCCCC(=O)N(C)CCCCCCCCN(C)C(=O)CCCCCN(C)Cc1ccccc1OC Show InChI InChI=1S/C40H66N4O4/c1-41(33-35-23-15-17-25-37(35)47-5)29-19-11-13-27-39(45)43(3)31-21-9-7-8-10-22-32-44(4)40(46)28-14-12-20-30-42(2)34-36-24-16-18-26-38(36)48-6/h15-18,23-26H,7-14,19-22,27-34H2,1-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50275721

(2,2'-Bis-{6-[ethyl-(2-methoxybenzyl)amino]hexyl}-[...)Show SMILES CCN(CCCCCCN1C(=O)c2ccc(cc2C1=O)-c1ccc2C(=O)N(CCCCCCN(CC)Cc3ccccc3OC)C(=O)c2c1)Cc1ccccc1OC Show InChI InChI=1S/C48H58N4O6/c1-5-49(33-37-19-11-13-21-43(37)57-3)27-15-7-9-17-29-51-45(53)39-25-23-35(31-41(39)47(51)55)36-24-26-40-42(32-36)48(56)52(46(40)54)30-18-10-8-16-28-50(6-2)34-38-20-12-14-22-44(38)58-4/h11-14,19-26,31-32H,5-10,15-18,27-30,33-34H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 293 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50275724

(2-{6-[Ethyl-(2-methoxybenzyl)amino]hexyl}benzo[de]...)Show SMILES CCN(CCCCCCN1C(=O)c2cccc3cccc(C1=O)c23)Cc1ccccc1OC Show InChI InChI=1S/C28H32N2O3/c1-3-29(20-22-12-6-7-17-25(22)33-2)18-8-4-5-9-19-30-27(31)23-15-10-13-21-14-11-16-24(26(21)23)28(30)32/h6-7,10-17H,3-5,8-9,18-20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 416 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50275720

(1,3-Bis-{6-[ethyl-(2-methoxybenzyl)amino]hexyl}imi...)Show SMILES CCN(CCCCCCN1CCN(CCCCCCN(CC)Cc2ccccc2OC)C1=O)Cc1ccccc1OC Show InChI InChI=1S/C35H56N4O3/c1-5-36(29-31-19-11-13-21-33(31)41-3)23-15-7-9-17-25-38-27-28-39(35(38)40)26-18-10-8-16-24-37(6-2)30-32-20-12-14-22-34(32)42-4/h11-14,19-22H,5-10,15-18,23-30H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 876 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50275716

(1,1'-(benzo[lmn][3,8]phenanthroline-2,7(1H,3H,6H,8...)Show SMILES CCN(CCCCCC(=O)N1Cc2ccc3CN(Cc4ccc(C1)c2c34)C(=O)CCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC Show InChI InChI=1S/C46H60N4O4/c1-5-47(29-35-17-11-13-19-41(35)53-3)27-15-7-9-21-43(51)49-31-37-23-25-39-33-50(34-40-26-24-38(32-49)45(37)46(39)40)44(52)22-10-8-16-28-48(6-2)30-36-18-12-14-20-42(36)54-4/h11-14,17-20,23-26H,5-10,15-16,21-22,27-34H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50275723

(2,7-bis(6-(ethyl(2-methoxybenzyl)amino)hexyl)benzo...)Show SMILES CCN(CCCCCCn1c(=O)c2ccc3c4c(ccc(c24)c1=O)c(=O)n(CCCCCCN(CC)Cc1ccccc1OC)c3=O)Cc1ccccc1OC Show InChI InChI=1S/C46H56N4O6/c1-5-47(31-33-19-11-13-21-39(33)55-3)27-15-7-9-17-29-49-43(51)35-23-25-37-42-38(26-24-36(41(35)42)44(49)52)46(54)50(45(37)53)30-18-10-8-16-28-48(6-2)32-34-20-12-14-22-40(34)56-4/h11-14,19-26H,5-10,15-18,27-32H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50275718

