 Found 14 hits of Enzyme Inhibition Constant Data
Found 14 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50278540
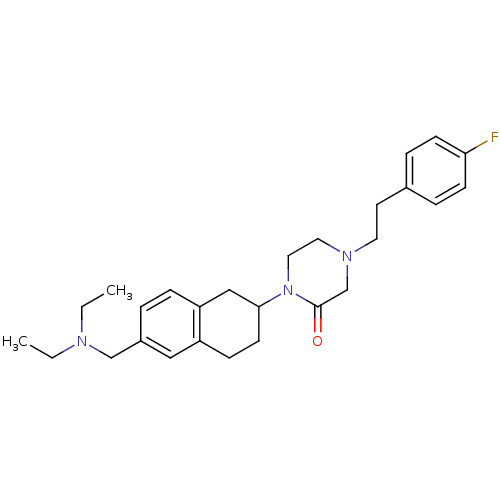
(1-(6-((diethylamino)methyl)-1,2,3,4-tetrahydronaph...)Show SMILES CCN(CC)Cc1ccc2CC(CCc2c1)N1CCN(CCc2ccc(F)cc2)CC1=O Show InChI InChI=1S/C27H36FN3O/c1-3-29(4-2)19-22-5-8-24-18-26(12-9-23(24)17-22)31-16-15-30(20-27(31)32)14-13-21-6-10-25(28)11-7-21/h5-8,10-11,17,26H,3-4,9,12-16,18-20H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at MCH1R (unknown origin) |
J Med Chem 52: 2076-89 (2009)
Article DOI: 10.1021/jm8016199
BindingDB Entry DOI: 10.7270/Q2QN66NZ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50278501
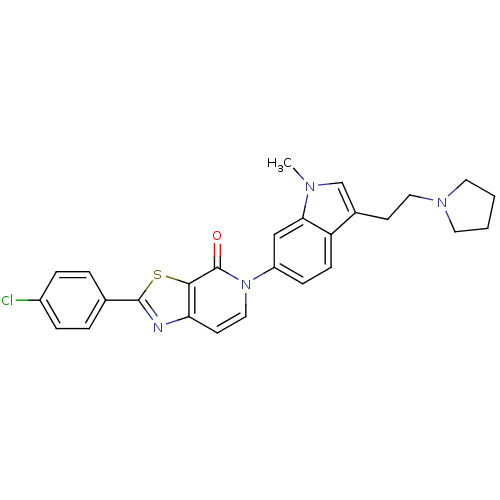
(2-(4-chlorophenyl)-5-(1-methyl-3-(2-(pyrrolidin-1-...)Show SMILES Cn1cc(CCN2CCCC2)c2ccc(cc12)-n1ccc2nc(sc2c1=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C27H25ClN4OS/c1-30-17-19(10-14-31-12-2-3-13-31)22-9-8-21(16-24(22)30)32-15-11-23-25(27(32)33)34-26(29-23)18-4-6-20(28)7-5-18/h4-9,11,15-17H,2-3,10,12-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at MCH1R (unknown origin) |
J Med Chem 52: 2076-89 (2009)
Article DOI: 10.1021/jm8016199
BindingDB Entry DOI: 10.7270/Q2QN66NZ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50278539
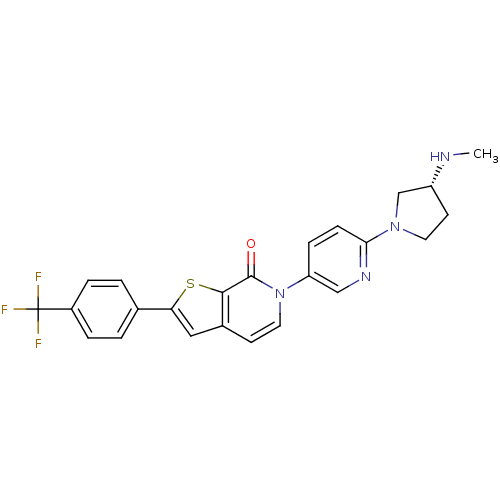
((R)-6-(6-(3-(methylamino)pyrrolidin-1-yl)pyridin-3...)Show SMILES CN[C@@H]1CCN(C1)c1ccc(cn1)-n1ccc2cc(sc2c1=O)-c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C24H21F3N4OS/c1-28-18-9-10-30(14-18)21-7-6-19(13-29-21)31-11-8-16-12-20(33-22(16)23(31)32)15-2-4-17(5-3-15)24(25,26)27/h2-8,11-13,18,28H,9-10,14H2,1H3/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at MCH1R (unknown origin) |
