

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50218786 (2-(4-(2-(2-cyclopentyl-5-((5,7-dimethyl-[1,2,4]tri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes | J Med Chem 52: 1255-8 (2010) Article DOI: 10.1021/jm8014537 BindingDB Entry DOI: 10.7270/Q2SN0901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50293132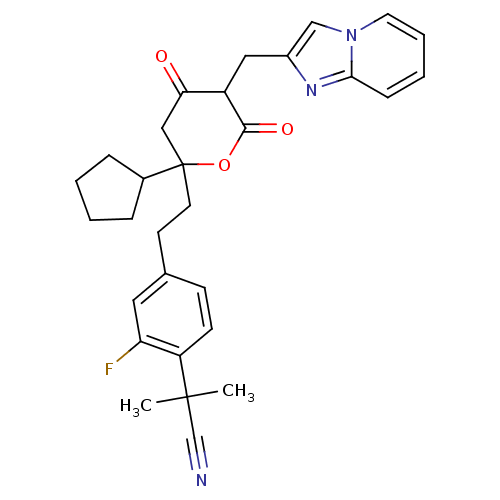 (2-(4-{2-[2-cyclopentyl-4-hydroxy-5-(imidazo[1,2-a]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes | J Med Chem 52: 1255-8 (2010) Article DOI: 10.1021/jm8014537 BindingDB Entry DOI: 10.7270/Q2SN0901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50293133 (2-(4-{2-[2-cyclopentyl-4-hydroxy-5-(3-methoxybenzy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes | J Med Chem 52: 1255-8 (2010) Article DOI: 10.1021/jm8014537 BindingDB Entry DOI: 10.7270/Q2SN0901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50218791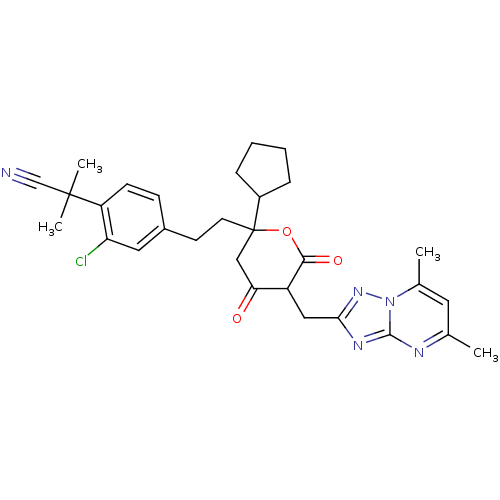 (2-(2-chloro-4-(2-(2-cyclopentyl-5-((5,7-dimethyl-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes | J Med Chem 52: 1255-8 (2010) Article DOI: 10.1021/jm8014537 BindingDB Entry DOI: 10.7270/Q2SN0901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50293134 (2-[4-(2-{2-cyclopentyl-5-[(5,7-dimethyl[1,2,4]tria...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes | J Med Chem 52: 1255-8 (2010) Article DOI: 10.1021/jm8014537 BindingDB Entry DOI: 10.7270/Q2SN0901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50293135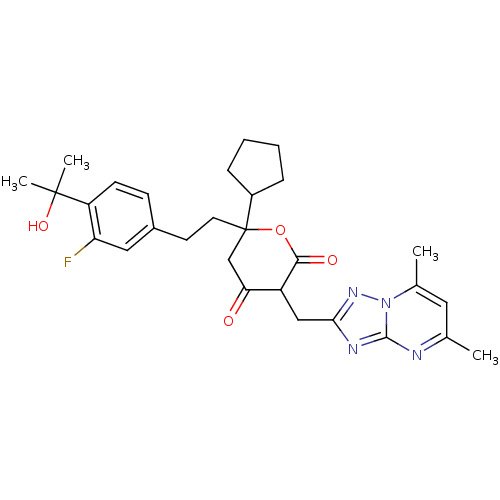 (6-cyclopentyl-3-[(5,7-dimethyl[1,2,4]triazolo[1,5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes | J Med Chem 52: 1255-8 (2010) Article DOI: 10.1021/jm8014537 BindingDB Entry DOI: 10.7270/Q2SN0901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50293131 ((6R)-6-cyclopentyl-6-[2-(2,6-diethylpyridin-4-yl)e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP1A2 | J Med Chem 52: 1255-8 (2010) Article DOI: 10.1021/jm8014537 BindingDB Entry DOI: 10.7270/Q2SN0901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50293131 ((6R)-6-cyclopentyl-6-[2-(2,6-diethylpyridin-4-yl)e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C9 | J Med Chem 52: 1255-8 (2010) Article DOI: 10.1021/jm8014537 BindingDB Entry DOI: 10.7270/Q2SN0901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50293131 ((6R)-6-cyclopentyl-6-[2-(2,6-diethylpyridin-4-yl)e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Med Chem 52: 1255-8 (2010) Article DOI: 10.1021/jm8014537 BindingDB Entry DOI: 10.7270/Q2SN0901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50293131 ((6R)-6-cyclopentyl-6-[2-(2,6-diethylpyridin-4-yl)e...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes | J Med Chem 52: 1255-8 (2010) Article DOI: 10.1021/jm8014537 BindingDB Entry DOI: 10.7270/Q2SN0901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50293131 ((6R)-6-cyclopentyl-6-[2-(2,6-diethylpyridin-4-yl)e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C19 | J Med Chem 52: 1255-8 (2010) Article DOI: 10.1021/jm8014537 BindingDB Entry DOI: 10.7270/Q2SN0901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50293131 ((6R)-6-cyclopentyl-6-[2-(2,6-diethylpyridin-4-yl)e...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C8 | J Med Chem 52: 1255-8 (2010) Article DOI: 10.1021/jm8014537 BindingDB Entry DOI: 10.7270/Q2SN0901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||