(1,4-Bis-{6-[ethyl-(2-methoxybenzyl)amino]hexyl}pip...)Show SMILES CCN(CCCCCCN1CC(=O)N(CCCCCCN(CC)Cc2ccccc2OC)CC1=O)Cc1ccccc1OC Show InChI InChI=1S/C36H56N4O4/c1-5-37(27-31-19-11-13-21-33(31)43-3)23-15-7-9-17-25-39-29-36(42)40(30-35(39)41)26-18-10-8-16-24-38(6-2)28-32-20-12-14-22-34(32)44-4/h11-14,19-22H,5-10,15-18,23-30H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50124570

(6-[Ethyl-(2-methoxy-benzyl)-amino]-hexanoic acid [...)Show SMILES CCN(CCCCCC(=O)N(C)CCCCCCCCN(C)C(=O)CCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC Show InChI InChI=1S/C42H70N4O4/c1-7-45(35-37-25-17-19-27-39(37)49-5)33-23-13-15-29-41(47)43(3)31-21-11-9-10-12-22-32-44(4)42(48)30-16-14-24-34-46(8-2)36-38-26-18-20-28-40(38)50-6/h17-20,25-28H,7-16,21-24,29-36H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50275722

(2,6-Bis-{6-[ethyl-(2-methoxybenzyl)amino]hexyl}pyr...)Show SMILES CCN(CCCCCCn1c(=O)c2cc3c(cc2c1=O)c(=O)n(CCCCCCN(CC)Cc1ccccc1OC)c3=O)Cc1ccccc1OC Show InChI InChI=1S/C42H54N4O6/c1-5-43(29-31-19-11-13-21-37(31)51-3)23-15-7-9-17-25-45-39(47)33-27-35-36(28-34(33)40(45)48)42(50)46(41(35)49)26-18-10-8-16-24-44(6-2)30-32-20-12-14-22-38(32)52-4/h11-14,19-22,27-28H,5-10,15-18,23-26,29-30H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50275713

(CHEMBL499825 | cis-6-[Ethyl-(2-methoxybenzyl)amino...)Show SMILES CCN(CCCCCC(=O)N[C@@H]1CCCC[C@@H]1NC(=O)CCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |r| Show InChI InChI=1S/C38H60N4O4/c1-5-41(29-31-19-11-15-23-35(31)45-3)27-17-7-9-25-37(43)39-33-21-13-14-22-34(33)40-38(44)26-10-8-18-28-42(6-2)30-32-20-12-16-24-36(32)46-4/h11-12,15-16,19-20,23-24,33-34H,5-10,13-14,17-18,21-22,25-30H2,1-4H3,(H,39,43)(H,40,44)/t33-,34+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50275714
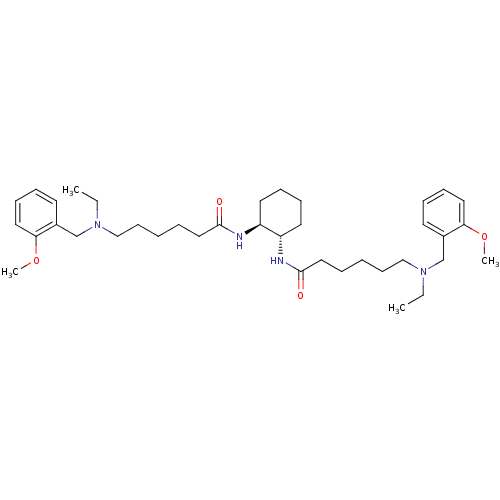
((+/-)-trans-6-[Ethyl-(2-methoxybenzyl)amino]hexano...)Show SMILES CCN(CCCCCC(=O)N[C@H]1CCCC[C@@H]1NC(=O)CCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |r| Show InChI InChI=1S/C38H60N4O4/c1-5-41(29-31-19-11-15-23-35(31)45-3)27-17-7-9-25-37(43)39-33-21-13-14-22-34(33)40-38(44)26-10-8-18-28-42(6-2)30-32-20-12-16-24-36(32)46-4/h11-12,15-16,19-20,23-24,33-34H,5-10,13-14,17-18,21-22,25-30H2,1-4H3,(H,39,43)(H,40,44)/t33-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50275723