J Med Chem 52: 2076-89 (2009)
Article DOI: 10.1021/jm8016199
BindingDB Entry DOI: 10.7270/Q2QN66NZ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50278541
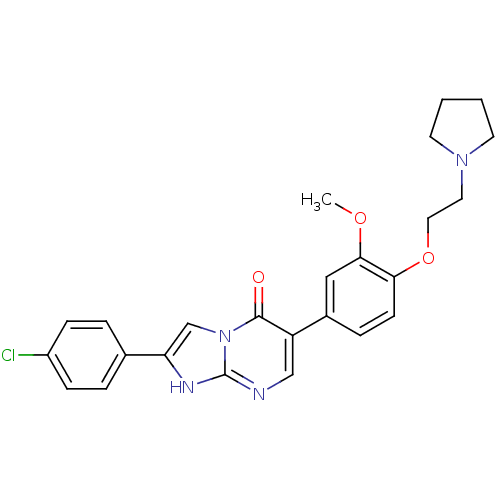
(2-(4-chlorophenyl)-6-(3-methoxy-4-(2-(pyrrolidin-1...)Show SMILES COc1cc(ccc1OCCN1CCCC1)-c1cnc2[nH]c(cn2c1=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H25ClN4O3/c1-32-23-14-18(6-9-22(23)33-13-12-29-10-2-3-11-29)20-15-27-25-28-21(16-30(25)24(20)31)17-4-7-19(26)8-5-17/h4-9,14-16H,2-3,10-13H2,1H3,(H,27,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at MCH1R (unknown origin) |
J Med Chem 52: 2076-89 (2009)
Article DOI: 10.1021/jm8016199
BindingDB Entry DOI: 10.7270/Q2QN66NZ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50211166

(1-(3-methoxy-4-(2-(pyrrolidin-1-yl)ethoxy)phenyl)-...)Show SMILES COc1cc(ccc1OCCN1CCCC1)-n1cc(O)n(-c2ccc(Oc3ccccc3)cc2)c1=O Show InChI InChI=1S/C28H29N3O5/c1-34-26-19-22(11-14-25(26)35-18-17-29-15-5-6-16-29)30-20-27(32)31(28(30)33)21-9-12-24(13-10-21)36-23-7-3-2-4-8-23/h2-4,7-14,19-20,32H,5-6,15-18H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at MCH1R (unknown origin) |
J Med Chem 52: 2076-89 (2009)
Article DOI: 10.1021/jm8016199
BindingDB Entry DOI: 10.7270/Q2QN66NZ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50278456
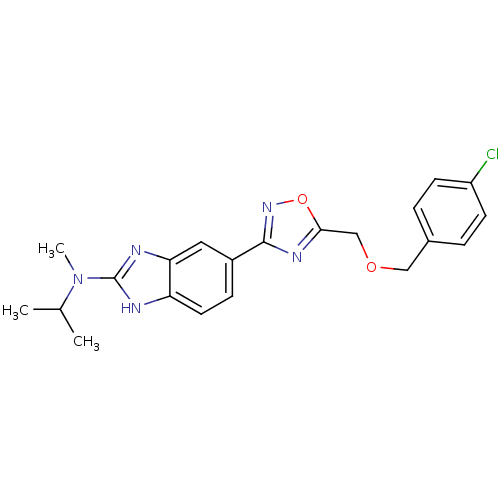
(5-(5-((4-chlorobenzyloxy)methyl)-1,2,4-oxadiazol-3...)Show SMILES CC(C)N(C)c1nc2cc(ccc2[nH]1)-c1noc(COCc2ccc(Cl)cc2)n1 Show InChI InChI=1S/C21H22ClN5O2/c1-13(2)27(3)21-23-17-9-6-15(10-18(17)24-21)20-25-19(29-26-20)12-28-11-14-4-7-16(22)8-5-14/h4-10,13H,11-12H2,1-3H3,(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at MCH1R (unknown origin) |
