 Found 81 hits of Enzyme Inhibition Constant Data
Found 81 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277718
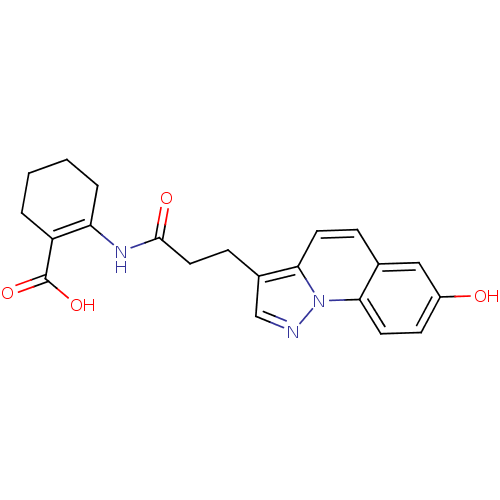
(2-(3-(7-hydroxypyrazolo[1,5-a]quinolin-3-yl)propan...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1cnn2c1ccc1cc(O)ccc21 |t:3| Show InChI InChI=1S/C21H21N3O4/c25-15-7-9-18-13(11-15)5-8-19-14(12-22-24(18)19)6-10-20(26)23-17-4-2-1-3-16(17)21(27)28/h5,7-9,11-12,25H,1-4,6,10H2,(H,23,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277717
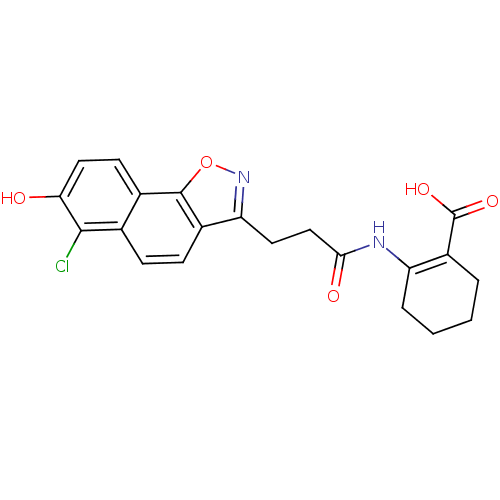
(2-(3-(6-chloro-7-hydroxynaphtho[2,1-d]isoxazol-3-y...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1noc2c1ccc1c(Cl)c(O)ccc21 |t:3| Show InChI InChI=1S/C21H19ClN2O5/c22-19-11-5-6-13-16(24-29-20(13)12(11)7-9-17(19)25)8-10-18(26)23-15-4-2-1-3-14(15)21(27)28/h5-7,9,25H,1-4,8,10H2,(H,23,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277581
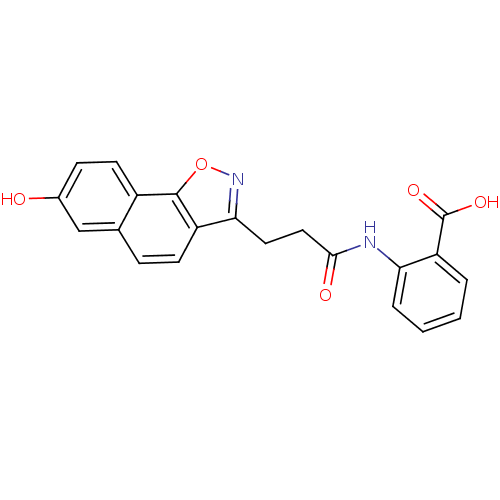
(2-(3-(7-hydroxynaphtho[2,1-d]isoxazol-3-yl)propana...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1noc2c1ccc1cc(O)ccc21 Show InChI InChI=1S/C21H16N2O5/c24-13-6-8-14-12(11-13)5-7-15-18(23-28-20(14)15)9-10-19(25)22-17-4-2-1-3-16(17)21(26)27/h1-8,11,24H,9-10H2,(H,22,25)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277715
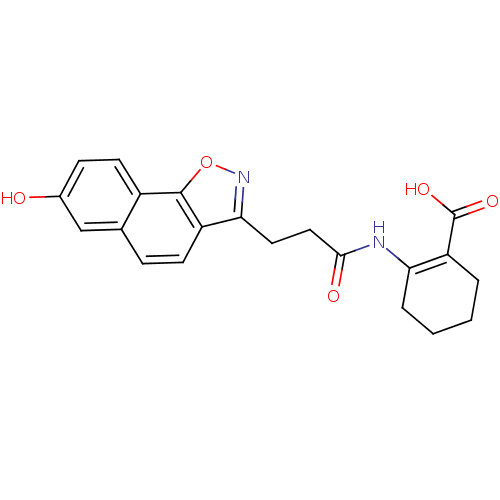
(2-(3-(7-hydroxynaphtho[2,1-d]isoxazol-3-yl)propana...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1noc2c1ccc1cc(O)ccc21 |t:3| Show InChI InChI=1S/C21H20N2O5/c24-13-6-8-14-12(11-13)5-7-15-18(23-28-20(14)15)9-10-19(25)22-17-4-2-1-3-16(17)21(26)27/h5-8,11,24H,1-4,9-10H2,(H,22,25)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277580

