

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28206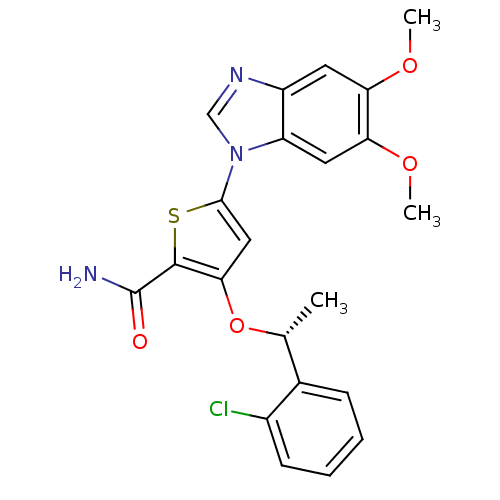 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(5,6-dimethoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28208 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(6-methoxy-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28210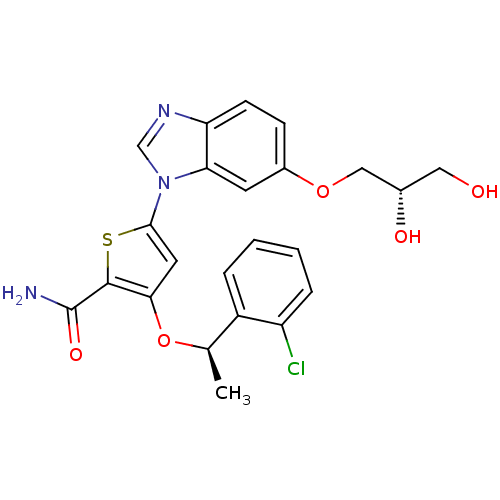 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(2S)-2,3-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28216 (5-{6-[(1-methylpiperidin-4-yl)oxy]-1H-1,3-benzodia...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28217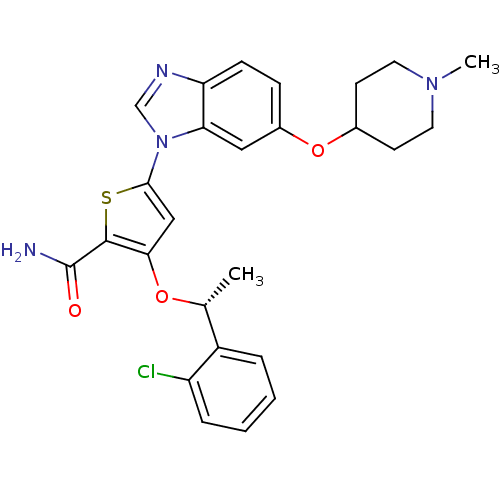 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(1-methylp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28178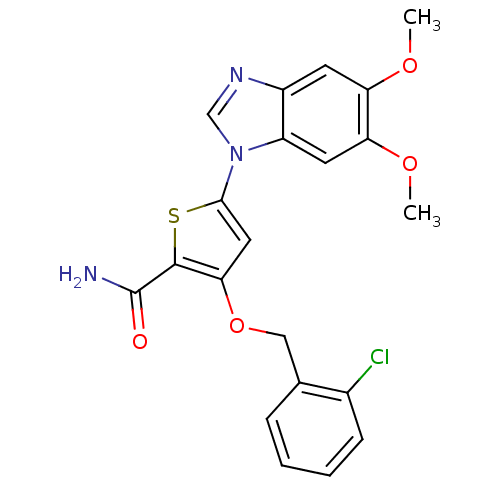 (3-[(2-chlorophenyl)methoxy]-5-(5,6-dimethoxy-1H-1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28218 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(6-{[(4S)-1-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28215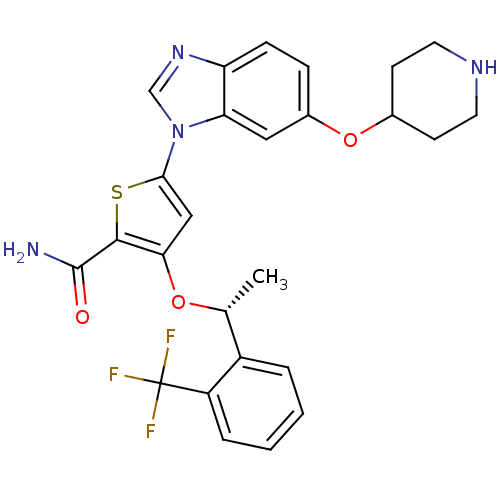 (5-[6-(piperidin-4-yloxy)-1H-1,3-benzodiazol-1-yl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28209 (5-(6-methoxy-1H-1,3-benzodiazol-1-yl)-3-[(1R)-1-[2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28212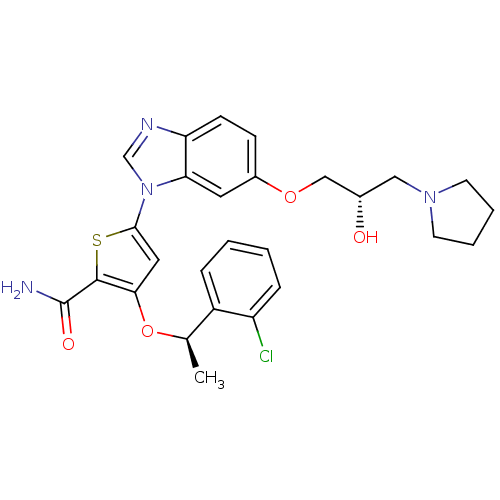 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(2S)-2-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28211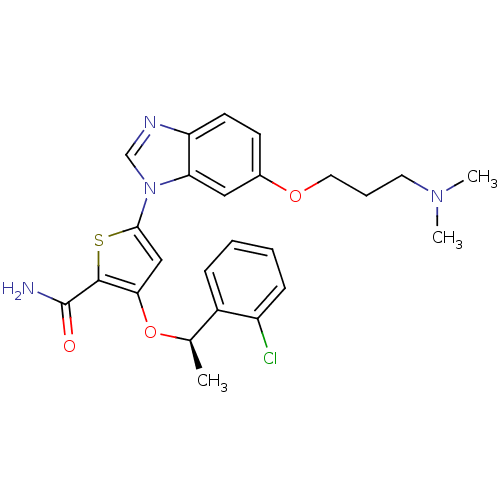 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[3-(dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28219 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(6-{[(4R)-1-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK3 (Homo sapiens (Human)) | BDBM28206 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(5,6-dimethoxy...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28214 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(1-methylp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28213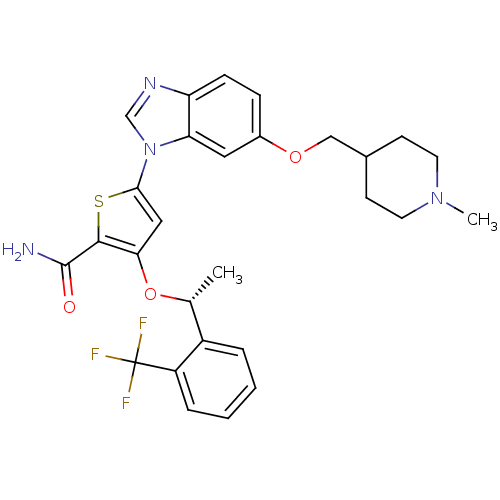 (5-{6-[(1-methylpiperidin-4-yl)methoxy]-1H-1,3-benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK3 (Homo sapiens (Human)) | BDBM28178 (3-[(2-chlorophenyl)methoxy]-5-(5,6-dimethoxy-1H-1,...