(2,7-bis(6-(ethyl(2-methoxybenzyl)amino)hexyl)benzo...)Show SMILES CCN(CCCCCCn1c(=O)c2ccc3c4c(ccc(c24)c1=O)c(=O)n(CCCCCCN(CC)Cc1ccccc1OC)c3=O)Cc1ccccc1OC Show InChI InChI=1S/C46H56N4O6/c1-5-47(31-33-19-11-13-21-39(33)55-3)27-15-7-9-17-29-49-43(51)35-23-25-37-42-38(26-24-36(41(35)42)44(49)52)46(54)50(45(37)53)30-18-10-8-16-28-48(6-2)32-34-20-12-14-22-40(34)56-4/h11-14,19-26H,5-10,15-18,27-32H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE-induced amyloid beta (1-40) aggregation by thioflavin T formation assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50157597

(6-[ethyl-(2-methoxy-benzyl)-amino]-1-(1'-{6-[ethyl...)Show SMILES CCN(CCCCCC(=O)N1CCC(CC1)C1CCN(CC1)C(=O)CCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC Show InChI InChI=1S/C42H66N4O4/c1-5-43(33-37-17-11-13-19-39(37)49-3)27-15-7-9-21-41(47)45-29-23-35(24-30-45)36-25-31-46(32-26-36)42(48)22-10-8-16-28-44(6-2)34-38-18-12-14-20-40(38)50-4/h11-14,17-20,35-36H,5-10,15-16,21-34H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50275715

(CHEMBL499722 | trans-6-[Ethyl-(2-methoxybenzyl)ami...)Show SMILES CCN(CCCCCC(=O)N[C@H]1CC[C@@H](CC1)NC(=O)CCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |r,wU:11.10,wD:14.17,(22.36,2.05,;23.69,1.29,;23.7,-.25,;22.37,-1.03,;21.03,-.26,;19.7,-1.04,;18.36,-.27,;17.03,-1.05,;15.7,-.28,;15.69,1.26,;14.36,-1.06,;13.03,-.29,;11.69,-1.06,;10.36,-.3,;10.37,1.24,;11.68,2.02,;13.02,1.25,;9.03,2,;7.7,1.22,;7.71,-.32,;6.36,1.99,;5.03,1.21,;3.7,1.98,;2.37,1.2,;1.03,1.97,;-.3,1.19,;-.3,-.35,;1.04,-1.11,;-1.64,1.96,;-2.97,1.18,;-2.96,-.37,;-4.3,-1.14,;-5.64,-.37,;-5.63,1.18,;-4.3,1.95,;-4.31,3.49,;-2.98,4.26,;25.03,-1.02,;26.36,-.24,;27.69,-1.02,;29.02,-.24,;29.02,1.3,;27.67,2.06,;26.35,1.29,;25.01,2.04,;24.99,3.58,)| Show InChI InChI=1S/C38H60N4O4/c1-5-41(29-31-17-11-13-19-35(31)45-3)27-15-7-9-21-37(43)39-33-23-25-34(26-24-33)40-38(44)22-10-8-16-28-42(6-2)30-32-18-12-14-20-36(32)46-4/h11-14,17-20,33-34H,5-10,15-16,21-30H2,1-4H3,(H,39,43)(H,40,44)/t33-,34- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50275723