(2-(3-(7-hydroxy-4,5-dihydronaphtho[2,1-d]isoxazol-...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1noc-2c1CCc1cc(O)ccc-21 Show InChI InChI=1S/C21H18N2O5/c24-13-6-8-14-12(11-13)5-7-15-18(23-28-20(14)15)9-10-19(25)22-17-4-2-1-3-16(17)21(26)27/h1-4,6,8,11,24H,5,7,9-10H2,(H,22,25)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277714
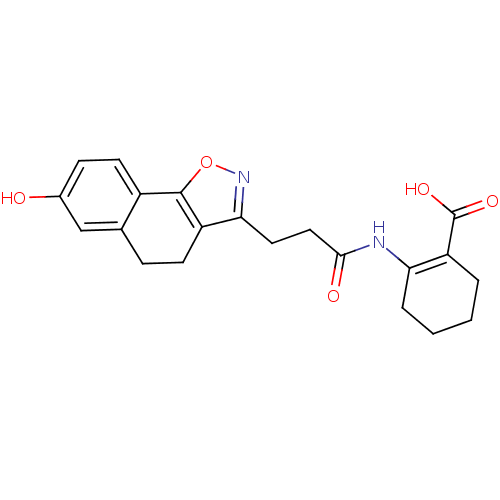
(2-(3-(7-hydroxy-4,5-dihydronaphtho[2,1-d]isoxazol-...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1noc-2c1CCc1cc(O)ccc-21 |t:3| Show InChI InChI=1S/C21H22N2O5/c24-13-6-8-14-12(11-13)5-7-15-18(23-28-20(14)15)9-10-19(25)22-17-4-2-1-3-16(17)21(26)27/h6,8,11,24H,1-5,7,9-10H2,(H,22,25)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277579
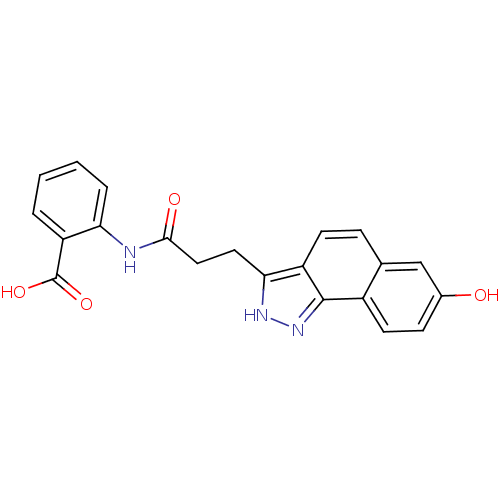
(2-(3-(7-hydroxy-2H-benzo[g]indazol-3-yl)propanamid...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1[nH]nc2c1ccc1cc(O)ccc21 Show InChI InChI=1S/C21H17N3O4/c25-13-6-8-14-12(11-13)5-7-15-18(23-24-20(14)15)9-10-19(26)22-17-4-2-1-3-16(17)21(27)28/h1-8,11,25H,9-10H2,(H,22,26)(H,23,24)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277753
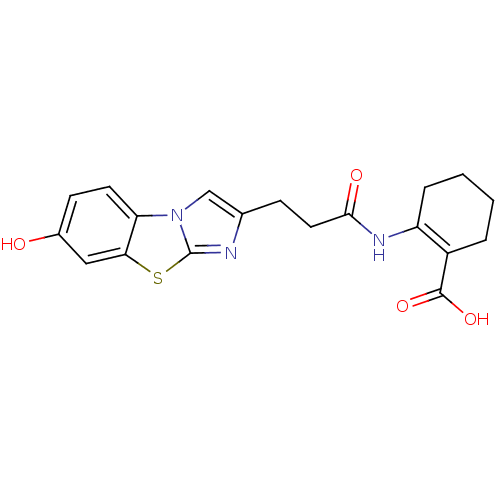
(2-[3-(7-Hydroxy-benzo[d]imidazo[2,1-b]thiazol-2-yl...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1cn2c(n1)sc1cc(O)ccc21 |t:3| Show InChI InChI=1S/C19H19N3O4S/c23-12-6-7-15-16(9-12)27-19-20-11(10-22(15)19)5-8-17(24)21-14-4-2-1-3-13(14)18(25)26/h6-7,9-10,23H,1-5,8H2,(H,21,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277716
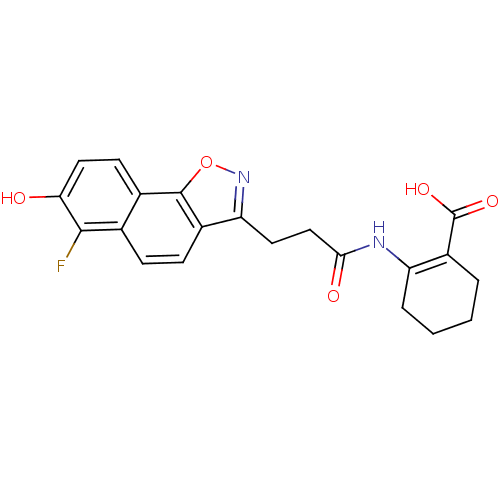
(2-(3-(6-fluoro-7-hydroxynaphtho[2,1-d]isoxazol-3-y...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1noc2c1ccc1c(F)c(O)ccc21 |t:3| Show InChI InChI=1S/C21H19FN2O5/c22-19-11-5-6-13-16(24-29-20(13)12(11)7-9-17(19)25)8-10-18(26)23-15-4-2-1-3-14(15)21(27)28/h5-7,9,25H,1-4,8,10H2,(H,23,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277633
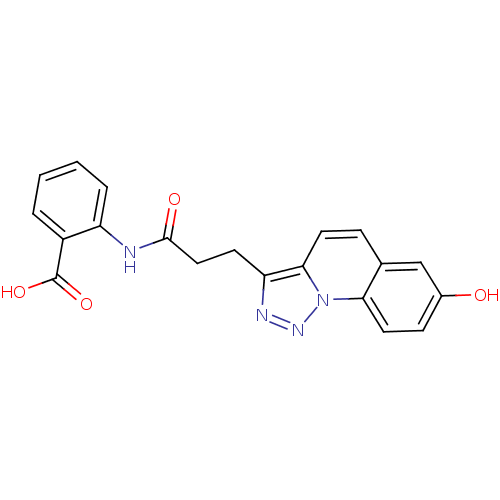
(2-(3-(7-hydroxy-[1,2,3]triazolo[1,5-a]quinolin-3-y...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1nnn2c1ccc1cc(O)ccc21 Show InChI InChI=1S/C20H16N4O4/c25-13-6-9-17-12(11-13)5-8-18-16(22-23-24(17)18)7-10-19(26)21-15-4-2-1-3-14(15)20(27)28/h1-6,8-9,11,25H,7,10H2,(H,21,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277635
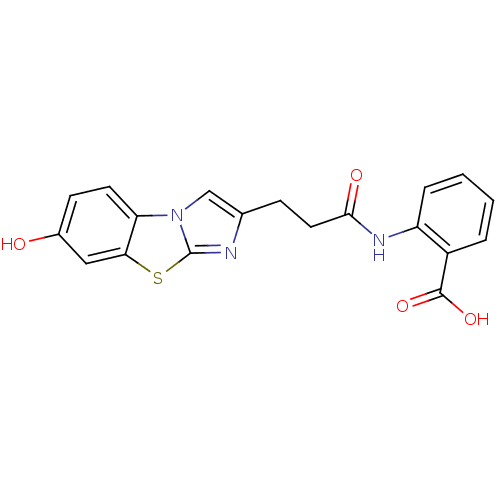
(2-[3-(7-Hydroxy-benzo[d]imidazo[2,1-b]thiazol-2-yl...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1cn2c(n1)sc1cc(O)ccc21 Show InChI InChI=1S/C19H15N3O4S/c23-12-6-7-15-16(9-12)27-19-20-11(10-22(15)19)5-8-17(24)21-14-4-2-1-3-13(14)18(25)26/h1-4,6-7,9-10,23H,5,8H2,(H,21,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277754
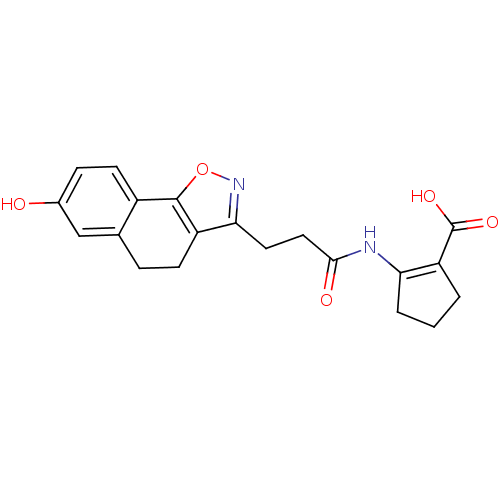
(2-(3-(7-hydroxy-4,5-dihydronaphtho[2,1-d]isoxazol-...)Show SMILES OC(=O)C1=C(CCC1)NC(=O)CCc1noc-2c1CCc1cc(O)ccc-21 |t:3| Show InChI InChI=1S/C20H20N2O5/c23-12-5-7-13-11(10-12)4-6-14-17(22-27-19(13)14)8-9-18(24)21-16-3-1-2-15(16)20(25)26/h5,7,10,23H,1-4,6,8-9H2,(H,21,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277582