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28207 (3-[(1S)-1-(2-chlorophenyl)ethoxy]-5-(5,6-dimethoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK3 (Homo sapiens (Human)) | BDBM28208 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(6-methoxy-1H-...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK3 (Homo sapiens (Human)) | BDBM28209 (5-(6-methoxy-1H-1,3-benzodiazol-1-yl)-3-[(1R)-1-[2...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK3 (Homo sapiens (Human)) | BDBM28207 (3-[(1S)-1-(2-chlorophenyl)ethoxy]-5-(5,6-dimethoxy...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK3 (Homo sapiens (Human)) | BDBM28210 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(2S)-2,3-d...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK3 (Homo sapiens (Human)) | BDBM28218 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(6-{[(4S)-1-me...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK3 (Homo sapiens (Human)) | BDBM28219 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(6-{[(4R)-1-me...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK3 (Homo sapiens (Human)) | BDBM28216 (5-{6-[(1-methylpiperidin-4-yl)oxy]-1H-1,3-benzodia...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK3 (Homo sapiens (Human)) | BDBM28215 (5-[6-(piperidin-4-yloxy)-1H-1,3-benzodiazol-1-yl]-...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK3 (Homo sapiens (Human)) | BDBM28211 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[3-(dimethy...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK3 (Homo sapiens (Human)) | BDBM28213 (5-{6-[(1-methylpiperidin-4-yl)methoxy]-1H-1,3-benz...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK3 (Homo sapiens (Human)) | BDBM28212 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(2S)-2-hyd...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK3 (Homo sapiens (Human)) | BDBM28217 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(1-methylp...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK3 (Homo sapiens (Human)) | BDBM28214 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(1-methylp...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||