J Med Chem 52: 2076-89 (2009)
Article DOI: 10.1021/jm8016199
BindingDB Entry DOI: 10.7270/Q2QN66NZ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50150715
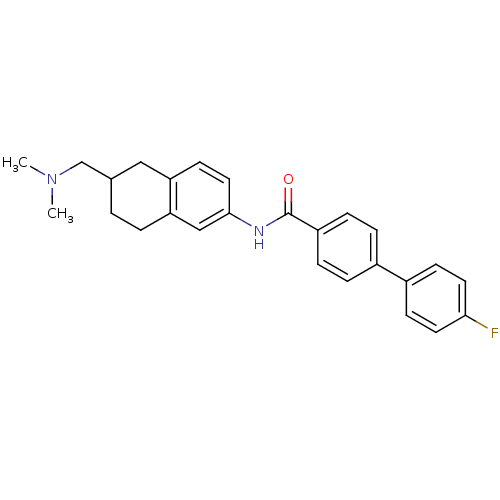
(4''-Fluoro-biphenyl-4-carboxylic acid (6-dimethyla...)Show SMILES CN(C)CC1CCc2cc(NC(=O)c3ccc(cc3)-c3ccc(F)cc3)ccc2C1 Show InChI InChI=1S/C26H27FN2O/c1-29(2)17-18-3-4-23-16-25(14-11-22(23)15-18)28-26(30)21-7-5-19(6-8-21)20-9-12-24(27)13-10-20/h5-14,16,18H,3-4,15,17H2,1-2H3,(H,28,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at MCH1R (unknown origin) by [35S]GTPgammaS binding assay |
J Med Chem 52: 2076-89 (2009)
Article DOI: 10.1021/jm8016199
BindingDB Entry DOI: 10.7270/Q2QN66NZ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50278502
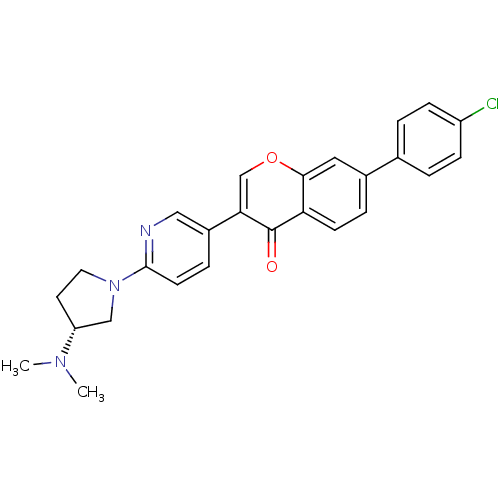
((R)-7-(4-chlorophenyl)-3-(6-(3-(dimethylamino)pyrr...)Show SMILES CN(C)[C@@H]1CCN(C1)c1ccc(cn1)-c1coc2cc(ccc2c1=O)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C26H24ClN3O2/c1-29(2)21-11-12-30(15-21)25-10-6-19(14-28-25)23-16-32-24-13-18(5-9-22(24)26(23)31)17-3-7-20(27)8-4-17/h3-10,13-14,16,21H,11-12,15H2,1-2H3/t21-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at MCH1R (unknown origin) by [35S]GTPgammaS binding assay |
J Med Chem 52: 2076-89 (2009)
Article DOI: 10.1021/jm8016199
BindingDB Entry DOI: 10.7270/Q2QN66NZ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50278457
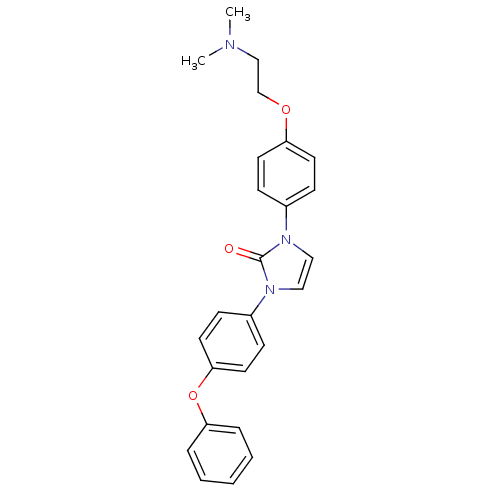
