

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247128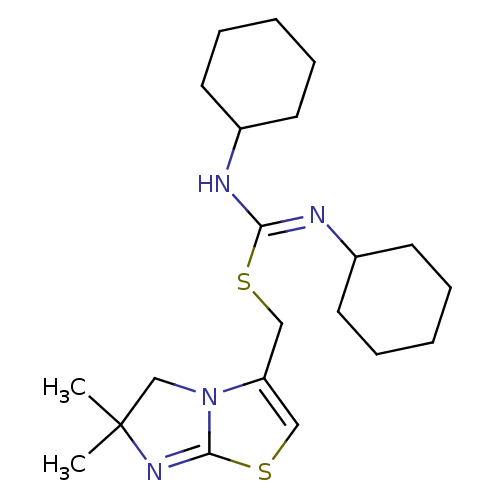 ((6,6-dimethyl-5,6-dihydroimidazo[2,1-b][1,3]thiazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilization | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247129 (1,3-dicycloheptyl-2-((6,6-dimethyl-5,6-dihydroimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilization | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247015 (1,3-dicycloheptyl-2-((5,6-dihydroimidazo[2,1-b]thi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilization | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247016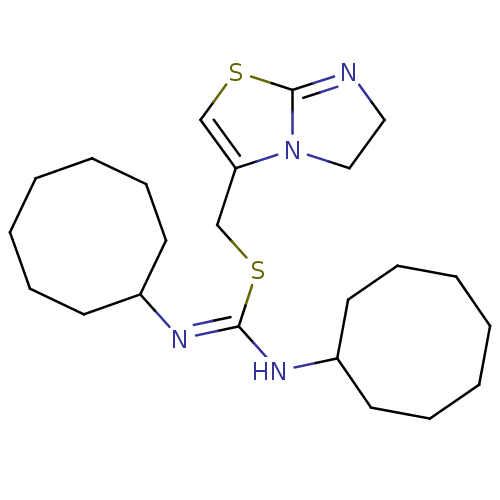 (1,3-dicyclooctyl-2-((5,6-dihydroimidazo[2,1-b]thia...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilization | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247017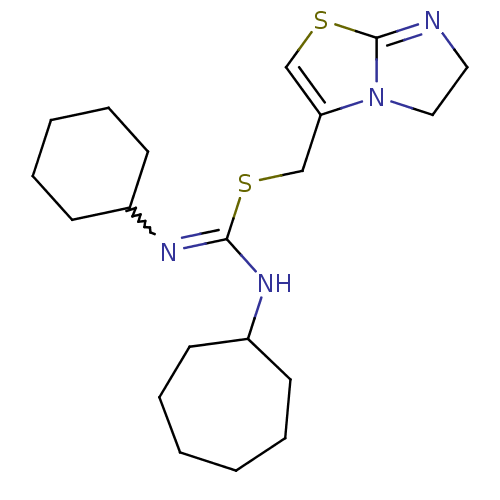 (1-cycloheptyl-3-cyclohexyl-2-((5,6-dihydroimidazo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilization | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247056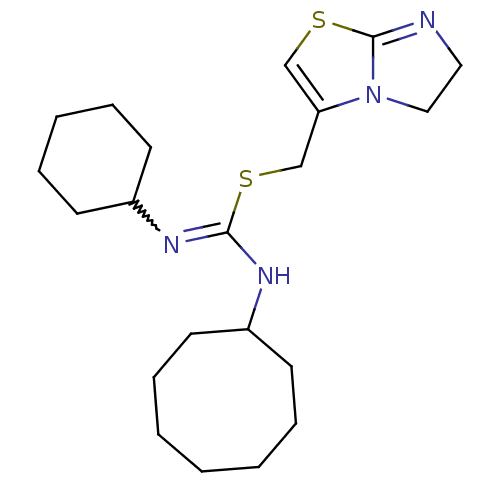 (1-cyclohexyl-3-cyclooctyl-2-((5,6-dihydroimidazo[2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilization | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247128 ((6,6-dimethyl-5,6-dihydroimidazo[2,1-b][1,3]thiazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in human CEM cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247129 (1,3-dicycloheptyl-2-((6,6-dimethyl-5,6-dihydroimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in human CEM cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Rattus norvegicus (Rat)) | BDBM50247128 ((6,6-dimethyl-5,6-dihydroimidazo[2,1-b][1,3]thiazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in rat IR983F cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50246956 (1,3-dicyclohexyl-2-((5,6-dihydroimidazo[2,1-b]thia...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilization | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247060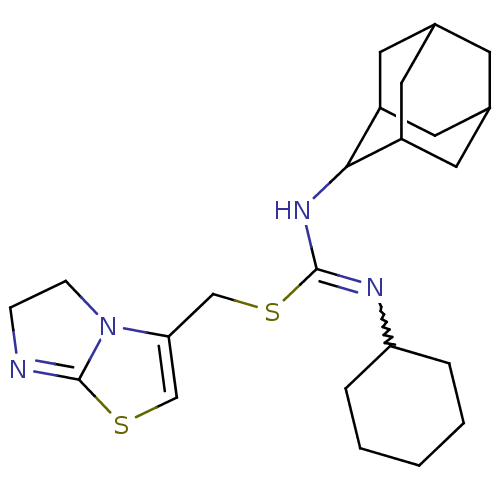 (1-Adamantan-2-yl-3-cyclohexyl-2-(5,6-dihydro-imida...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilization | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247015 (1,3-dicycloheptyl-2-((5,6-dihydroimidazo[2,1-b]thi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in human CEM cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247016 (1,3-dicyclooctyl-2-((5,6-dihydroimidazo[2,1-b]thia...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in human CEM cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247017 (1-cycloheptyl-3-cyclohexyl-2-((5,6-dihydroimidazo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in human CEM cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247056 (1-cyclohexyl-3-cyclooctyl-2-((5,6-dihydroimidazo[2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in human CEM cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Rattus norvegicus (Rat)) | BDBM50246956 (1,3-dicyclohexyl-2-((5,6-dihydroimidazo[2,1-b]thia...