(2-(3-(7-hydroxy-4,5-dihydropyrazolo[1,5-a]quinolin...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1cnn-2c1CCc1cc(O)ccc-21 Show InChI InChI=1S/C21H19N3O4/c25-15-7-9-18-13(11-15)5-8-19-14(12-22-24(18)19)6-10-20(26)23-17-4-2-1-3-16(17)21(27)28/h1-4,7,9,11-12,25H,5-6,8,10H2,(H,23,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277543
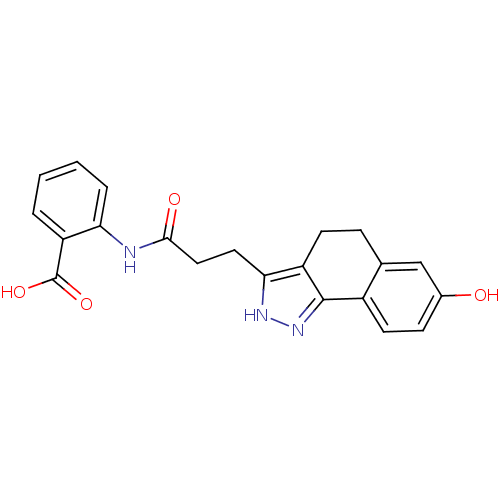
(2-(3-(7-hydroxy-4,5-dihydro-2H-benzo[g]indazol-3-y...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1[nH]nc-2c1CCc1cc(O)ccc-21 Show InChI InChI=1S/C21H19N3O4/c25-13-6-8-14-12(11-13)5-7-15-18(23-24-20(14)15)9-10-19(26)22-17-4-2-1-3-16(17)21(27)28/h1-4,6,8,11,25H,5,7,9-10H2,(H,22,26)(H,23,24)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277677
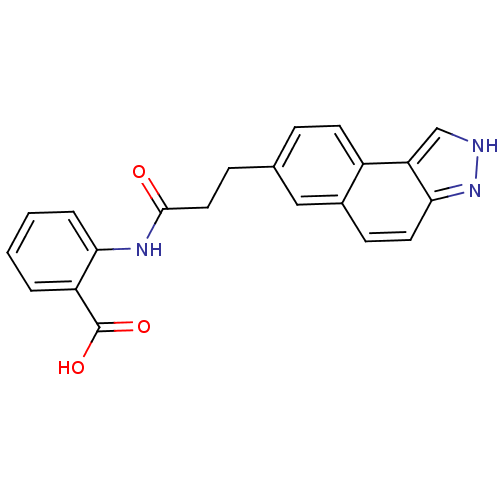
(2-(3-(3H-benzo[e]indazol-7-yl)propanamido)benzoic ...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1ccc2c3c[nH]nc3ccc2c1 Show InChI InChI=1S/C21H17N3O3/c25-20(23-18-4-2-1-3-16(18)21(26)27)10-6-13-5-8-15-14(11-13)7-9-19-17(15)12-22-24-19/h1-5,7-9,11-12H,6,10H2,(H,22,24)(H,23,25)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50264232
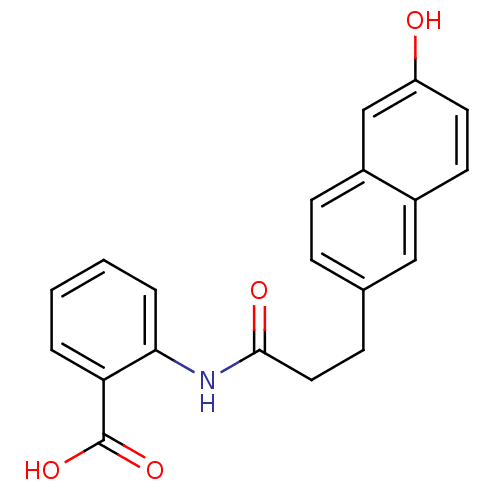
(2-(3-(6-hydroxynaphthalen-2-yl)propanamido)benzoic...)Show InChI InChI=1S/C20H17NO4/c22-16-9-8-14-11-13(5-7-15(14)12-16)6-10-19(23)21-18-4-2-1-3-17(18)20(24)25/h1-5,7-9,11-12,22H,6,10H2,(H,21,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277755

