

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM26226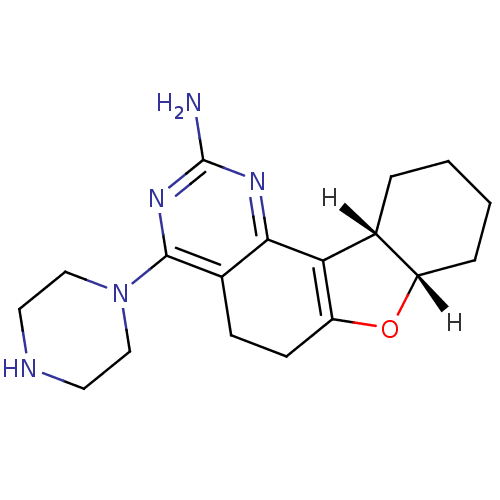 ((12S,17S)-6-(piperazin-1-yl)-11-oxa-3,5-diazatetra...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 3.40 | -48.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 7094-8 (2008) Article DOI: 10.1021/jm8007618 BindingDB Entry DOI: 10.7270/Q2862DS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 7094-8 (2008) Article DOI: 10.1021/jm8007618 BindingDB Entry DOI: 10.7270/Q2862DS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM26226 ((12S,17S)-6-(piperazin-1-yl)-11-oxa-3,5-diazatetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 5.80 | -47.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 7094-8 (2008) Article DOI: 10.1021/jm8007618 BindingDB Entry DOI: 10.7270/Q2862DS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 7094-8 (2008) Article DOI: 10.1021/jm8007618 BindingDB Entry DOI: 10.7270/Q2862DS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM26226 ((12S,17S)-6-(piperazin-1-yl)-11-oxa-3,5-diazatetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 13.5 | -44.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 7094-8 (2008) Article DOI: 10.1021/jm8007618 BindingDB Entry DOI: 10.7270/Q2862DS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM26226 ((12S,17S)-6-(piperazin-1-yl)-11-oxa-3,5-diazatetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 933 | -34.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 7094-8 (2008) Article DOI: 10.1021/jm8007618 BindingDB Entry DOI: 10.7270/Q2862DS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 977 | -34.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 7094-8 (2008) Article DOI: 10.1021/jm8007618 BindingDB Entry DOI: 10.7270/Q2862DS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.45E+3 | -33.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 7094-8 (2008) Article DOI: 10.1021/jm8007618 BindingDB Entry DOI: 10.7270/Q2862DS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.24E+3 | -32.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 7094-8 (2008) Article DOI: 10.1021/jm8007618 BindingDB Entry DOI: 10.7270/Q2862DS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM26226 ((12S,17S)-6-(piperazin-1-yl)-11-oxa-3,5-diazatetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 3.55E+3 | -31.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 7094-8 (2008) Article DOI: 10.1021/jm8007618 BindingDB Entry DOI: 10.7270/Q2862DS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 8.51E+3 | -28.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 7094-8 (2008) Article DOI: 10.1021/jm8007618 BindingDB Entry DOI: 10.7270/Q2862DS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM26226 ((12S,17S)-6-(piperazin-1-yl)-11-oxa-3,5-diazatetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | >9.12E+3 | >-28.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 7094-8 (2008) Article DOI: 10.1021/jm8007618 BindingDB Entry DOI: 10.7270/Q2862DS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||