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in rat IR983F cells assessed as inhibition of CXCL12-induced cell migration | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50246956 (1,3-dicyclohexyl-2-((5,6-dihydroimidazo[2,1-b]thia...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in human CEM cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Rattus norvegicus (Rat)) | BDBM50246956 (1,3-dicyclohexyl-2-((5,6-dihydroimidazo[2,1-b]thia...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in rat IR983F cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247127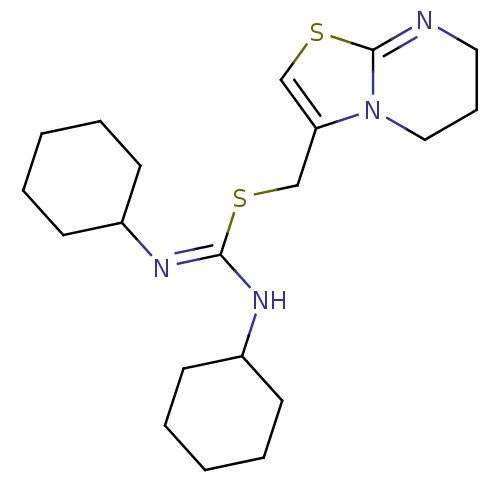 (1,3-dicyclohexyl-2-((6,7-dihydro-5H-thiazolo[3,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilization | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50246956 (1,3-dicyclohexyl-2-((5,6-dihydroimidazo[2,1-b]thia...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human Jurkat T cells assessed as inhibition of CXCL12-induced cell migration | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247058 (1-cyclohexyl-2-((5,6-dihydroimidazo[2,1-b]thiazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilization | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247060 (1-Adamantan-2-yl-3-cyclohexyl-2-(5,6-dihydro-imida...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in human CEM cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Rattus norvegicus (Rat)) | BDBM50035696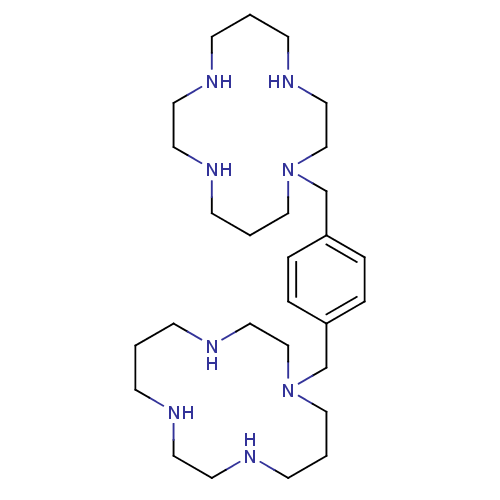 (1,1''-{1,4-phenylenebis(methylene)}-bis{1,4,8,11-t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in rat IR983F cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50246955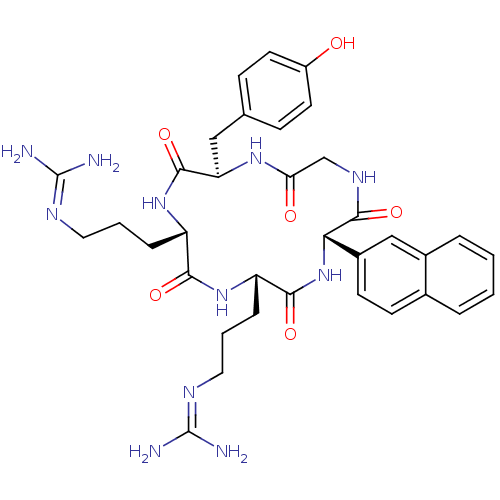 (CHEMBL506505 | N-{3-[(2S,5S,8S,14R)-5-(3-Guanidino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in human CEM cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247057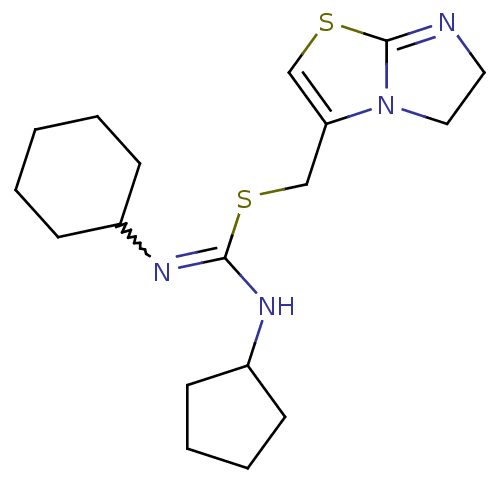 (1-cyclohexyl-3-cyclopentyl-2-((5,6-dihydroimidazo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilization | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247127 (1,3-dicyclohexyl-2-((6,7-dihydro-5H-thiazolo[3,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in human CEM cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50035696 (1,1''-{1,4-phenylenebis(methylene)}-bis{1,4,8,11-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in human CEM cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247059 (1-Adamantan-1-yl-3-cyclohexyl-2-(5,6-dihydro-imida...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 304 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilization | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247057 (1-cyclohexyl-3-cyclopentyl-2-((5,6-dihydroimidazo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 372 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in human CEM cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50247128 ((6,6-dimethyl-5,6-dihydroimidazo[2,1-b][1,3]thiazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247095 (1-(4-tert-butylcyclohexyl)-3-cyclohexyl-2-((5,6-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 437 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilization | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247013 (1,3-dicyclopentyl-2-((5,6-dihydroimidazo[2,1-b]thi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 493 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilization | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247058 (1-cyclohexyl-2-((5,6-dihydroimidazo[2,1-b]thiazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 855 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in human CEM cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50246957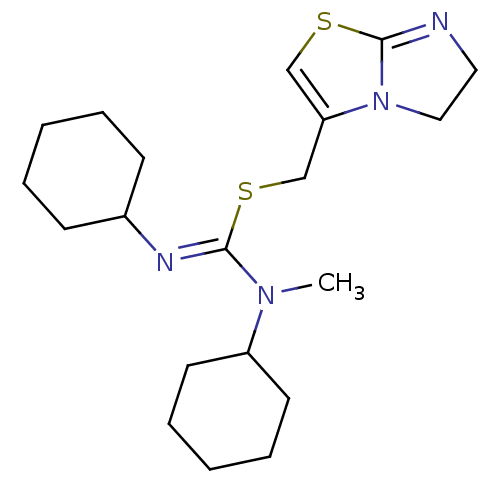 (1,3-dicyclohexyl-2-((5,6-dihydroimidazo[2,1-b]thia...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 931 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilization | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Rattus norvegicus (Rat)) | BDBM50246955 (CHEMBL506505 | N-{3-[(2S,5S,8S,14R)-5-(3-Guanidino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in rat IR983F cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247095 (1-(4-tert-butylcyclohexyl)-3-cyclohexyl-2-((5,6-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in human CEM cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247059 (1-Adamantan-1-yl-3-cyclohexyl-2-(5,6-dihydro-imida...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in human CEM cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247097 (1-benzyl-3-cyclohexyl-2-((2,3,5,6-tetrahydroimidaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilization | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 7 (Homo sapiens (Human)) | BDBM50246956 (1,3-dicyclohexyl-2-((5,6-dihydroimidazo[2,1-b]thia...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CCR7 (unknown origin) | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50246957 (1,3-dicyclohexyl-2-((5,6-dihydroimidazo[2,1-b]thia...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in human CEM cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247013 (1,3-dicyclopentyl-2-((5,6-dihydroimidazo[2,1-b]thi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in human CEM cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50246956 (1,3-dicyclohexyl-2-((5,6-dihydroimidazo[2,1-b]thia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50247128 ((6,6-dimethyl-5,6-dihydroimidazo[2,1-b][1,3]thiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247096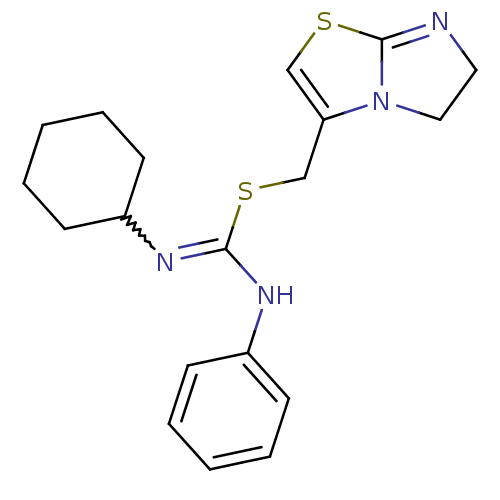 (1-cyclohexyl-2-((5,6-dihydroimidazo[2,1-b]thiazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilization | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50246956 (1,3-dicyclohexyl-2-((5,6-dihydroimidazo[2,1-b]thia...