(2-[3-(7-Hydroxy-benzo[d]imidazo[2,1-b]thiazol-2-yl...)Show SMILES OC(=O)C1=C(CCC1)NC(=O)CCc1cn2c(n1)sc1cc(O)ccc21 |t:3| Show InChI InChI=1S/C18H17N3O4S/c22-11-5-6-14-15(8-11)26-18-19-10(9-21(14)18)4-7-16(23)20-13-3-1-2-12(13)17(24)25/h5-6,8-9,22H,1-4,7H2,(H,20,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277636
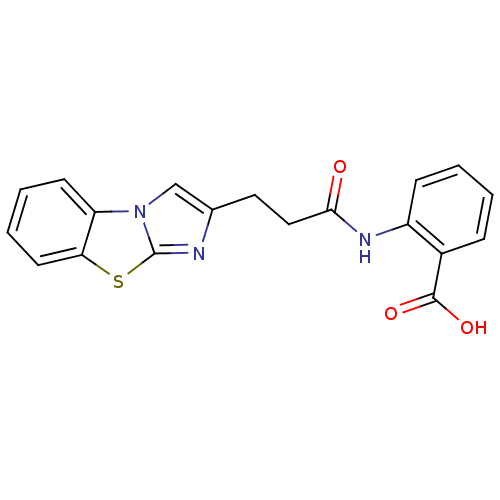
(2-(3-Benzo[d]imidazo[2,1-b]thiazol-2-yl-propionyla...)Show InChI InChI=1S/C19H15N3O3S/c23-17(21-14-6-2-1-5-13(14)18(24)25)10-9-12-11-22-15-7-3-4-8-16(15)26-19(22)20-12/h1-8,11H,9-10H2,(H,21,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277544
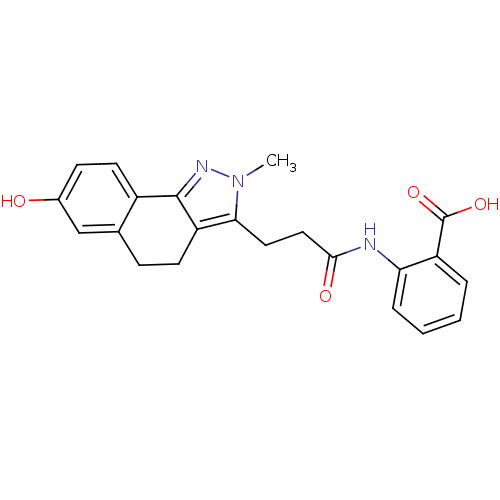
(2-(3-(7-hydroxy-2-methyl-4,5-dihydro-2H-benzo[g]in...)Show SMILES Cn1nc-2c(CCc3cc(O)ccc-23)c1CCC(=O)Nc1ccccc1C(O)=O Show InChI InChI=1S/C22H21N3O4/c1-25-19(10-11-20(27)23-18-5-3-2-4-16(18)22(28)29)17-8-6-13-12-14(26)7-9-15(13)21(17)24-25/h2-5,7,9,12,26H,6,8,10-11H2,1H3,(H,23,27)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277578
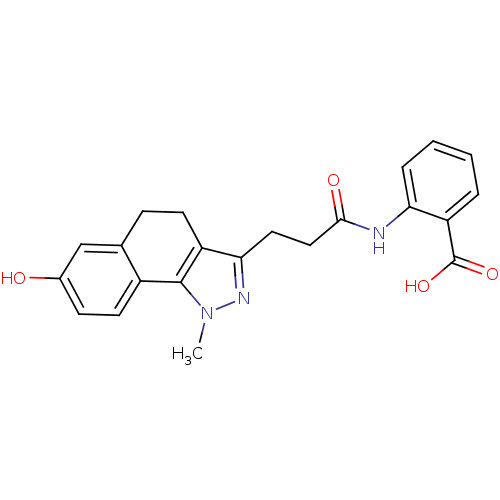
(2-(3-(7-hydroxy-1-methyl-4,5-dihydro-1H-benzo[g]in...)Show SMILES Cn1nc(CCC(=O)Nc2ccccc2C(O)=O)c2CCc3cc(O)ccc3-c12 Show InChI InChI=1S/C22H21N3O4/c1-25-21-15-9-7-14(26)12-13(15)6-8-16(21)19(24-25)10-11-20(27)23-18-5-3-2-4-17(18)22(28)29/h2-5,7,9,12,26H,6,8,10-11H2,1H3,(H,23,27)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23515

(CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...)Show InChI InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277676
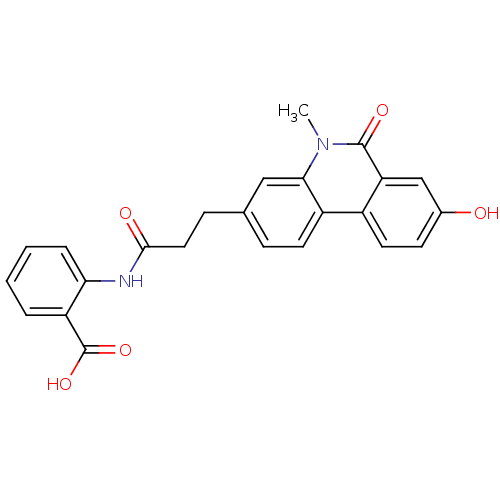
(2-(3-(8-hydroxy-5-methyl-6-oxo-5,6-dihydrophenanth...)Show SMILES Cn1c2cc(CCC(=O)Nc3ccccc3C(O)=O)ccc2c2ccc(O)cc2c1=O Show InChI InChI=1S/C24H20N2O5/c1-26-21-12-14(6-9-17(21)16-10-8-15(27)13-19(16)23(26)29)7-11-22(28)25-20-5-3-2-4-18(20)24(30)31/h2-6,8-10,12-13,27H,7,11H2,1H3,(H,25,28)(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277634
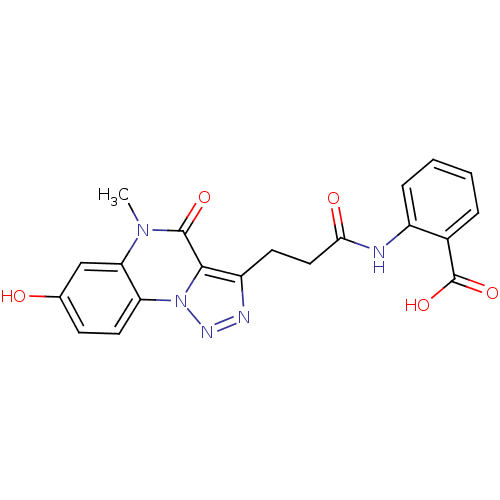
(2-(3-(7-hydroxy-5-methyl-4-oxo-4,5-dihydro-[1,2,3]...)Show SMILES Cn1c2cc(O)ccc2n2nnc(CCC(=O)Nc3ccccc3C(O)=O)c2c1=O Show InChI InChI=1S/C20H17N5O5/c1-24-16-10-11(26)6-8-15(16)25-18(19(24)28)14(22-23-25)7-9-17(27)21-13-5-3-2-4-12(13)20(29)30/h2-6,8,10,26H,7,9H2,1H3,(H,21,27)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277674
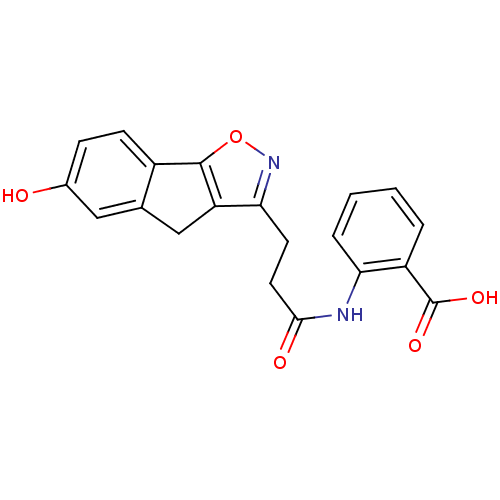
(2-(3-(6-hydroxy-4H-indeno[2,1-d]isoxazol-3-yl)prop...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1noc-2c1Cc1cc(O)ccc-21 Show InChI InChI=1S/C20H16N2O5/c23-12-5-6-13-11(9-12)10-15-17(22-27-19(13)15)7-8-18(24)21-16-4-2-1-3-14(16)20(25)26/h1-6,9,23H,7-8,10H2,(H,21,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50277581