(2,7-bis(6-(ethyl(2-methoxybenzyl)amino)hexyl)benzo...)Show SMILES CCN(CCCCCCn1c(=O)c2ccc3c4c(ccc(c24)c1=O)c(=O)n(CCCCCCN(CC)Cc1ccccc1OC)c3=O)Cc1ccccc1OC Show InChI InChI=1S/C46H56N4O6/c1-5-47(31-33-19-11-13-21-39(33)55-3)27-15-7-9-17-29-49-43(51)35-23-25-37-42-38(26-24-36(41(35)42)44(49)52)46(54)50(45(37)53)30-18-10-8-16-28-48(6-2)32-34-20-12-14-22-40(34)56-4/h11-14,19-26H,5-10,15-18,27-32H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of self-induced amyloid beta (1-40) aggregation by thioflavin T formation assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50275717

(1,4-Bis-{6-[ethyl-(2-methoxybenzyl)amino]hexyl}pip...)Show SMILES CCN(CCCCCCN1CCN(CCCCCCN(CC)Cc2ccccc2OC)C(=O)C1=O)Cc1ccccc1OC Show InChI InChI=1S/C36H56N4O4/c1-5-37(29-31-19-11-13-21-33(31)43-3)23-15-7-9-17-25-39-27-28-40(36(42)35(39)41)26-18-10-8-16-24-38(6-2)30-32-20-12-14-22-34(32)44-4/h11-14,19-22H,5-10,15-18,23-30H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50275712

(6-[Ethyl-(2-methoxybenzyl)amino]-1-(4-{6-[ethyl-(2...)Show SMILES CCN(CCCCCC(=O)N1CCN(CC1)C(=O)CCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC Show InChI InChI=1S/C36H56N4O4/c1-5-37(29-31-17-11-13-19-33(31)43-3)23-15-7-9-21-35(41)39-25-27-40(28-26-39)36(42)22-10-8-16-24-38(6-2)30-32-18-12-14-20-34(32)44-4/h11-14,17-20H,5-10,15-16,21-30H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50067482

(6-[(2-Methoxy-benzyl)-methyl-amino]-hexanoic acid ...)Show SMILES COc1ccccc1CN(C)CCCCCC(=O)N(C)CCCCCCCCN(C)C(=O)CCCCCN(C)Cc1ccccc1OC Show InChI InChI=1S/C40H66N4O4/c1-41(33-35-23-15-17-25-37(35)47-5)29-19-11-13-27-39(45)43(3)31-21-9-7-8-10-22-32-44(4)40(46)28-14-12-20-30-42(2)34-36-24-16-18-26-38(36)48-6/h15-18,23-26H,7-14,19-22,27-34H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM31904
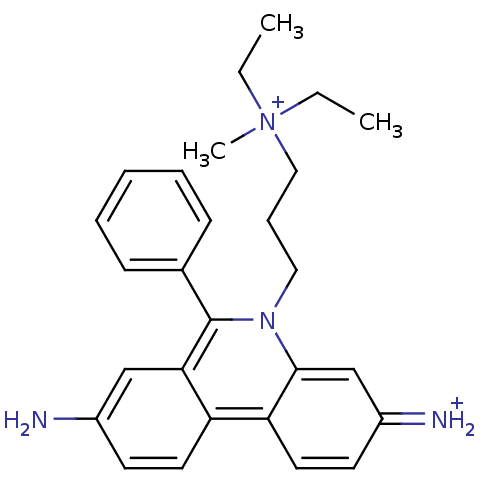
(CHEMBL345124 | Propidium | Propidium Iodide, 2 | p...)Show SMILES CC[N+](C)(CC)CCCn1c(-c2ccccc2)c2cc(N)ccc2c2ccc(=[NH2+])cc12 Show InChI InChI=1S/C27H33N4/c1-4-31(3,5-2)17-9-16-30-26-19-22(29)13-15-24(26)23-14-12-21(28)18-25(23)27(30)20-10-7-6-8-11-20/h6-8,10-15,18-19,29H,4-5,9,16-17,28H2,1-3H3/q+1/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 2.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50275715

