

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50054470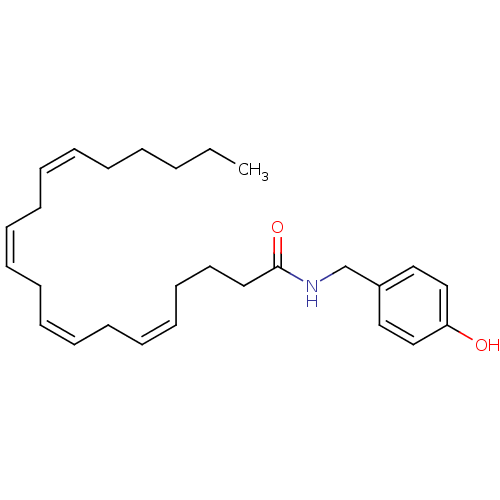 ((5Z,8Z,11Z,14Z)-Icosa-5,8,11,14-tetraenoic acid 4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells | J Med Chem 51: 7800-5 (2008) Article DOI: 10.1021/jm800807k BindingDB Entry DOI: 10.7270/Q2ST7PPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50054470 ((5Z,8Z,11Z,14Z)-Icosa-5,8,11,14-tetraenoic acid 4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells | J Med Chem 51: 7800-5 (2008) Article DOI: 10.1021/jm800807k BindingDB Entry DOI: 10.7270/Q2ST7PPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50246638 (CHEMBL472897 | N-(1H-indazol-5-yl)icosa-5,8,11,14-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells | J Med Chem 51: 7800-5 (2008) Article DOI: 10.1021/jm800807k BindingDB Entry DOI: 10.7270/Q2ST7PPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50246638 (CHEMBL472897 | N-(1H-indazol-5-yl)icosa-5,8,11,14-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells | J Med Chem 51: 7800-5 (2008) Article DOI: 10.1021/jm800807k BindingDB Entry DOI: 10.7270/Q2ST7PPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50246639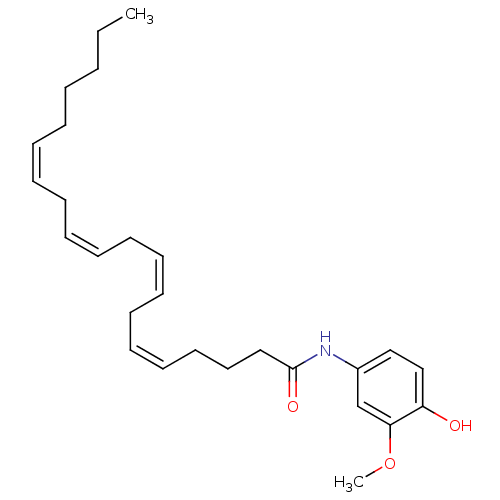 (CHEMBL472898 | N-(4-hydroxy-3-methoxyphenyl)icosa-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells | J Med Chem 51: 7800-5 (2008) Article DOI: 10.1021/jm800807k BindingDB Entry DOI: 10.7270/Q2ST7PPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50054471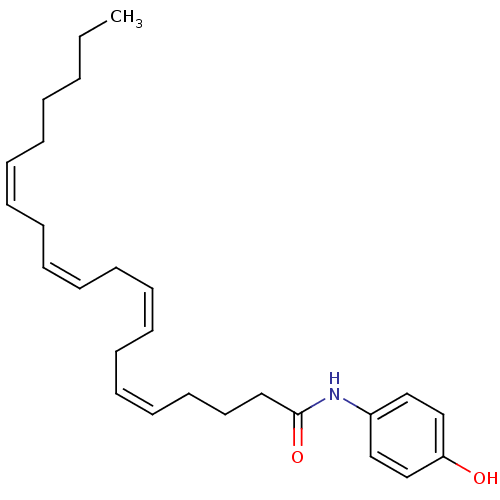 ((5Z,8Z)-Icosa-5,8,11,14-tetraenoic acid (4-hydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells | J Med Chem 51: 7800-5 (2008) Article DOI: 10.1021/jm800807k BindingDB Entry DOI: 10.7270/Q2ST7PPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50246639 (CHEMBL472898 | N-(4-hydroxy-3-methoxyphenyl)icosa-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells | J Med Chem 51: 7800-5 (2008) Article DOI: 10.1021/jm800807k BindingDB Entry DOI: 10.7270/Q2ST7PPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50054471 ((5Z,8Z)-Icosa-5,8,11,14-tetraenoic acid (4-hydroxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells | J Med Chem 51: 7800-5 (2008) Article DOI: 10.1021/jm800807k BindingDB Entry DOI: 10.7270/Q2ST7PPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50246684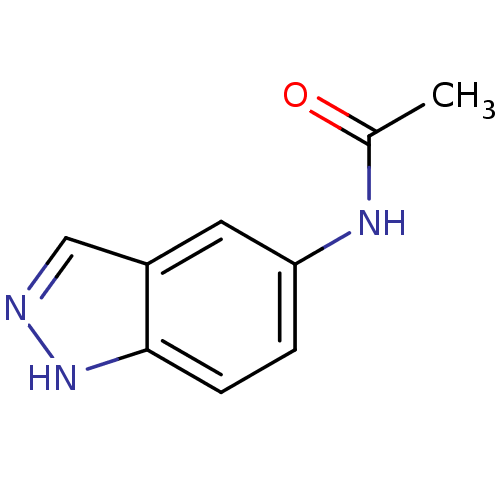 (CHEMBL503641 | N-(1H-indazol-5-yl)acetamide) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet Curated by ChEMBL | Assay Description Inhibition of cyclooxygenase 2 in human whole blood assessed as prostaglandin H2 level | J Med Chem 51: 7800-5 (2008) Article DOI: 10.1021/jm800807k BindingDB Entry DOI: 