(2-(3-(7-hydroxynaphtho[2,1-d]isoxazol-3-yl)propana...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1noc2c1ccc1cc(O)ccc21 Show InChI InChI=1S/C21H16N2O5/c24-13-6-8-14-12(11-13)5-7-15-18(23-28-20(14)15)9-10-19(25)22-17-4-2-1-3-16(17)21(26)27/h1-8,11,24H,9-10H2,(H,22,25)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50277633

(2-(3-(7-hydroxy-[1,2,3]triazolo[1,5-a]quinolin-3-y...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1nnn2c1ccc1cc(O)ccc21 Show InChI InChI=1S/C20H16N4O4/c25-13-6-9-17-12(11-13)5-8-18-16(22-23-24(17)18)7-10-19(26)21-15-4-2-1-3-14(15)20(27)28/h1-6,8-9,11,25H,7,10H2,(H,21,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50277717

(2-(3-(6-chloro-7-hydroxynaphtho[2,1-d]isoxazol-3-y...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1noc2c1ccc1c(Cl)c(O)ccc21 |t:3| Show InChI InChI=1S/C21H19ClN2O5/c22-19-11-5-6-13-16(24-29-20(13)12(11)7-9-17(19)25)8-10-18(26)23-15-4-2-1-3-14(15)21(27)28/h5-7,9,25H,1-4,8,10H2,(H,23,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50277716

(2-(3-(6-fluoro-7-hydroxynaphtho[2,1-d]isoxazol-3-y...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1noc2c1ccc1c(F)c(O)ccc21 |t:3| Show InChI InChI=1S/C21H19FN2O5/c22-19-11-5-6-13-16(24-29-20(13)12(11)7-9-17(19)25)8-10-18(26)23-15-4-2-1-3-14(15)21(27)28/h5-7,9,25H,1-4,8,10H2,(H,23,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277675
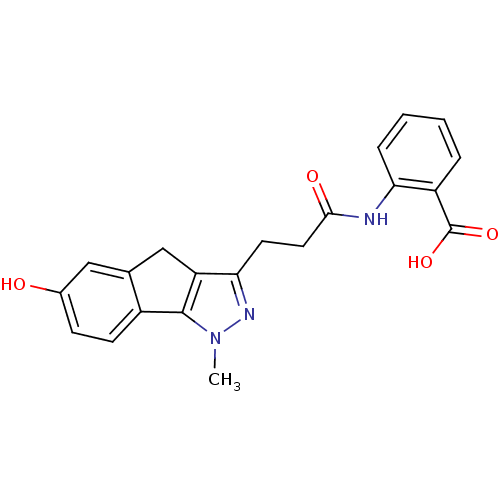
(2-(3-(6-hydroxy-1-methyl-1,4-dihydroindeno[1,2-c]p...)Show SMILES Cn1nc(CCC(=O)Nc2ccccc2C(O)=O)c2Cc3cc(O)ccc3-c12 Show InChI InChI=1S/C21H19N3O4/c1-24-20-14-7-6-13(25)10-12(14)11-16(20)18(23-24)8-9-19(26)22-17-5-3-2-4-15(17)21(27)28/h2-7,10,25H,8-9,11H2,1H3,(H,22,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A expressed in CHO cells |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50277580

(2-(3-(7-hydroxy-4,5-dihydronaphtho[2,1-d]isoxazol-...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1noc-2c1CCc1cc(O)ccc-21 Show InChI InChI=1S/C21H18N2O5/c24-13-6-8-14-12(11-13)5-7-15-18(23-28-20(14)15)9-10-19(25)22-17-4-2-1-3-16(17)21(26)27/h1-4,6,8,11,24H,5,7,9-10H2,(H,22,25)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50277636

(2-(3-Benzo[d]imidazo[2,1-b]thiazol-2-yl-propionyla...)Show InChI InChI=1S/C19H15N3O3S/c23-17(21-14-6-2-1-5-13(14)18(24)25)10-9-12-11-22-15-7-3-4-8-16(15)26-19(22)20-12/h1-8,11H,9-10H2,(H,21,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50277676

(2-(3-(8-hydroxy-5-methyl-6-oxo-5,6-dihydrophenanth...)Show SMILES Cn1c2cc(CCC(=O)Nc3ccccc3C(O)=O)ccc2c2ccc(O)cc2c1=O Show InChI InChI=1S/C24H20N2O5/c1-26-21-12-14(6-9-17(21)16-10-8-15(27)13-19(16)23(26)29)7-11-22(28)25-20-5-3-2-4-18(20)24(30)31/h2-6,8-10,12-13,27H,7,11H2,1H3,(H,25,28)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50277579

(2-(3-(7-hydroxy-2H-benzo[g]indazol-3-yl)propanamid...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1[nH]nc2c1ccc1cc(O)ccc21 Show InChI InChI=1S/C21H17N3O4/c25-13-6-8-14-12(11-13)5-7-15-18(23-24-20(14)15)9-10-19(26)22-17-4-2-1-3-16(17)21(27)28/h1-8,11,25H,9-10H2,(H,22,26)(H,23,24)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using montelukast substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50277580

(2-(3-(7-hydroxy-4,5-dihydronaphtho[2,1-d]isoxazol-...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1noc-2c1CCc1cc(O)ccc-21 Show InChI InChI=1S/C21H18N2O5/c24-13-6-8-14-12(11-13)5-7-15-18(23-28-20(14)15)9-10-19(25)22-17-4-2-1-3-16(17)21(26)27/h1-4,6,8,11,24H,5,7,9-10H2,(H,22,25)(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using montelukast substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50277674

(2-(3-(6-hydroxy-4H-indeno[2,1-d]isoxazol-3-yl)prop...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1noc-2c1Cc1cc(O)ccc-21 Show InChI InChI=1S/C20H16N2O5/c23-12-5-6-13-11(9-12)10-15-17(22-27-19(13)15)7-8-18(24)21-16-4-2-1-3-14(16)20(25)26/h1-6,9,23H,7-8,10H2,(H,21,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50277579