(CHEMBL499722 | trans-6-[Ethyl-(2-methoxybenzyl)ami...)Show SMILES CCN(CCCCCC(=O)N[C@H]1CC[C@@H](CC1)NC(=O)CCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |r,wU:11.10,wD:14.17,(22.36,2.05,;23.69,1.29,;23.7,-.25,;22.37,-1.03,;21.03,-.26,;19.7,-1.04,;18.36,-.27,;17.03,-1.05,;15.7,-.28,;15.69,1.26,;14.36,-1.06,;13.03,-.29,;11.69,-1.06,;10.36,-.3,;10.37,1.24,;11.68,2.02,;13.02,1.25,;9.03,2,;7.7,1.22,;7.71,-.32,;6.36,1.99,;5.03,1.21,;3.7,1.98,;2.37,1.2,;1.03,1.97,;-.3,1.19,;-.3,-.35,;1.04,-1.11,;-1.64,1.96,;-2.97,1.18,;-2.96,-.37,;-4.3,-1.14,;-5.64,-.37,;-5.63,1.18,;-4.3,1.95,;-4.31,3.49,;-2.98,4.26,;25.03,-1.02,;26.36,-.24,;27.69,-1.02,;29.02,-.24,;29.02,1.3,;27.67,2.06,;26.35,1.29,;25.01,2.04,;24.99,3.58,)| Show InChI InChI=1S/C38H60N4O4/c1-5-41(29-31-17-11-13-19-35(31)45-3)27-15-7-9-21-37(43)39-33-23-25-34(26-24-33)40-38(44)22-10-8-16-28-42(6-2)30-32-18-12-14-20-36(32)46-4/h11-14,17-20,33-34H,5-10,15-16,21-30H2,1-4H3,(H,39,43)(H,40,44)/t33-,34- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50275713

(CHEMBL499825 | cis-6-[Ethyl-(2-methoxybenzyl)amino...)Show SMILES CCN(CCCCCC(=O)N[C@@H]1CCCC[C@@H]1NC(=O)CCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |r| Show InChI InChI=1S/C38H60N4O4/c1-5-41(29-31-19-11-15-23-35(31)45-3)27-17-7-9-25-37(43)39-33-21-13-14-22-34(33)40-38(44)26-10-8-18-28-42(6-2)30-32-20-12-16-24-36(32)46-4/h11-12,15-16,19-20,23-24,33-34H,5-10,13-14,17-18,21-22,25-30H2,1-4H3,(H,39,43)(H,40,44)/t33-,34+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM31904

(CHEMBL345124 | Propidium | Propidium Iodide, 2 | p...)Show SMILES CC[N+](C)(CC)CCCn1c(-c2ccccc2)c2cc(N)ccc2c2ccc(=[NH2+])cc12 Show InChI InChI=1S/C27H33N4/c1-4-31(3,5-2)17-9-16-30-26-19-22(29)13-15-24(26)23-14-12-21(28)18-25(23)27(30)20-10-7-6-8-11-20/h6-8,10-15,18-19,29H,4-5,9,16-17,28H2,1-3H3/q+1/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50275714

((+/-)-trans-6-[Ethyl-(2-methoxybenzyl)amino]hexano...)Show SMILES CCN(CCCCCC(=O)N[C@H]1CCCC[C@@H]1NC(=O)CCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |r| Show InChI InChI=1S/C38H60N4O4/c1-5-41(29-31-19-11-15-23-35(31)45-3)27-17-7-9-25-37(43)39-33-21-13-14-22-34(33)40-38(44)26-10-8-18-28-42(6-2)30-32-20-12-16-24-36(32)46-4/h11-12,15-16,19-20,23-24,33-34H,5-10,13-14,17-18,21-22,25-30H2,1-4H3,(H,39,43)(H,40,44)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's assay |
J Med Chem 51: 7308-12 (2009)
Article DOI: 10.1021/jm8009684
BindingDB Entry DOI: 10.7270/Q2MW2J3W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data