10.7270/Q2ST7PPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50246684 (CHEMBL503641 | N-(1H-indazol-5-yl)acetamide) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet Curated by ChEMBL | Assay Description Inhibition of cyclooxygenase 2 in human whole blood assessed as prostaglandin H2 level | J Med Chem 51: 7800-5 (2008) Article DOI: 10.1021/jm800807k BindingDB Entry DOI: 10.7270/Q2ST7PPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50246685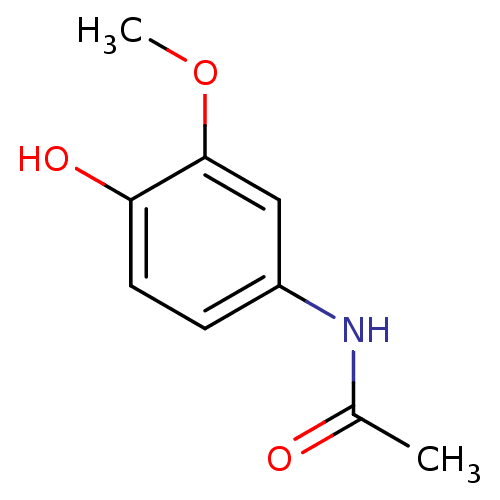 (CHEMBL498882 | N-(4-hydroxy-3-methoxyphenyl)acetam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet Curated by ChEMBL | Assay Description Inhibition of cyclooxygenase 2 in human whole blood assessed as prostaglandin H2 level | J Med Chem 51: 7800-5 (2008) Article DOI: 10.1021/jm800807k BindingDB Entry DOI: 10.7270/Q2ST7PPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50246685 (CHEMBL498882 | N-(4-hydroxy-3-methoxyphenyl)acetam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet Curated by ChEMBL | Assay Description Inhibition of cyclooxygenase 2 in human whole blood assessed as prostaglandin H2 level | J Med Chem 51: 7800-5 (2008) Article DOI: 10.1021/jm800807k BindingDB Entry DOI: 10.7270/Q2ST7PPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM26197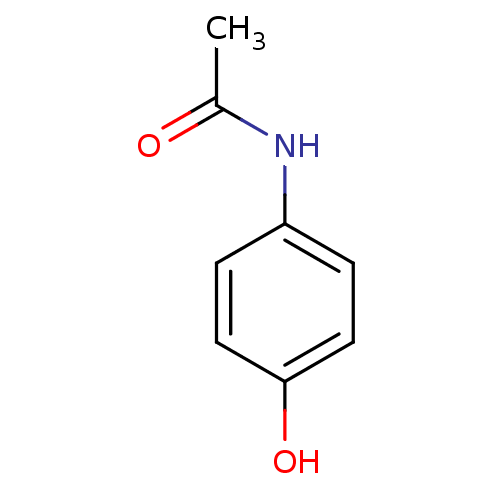 (CHEMBL112 | N-(4-hydroxyphenyl)acetamide | Norco |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | DrugBank Article PubMed | n/a | n/a | 1.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet Curated by ChEMBL | Assay Description Inhibition of cyclooxygenase 2 in human whole blood assessed as prostaglandin H2 level | J Med Chem 51: 7800-5 (2008) Article DOI: 10.1021/jm800807k BindingDB Entry DOI: 10.7270/Q2ST7PPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM26197 (CHEMBL112 | N-(4-hydroxyphenyl)acetamide | Norco |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | DrugBank Article PubMed | n/a | n/a | 1.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet Curated by ChEMBL | Assay Description Inhibition of cyclooxygenase 2 in human whole blood assessed as prostaglandin H2 level | J Med Chem 51: 7800-5 (2008) Article DOI: 10.1021/jm800807k BindingDB Entry DOI: 10.7270/Q2ST7PPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50246685 (CHEMBL498882 | N-(4-hydroxy-3-methoxyphenyl)acetam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet Curated by ChEMBL | Assay Description Inhibition of cyclooxygenase 1 in human whole blood assessed as thromboxane B2 level | J Med Chem 51: 7800-5 (2008) Article DOI: 10.1021/jm800807k BindingDB Entry DOI: 10.7270/Q2ST7PPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||