(2-(3-(7-hydroxy-2H-benzo[g]indazol-3-yl)propanamid...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1[nH]nc2c1ccc1cc(O)ccc21 Show InChI InChI=1S/C21H17N3O4/c25-13-6-8-14-12(11-13)5-7-15-18(23-24-20(14)15)9-10-19(26)22-17-4-2-1-3-16(17)21(27)28/h1-8,11,25H,9-10H2,(H,22,26)(H,23,24)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50277714

(2-(3-(7-hydroxy-4,5-dihydronaphtho[2,1-d]isoxazol-...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1noc-2c1CCc1cc(O)ccc-21 |t:3| Show InChI InChI=1S/C21H22N2O5/c24-13-6-8-14-12(11-13)5-7-15-18(23-28-20(14)15)9-10-19(25)22-17-4-2-1-3-16(17)21(26)27/h6,8,11,24H,1-5,7,9-10H2,(H,22,25)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50277582

(2-(3-(7-hydroxy-4,5-dihydropyrazolo[1,5-a]quinolin...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1cnn-2c1CCc1cc(O)ccc-21 Show InChI InChI=1S/C21H19N3O4/c25-15-7-9-18-13(11-15)5-8-19-14(12-22-24(18)19)6-10-20(26)23-17-4-2-1-3-16(17)21(27)28/h1-4,7,9,11-12,25H,5-6,8,10H2,(H,23,26)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using montelukast substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50277755

(2-[3-(7-Hydroxy-benzo[d]imidazo[2,1-b]thiazol-2-yl...)Show SMILES OC(=O)C1=C(CCC1)NC(=O)CCc1cn2c(n1)sc1cc(O)ccc21 |t:3| Show InChI InChI=1S/C18H17N3O4S/c22-11-5-6-14-15(8-11)26-18-19-10(9-21(14)18)4-7-16(23)20-13-3-1-2-12(13)17(24)25/h5-6,8-9,22H,1-4,7H2,(H,20,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50277753

(2-[3-(7-Hydroxy-benzo[d]imidazo[2,1-b]thiazol-2-yl...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1cn2c(n1)sc1cc(O)ccc21 |t:3| Show InChI InChI=1S/C19H19N3O4S/c23-12-6-7-15-16(9-12)27-19-20-11(10-22(15)19)5-8-17(24)21-14-4-2-1-3-13(14)18(25)26/h6-7,9-10,23H,1-5,8H2,(H,21,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50277715

(2-(3-(7-hydroxynaphtho[2,1-d]isoxazol-3-yl)propana...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1noc2c1ccc1cc(O)ccc21 |t:3| Show InChI InChI=1S/C21H20N2O5/c24-13-6-8-14-12(11-13)5-7-15-18(23-28-20(14)15)9-10-19(25)22-17-4-2-1-3-16(17)21(26)27/h5-8,11,24H,1-4,9-10H2,(H,22,25)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50277578

(2-(3-(7-hydroxy-1-methyl-4,5-dihydro-1H-benzo[g]in...)Show SMILES Cn1nc(CCC(=O)Nc2ccccc2C(O)=O)c2CCc3cc(O)ccc3-c12 Show InChI InChI=1S/C22H21N3O4/c1-25-21-15-9-7-14(26)12-13(15)6-8-16(21)19(24-25)10-11-20(27)23-18-5-3-2-4-17(18)22(28)29/h2-5,7,9,12,26H,6,8,10-11H2,1H3,(H,23,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50277582

(2-(3-(7-hydroxy-4,5-dihydropyrazolo[1,5-a]quinolin...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1cnn-2c1CCc1cc(O)ccc-21 Show InChI InChI=1S/C21H19N3O4/c25-15-7-9-18-13(11-15)5-8-19-14(12-22-24(18)19)6-10-20(26)23-17-4-2-1-3-16(17)21(27)28/h1-4,7,9,11-12,25H,5-6,8,10H2,(H,23,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50277718

(2-(3-(7-hydroxypyrazolo[1,5-a]quinolin-3-yl)propan...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1cnn2c1ccc1cc(O)ccc21 |t:3| Show InChI InChI=1S/C21H21N3O4/c25-15-7-9-18-13(11-15)5-8-19-14(12-22-24(18)19)6-10-20(26)23-17-4-2-1-3-16(17)21(27)28/h5,7-9,11-12,25H,1-4,6,10H2,(H,23,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50277674

(2-(3-(6-hydroxy-4H-indeno[2,1-d]isoxazol-3-yl)prop...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1noc-2c1Cc1cc(O)ccc-21 Show InChI InChI=1S/C20H16N2O5/c23-12-5-6-13-11(9-12)10-15-17(22-27-19(13)15)7-8-18(24)21-16-4-2-1-3-14(16)20(25)26/h1-6,9,23H,7-8,10H2,(H,21,24)(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using montelukast substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50277543

(2-(3-(7-hydroxy-4,5-dihydro-2H-benzo[g]indazol-3-y...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1[nH]nc-2c1CCc1cc(O)ccc-21 Show InChI InChI=1S/C21H19N3O4/c25-13-6-8-14-12(11-13)5-7-15-18(23-24-20(14)15)9-10-19(26)22-17-4-2-1-3-16(17)21(27)28/h1-4,6,8,11,25H,5,7,9-10H2,(H,22,26)(H,23,24)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using montelukast substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50277543

(2-(3-(7-hydroxy-4,5-dihydro-2H-benzo[g]indazol-3-y...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1[nH]nc-2c1CCc1cc(O)ccc-21 Show InChI InChI=1S/C21H19N3O4/c25-13-6-8-14-12(11-13)5-7-15-18(23-24-20(14)15)9-10-19(26)22-17-4-2-1-3-16(17)21(27)28/h1-4,6,8,11,25H,5,7,9-10H2,(H,22,26)(H,23,24)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50277714

(2-(3-(7-hydroxy-4,5-dihydronaphtho[2,1-d]isoxazol-...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1noc-2c1CCc1cc(O)ccc-21 |t:3| Show InChI InChI=1S/C21H22N2O5/c24-13-6-8-14-12(11-13)5-7-15-18(23-28-20(14)15)9-10-19(25)22-17-4-2-1-3-16(17)21(26)27/h6,8,11,24H,1-5,7,9-10H2,(H,22,25)(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using montelukast substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50277754

(2-(3-(7-hydroxy-4,5-dihydronaphtho[2,1-d]isoxazol-...)Show SMILES OC(=O)C1=C(CCC1)NC(=O)CCc1noc-2c1CCc1cc(O)ccc-21 |t:3| Show InChI InChI=1S/C20H20N2O5/c23-12-5-7-13-11(10-12)4-6-14-17(22-27-19(13)14)8-9-18(24)21-16-3-1-2-15(16)20(25)26/h5,7,10,23H,1-4,6,8-9H2,(H,21,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50277754