(1-(4-(2-(dimethylamino)ethoxy)phenyl)-3-(4-phenoxy...)Show SMILES CN(C)CCOc1ccc(cc1)-n1ccn(-c2ccc(Oc3ccccc3)cc2)c1=O Show InChI InChI=1S/C25H25N3O3/c1-26(2)18-19-30-22-12-8-20(9-13-22)27-16-17-28(25(27)29)21-10-14-24(15-11-21)31-23-6-4-3-5-7-23/h3-17H,18-19H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at MCH1R (unknown origin) |
J Med Chem 52: 2076-89 (2009)
Article DOI: 10.1021/jm8016199
BindingDB Entry DOI: 10.7270/Q2QN66NZ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50150711
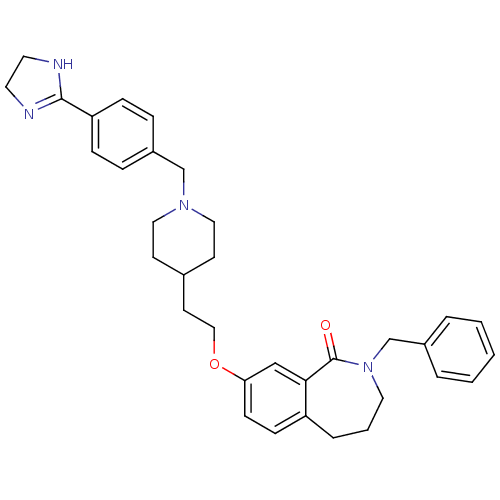
(2-Benzyl-8-(2-{1-[4-(4,5-dihydro-1H-imidazol-2-yl)...)Show SMILES O=C1N(Cc2ccccc2)CCCc2ccc(OCCC3CCN(Cc4ccc(cc4)C4=NCCN4)CC3)cc12 |t:33| Show InChI InChI=1S/C34H40N4O2/c39-34-32-23-31(13-12-29(32)7-4-19-38(34)25-27-5-2-1-3-6-27)40-22-16-26-14-20-37(21-15-26)24-28-8-10-30(11-9-28)33-35-17-18-36-33/h1-3,5-6,8-13,23,26H,4,7,14-22,24-25H2,(H,35,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at MCH1R (unknown origin) by [35S]GTPgammaS binding assay |
J Med Chem 52: 2076-89 (2009)
Article DOI: 10.1021/jm8016199
BindingDB Entry DOI: 10.7270/Q2QN66NZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50241083
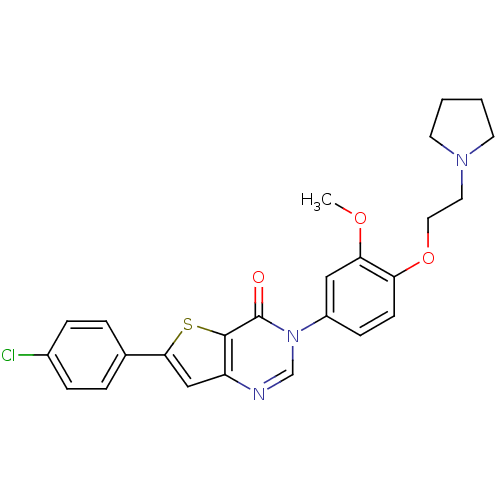
(6-(4-chlorophenyl)-3-(3-methoxy-4-(2-(pyrrolidin-1...)Show SMILES COc1cc(ccc1OCCN1CCCC1)-n1cnc2cc(sc2c1=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H24ClN3O3S/c1-31-22-14-19(8-9-21(22)32-13-12-28-10-2-3-11-28)29-16-27-20-15-23(33-24(20)25(29)30)17-4-6-18(26)7-5-17/h4-9,14-16H,2-3,10-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]astemizole from human ERG expressed in HEK293 cells |
J Med Chem 52: 2076-89 (2009)
Article DOI: 10.1021/jm8016199
BindingDB Entry DOI: 10.7270/Q2QN66NZ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50278542
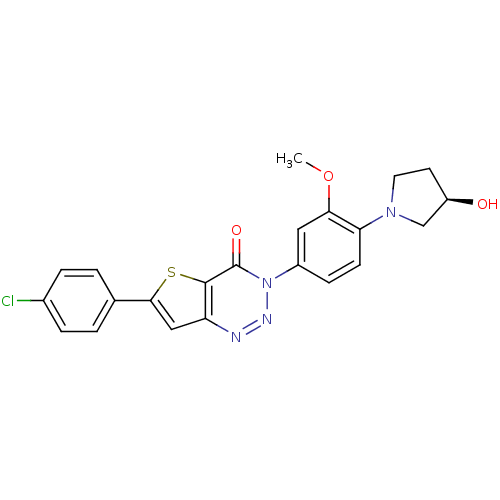