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247126 (2-Benzo[4,5]imidazo[2,1-b]thiazol-3-ylmethyl-1,3-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilization | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50246956 (1,3-dicyclohexyl-2-((5,6-dihydroimidazo[2,1-b]thia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human recombinant ERG expressed in HEK293 cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247099 (1,3-dicyclohexyl-2-(((E)-3-methyl-2-(methylimino)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilization | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247098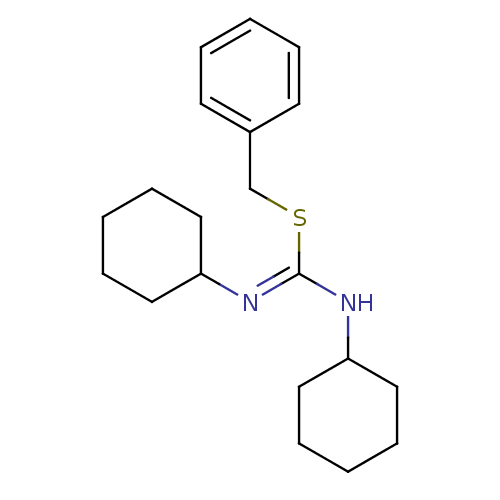 (2-Benzyl-1,3-dicyclohexyl-isothiourea | CHEMBL4604...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in human CEM cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247097 (1-benzyl-3-cyclohexyl-2-((2,3,5,6-tetrahydroimidaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in human CEM cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247098 (2-Benzyl-1,3-dicyclohexyl-isothiourea | CHEMBL4604...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilization | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247099 (1,3-dicyclohexyl-2-(((E)-3-methyl-2-(methylimino)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in human CEM cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247126 (2-Benzo[4,5]imidazo[2,1-b]thiazol-3-ylmethyl-1,3-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in human CEM cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50246956 (1,3-dicyclohexyl-2-((5,6-dihydroimidazo[2,1-b]thia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247096 (1-cyclohexyl-2-((5,6-dihydroimidazo[2,1-b]thiazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in human CEM cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50247128 ((6,6-dimethyl-5,6-dihydroimidazo[2,1-b][1,3]thiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50246956 (1,3-dicyclohexyl-2-((5,6-dihydroimidazo[2,1-b]thia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247014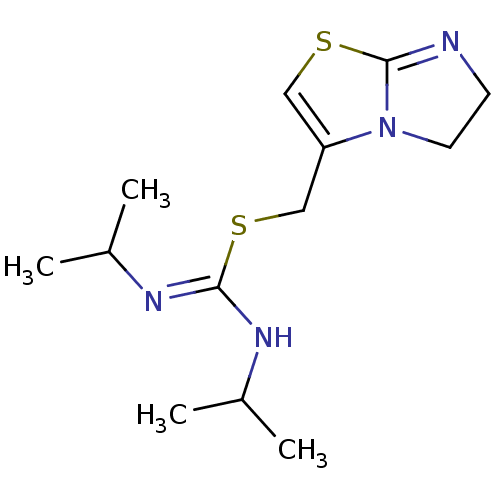 (2-(5,6-Dihydro-imidazo[2,1-b]thiazol-3-ylmethyl)-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from CXCR4 in human CEM cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50247128 ((6,6-dimethyl-5,6-dihydroimidazo[2,1-b][1,3]thiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50247128 ((6,6-dimethyl-5,6-dihydroimidazo[2,1-b][1,3]thiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50246956 (1,3-dicyclohexyl-2-((5,6-dihydroimidazo[2,1-b]thia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50247128 ((6,6-dimethyl-5,6-dihydroimidazo[2,1-b][1,3]thiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human recombinant ERG expressed in HEK293 cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50246956 (1,3-dicyclohexyl-2-((5,6-dihydroimidazo[2,1-b]thia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant ERG in CHOK1 cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50247128 ((6,6-dimethyl-5,6-dihydroimidazo[2,1-b][1,3]thiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant ERG in CHOK1 cells | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||