(2-(3-(7-hydroxy-4,5-dihydronaphtho[2,1-d]isoxazol-...)Show SMILES OC(=O)C1=C(CCC1)NC(=O)CCc1noc-2c1CCc1cc(O)ccc-21 |t:3| Show InChI InChI=1S/C20H20N2O5/c23-12-5-7-13-11(10-12)4-6-14-17(22-27-19(13)14)8-9-18(24)21-16-3-1-2-15(16)20(25)26/h5,7,10,23H,1-4,6,8-9H2,(H,21,24)(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using montelukast substrate |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23519

(2-[3-(4-phenylphenyl)propanamido]benzoic acid | Bi...)Show InChI InChI=1S/C22H19NO3/c24-21(23-20-9-5-4-8-19(20)22(25)26)15-12-16-10-13-18(14-11-16)17-6-2-1-3-7-17/h1-11,13-14H,12,15H2,(H,23,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 590 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277633

(2-(3-(7-hydroxy-[1,2,3]triazolo[1,5-a]quinolin-3-y...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1nnn2c1ccc1cc(O)ccc21 Show InChI InChI=1S/C20H16N4O4/c25-13-6-9-17-12(11-13)5-8-18-16(22-23-24(17)18)7-10-19(26)21-15-4-2-1-3-14(15)20(27)28/h1-6,8-9,11,25H,7,10H2,(H,21,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277578

(2-(3-(7-hydroxy-1-methyl-4,5-dihydro-1H-benzo[g]in...)Show SMILES Cn1nc(CCC(=O)Nc2ccccc2C(O)=O)c2CCc3cc(O)ccc3-c12 Show InChI InChI=1S/C22H21N3O4/c1-25-21-15-9-7-14(26)12-13(15)6-8-16(21)19(24-25)10-11-20(27)23-18-5-3-2-4-17(18)22(28)29/h2-5,7,9,12,26H,6,8,10-11H2,1H3,(H,23,27)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 840 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277581

(2-(3-(7-hydroxynaphtho[2,1-d]isoxazol-3-yl)propana...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1noc2c1ccc1cc(O)ccc21 Show InChI InChI=1S/C21H16N2O5/c24-13-6-8-14-12(11-13)5-7-15-18(23-28-20(14)15)9-10-19(25)22-17-4-2-1-3-16(17)21(26)27/h1-8,11,24H,9-10H2,(H,22,25)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 51 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277636

(2-(3-Benzo[d]imidazo[2,1-b]thiazol-2-yl-propionyla...)Show InChI InChI=1S/C19H15N3O3S/c23-17(21-14-6-2-1-5-13(14)18(24)25)10-9-12-11-22-15-7-3-4-8-16(15)26-19(22)20-12/h1-8,11H,9-10H2,(H,21,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 690 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277754

(2-(3-(7-hydroxy-4,5-dihydronaphtho[2,1-d]isoxazol-...)Show SMILES OC(=O)C1=C(CCC1)NC(=O)CCc1noc-2c1CCc1cc(O)ccc-21 |t:3| Show InChI InChI=1S/C20H20N2O5/c23-12-5-7-13-11(10-12)4-6-14-17(22-27-19(13)14)8-9-18(24)21-16-3-1-2-15(16)20(25)26/h5,7,10,23H,1-4,6,8-9H2,(H,21,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277542
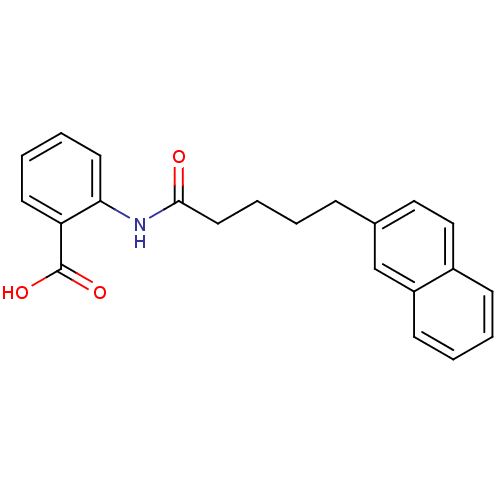
(2-(5-(naphthalen-2-yl)pentanamido)benzoic acid | C...)Show InChI InChI=1S/C22H21NO3/c24-21(23-20-11-5-4-10-19(20)22(25)26)12-6-1-7-16-13-14-17-8-2-3-9-18(17)15-16/h2-5,8-11,13-15H,1,6-7,12H2,(H,23,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23515

(CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...)Show InChI InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277755

(2-[3-(7-Hydroxy-benzo[d]imidazo[2,1-b]thiazol-2-yl...)Show SMILES OC(=O)C1=C(CCC1)NC(=O)CCc1cn2c(n1)sc1cc(O)ccc21 |t:3| Show InChI InChI=1S/C18H17N3O4S/c22-11-5-6-14-15(8-11)26-18-19-10(9-21(14)18)4-7-16(23)20-13-3-1-2-12(13)17(24)25/h5-6,8-9,22H,1-4,7H2,(H,20,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 620 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Rattus norvegicus) | BDBM23515

(CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...)Show InChI InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 670 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at rat GPR109A expressed by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Rattus norvegicus) | BDBM50277582

(2-(3-(7-hydroxy-4,5-dihydropyrazolo[1,5-a]quinolin...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1cnn-2c1CCc1cc(O)ccc-21 Show InChI InChI=1S/C21H19N3O4/c25-15-7-9-18-13(11-15)5-8-19-14(12-22-24(18)19)6-10-20(26)23-17-4-2-1-3-16(17)21(27)28/h1-4,7,9,11-12,25H,5-6,8,10H2,(H,23,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at rat GPR109A expressed by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23518

(2-[3-(naphthalen-2-yl)propanamido]benzoic acid | B...)Show InChI InChI=1S/C20H17NO3/c22-19(21-18-8-4-3-7-17(18)20(23)24)12-10-14-9-11-15-5-1-2-6-16(15)13-14/h1-9,11,13H,10,12H2,(H,21,22)(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277674

(2-(3-(6-hydroxy-4H-indeno[2,1-d]isoxazol-3-yl)prop...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1noc-2c1Cc1cc(O)ccc-21 Show InChI InChI=1S/C20H16N2O5/c23-12-5-6-13-11(9-12)10-15-17(22-27-19(13)15)7-8-18(24)21-16-4-2-1-3-14(16)20(25)26/h1-6,9,23H,7-8,10H2,(H,21,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277714