((R)-6-(4-chlorophenyl)-3-(4-(3-hydroxypyrrolidin-1...)Show SMILES COc1cc(ccc1N1CC[C@@H](O)C1)-n1nnc2cc(sc2c1=O)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C22H19ClN4O3S/c1-30-19-10-15(6-7-18(19)26-9-8-16(28)12-26)27-22(29)21-17(24-25-27)11-20(31-21)13-2-4-14(23)5-3-13/h2-7,10-11,16,28H,8-9,12H2,1H3/t16-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at MCH1R (unknown origin) |
J Med Chem 52: 2076-89 (2009)
Article DOI: 10.1021/jm8016199
BindingDB Entry DOI: 10.7270/Q2QN66NZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50278611

(1-[3-Methoxy-4-(2-pyrrolidin-1-ylethoxy)phenyl]-3-...)Show SMILES COc1cc(ccc1OCCN1CCCC1)-n1ccnc(NCCc2ccccc2)c1=O Show InChI InChI=1S/C25H30N4O3/c1-31-23-19-21(9-10-22(23)32-18-17-28-14-5-6-15-28)29-16-13-27-24(25(29)30)26-12-11-20-7-3-2-4-8-20/h2-4,7-10,13,16,19H,5-6,11-12,14-15,17-18H2,1H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]astemizole from human ERG expressed in HEK293 cells |
J Med Chem 52: 2076-89 (2009)
Article DOI: 10.1021/jm8016199
BindingDB Entry DOI: 10.7270/Q2QN66NZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50278574
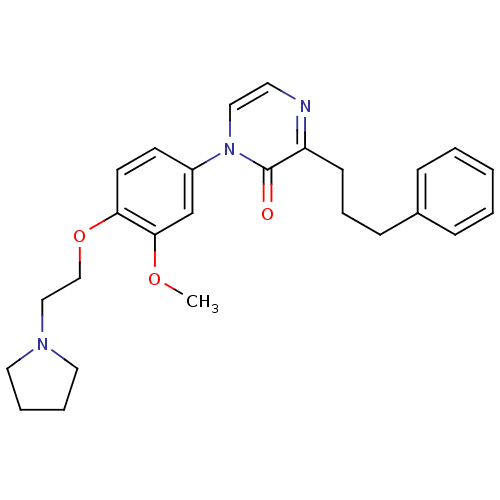
(1-[3-Methoxy-4-(2-pyrrolidin-1-ylethoxy)phenyl]-3-...)Show SMILES COc1cc(ccc1OCCN1CCCC1)-n1ccnc(CCCc2ccccc2)c1=O Show InChI InChI=1S/C26H31N3O3/c1-31-25-20-22(12-13-24(25)32-19-18-28-15-5-6-16-28)29-17-14-27-23(26(29)30)11-7-10-21-8-3-2-4-9-21/h2-4,8-9,12-14,17,20H,5-7,10-11,15-16,18-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]astemizole from human ERG expressed in HEK293 cells |
J Med Chem 52: 2076-89 (2009)
Article DOI: 10.1021/jm8016199
BindingDB Entry DOI: 10.7270/Q2QN66NZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data