(2-(3-(7-hydroxy-4,5-dihydronaphtho[2,1-d]isoxazol-...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1noc-2c1CCc1cc(O)ccc-21 |t:3| Show InChI InChI=1S/C21H22N2O5/c24-13-6-8-14-12(11-13)5-7-15-18(23-28-20(14)15)9-10-19(25)22-17-4-2-1-3-16(17)21(26)27/h6,8,11,24H,1-5,7,9-10H2,(H,22,25)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277582

(2-(3-(7-hydroxy-4,5-dihydropyrazolo[1,5-a]quinolin...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1cnn-2c1CCc1cc(O)ccc-21 Show InChI InChI=1S/C21H19N3O4/c25-15-7-9-18-13(11-15)5-8-19-14(12-22-24(18)19)6-10-20(26)23-17-4-2-1-3-16(17)21(27)28/h1-4,7,9,11-12,25H,5-6,8,10H2,(H,23,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 64 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50264232

(2-(3-(6-hydroxynaphthalen-2-yl)propanamido)benzoic...)Show InChI InChI=1S/C20H17NO4/c22-16-9-8-14-11-13(5-7-15(14)12-16)6-10-19(23)21-18-4-2-1-3-17(18)20(24)25/h1-5,7-9,11-12,22H,6,10H2,(H,21,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 340 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277634

(2-(3-(7-hydroxy-5-methyl-4-oxo-4,5-dihydro-[1,2,3]...)Show SMILES Cn1c2cc(O)ccc2n2nnc(CCC(=O)Nc3ccccc3C(O)=O)c2c1=O Show InChI InChI=1S/C20H17N5O5/c1-24-16-10-11(26)6-8-15(16)25-18(19(24)28)14(22-23-25)7-9-17(27)21-13-5-3-2-4-12(13)20(29)30/h2-6,8,10,26H,7,9H2,1H3,(H,21,27)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277635

(2-[3-(7-Hydroxy-benzo[d]imidazo[2,1-b]thiazol-2-yl...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1cn2c(n1)sc1cc(O)ccc21 Show InChI InChI=1S/C19H15N3O4S/c23-12-6-7-15-16(9-12)27-19-20-11(10-22(15)19)5-8-17(24)21-14-4-2-1-3-13(14)18(25)26/h1-4,6-7,9-10,23H,5,8H2,(H,21,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277676

(2-(3-(8-hydroxy-5-methyl-6-oxo-5,6-dihydrophenanth...)Show SMILES Cn1c2cc(CCC(=O)Nc3ccccc3C(O)=O)ccc2c2ccc(O)cc2c1=O Show InChI InChI=1S/C24H20N2O5/c1-26-21-12-14(6-9-17(21)16-10-8-15(27)13-19(16)23(26)29)7-11-22(28)25-20-5-3-2-4-18(20)24(30)31/h2-6,8-10,12-13,27H,7,11H2,1H3,(H,25,28)(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277715

(2-(3-(7-hydroxynaphtho[2,1-d]isoxazol-3-yl)propana...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1noc2c1ccc1cc(O)ccc21 |t:3| Show InChI InChI=1S/C21H20N2O5/c24-13-6-8-14-12(11-13)5-7-15-18(23-28-20(14)15)9-10-19(25)22-17-4-2-1-3-16(17)21(26)27/h5-8,11,24H,1-4,9-10H2,(H,22,25)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277717

(2-(3-(6-chloro-7-hydroxynaphtho[2,1-d]isoxazol-3-y...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1noc2c1ccc1c(Cl)c(O)ccc21 |t:3| Show InChI InChI=1S/C21H19ClN2O5/c22-19-11-5-6-13-16(24-29-20(13)12(11)7-9-17(19)25)8-10-18(26)23-15-4-2-1-3-14(15)21(27)28/h5-7,9,25H,1-4,8,10H2,(H,23,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 43 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23517
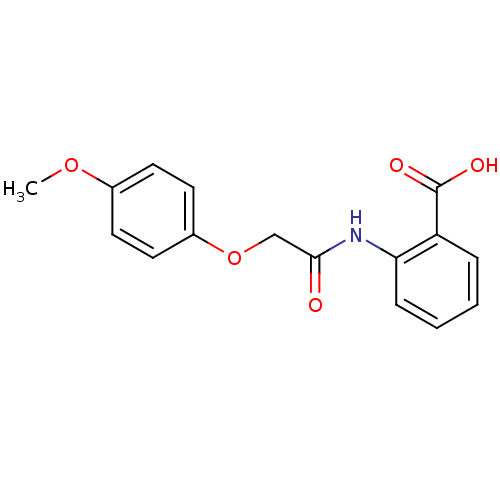
(2-[2-(4-methoxyphenoxy)acetamido]benzoic acid | Bi...)Show InChI InChI=1S/C16H15NO5/c1-21-11-6-8-12(9-7-11)22-10-15(18)17-14-5-3-2-4-13(14)16(19)20/h2-9H,10H2,1H3,(H,17,18)(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277544

(2-(3-(7-hydroxy-2-methyl-4,5-dihydro-2H-benzo[g]in...)Show SMILES Cn1nc-2c(CCc3cc(O)ccc-23)c1CCC(=O)Nc1ccccc1C(O)=O Show InChI InChI=1S/C22H21N3O4/c1-25-19(10-11-20(27)23-18-5-3-2-4-16(18)22(28)29)17-8-6-13-12-14(26)7-9-15(13)21(17)24-25/h2-5,7,9,12,26H,6,8,10-11H2,1H3,(H,23,27)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277716

(2-(3-(6-fluoro-7-hydroxynaphtho[2,1-d]isoxazol-3-y...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1noc2c1ccc1c(F)c(O)ccc21 |t:3| Show InChI InChI=1S/C21H19FN2O5/c22-19-11-5-6-13-16(24-29-20(13)12(11)7-9-17(19)25)8-10-18(26)23-15-4-2-1-3-14(15)21(27)28/h5-7,9,25H,1-4,8,10H2,(H,23,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277718

(2-(3-(7-hydroxypyrazolo[1,5-a]quinolin-3-yl)propan...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1cnn2c1ccc1cc(O)ccc21 |t:3| Show InChI InChI=1S/C21H21N3O4/c25-15-7-9-18-13(11-15)5-8-19-14(12-22-24(18)19)6-10-20(26)23-17-4-2-1-3-16(17)21(27)28/h5,7-9,11-12,25H,1-4,6,10H2,(H,23,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277580

(2-(3-(7-hydroxy-4,5-dihydronaphtho[2,1-d]isoxazol-...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1noc-2c1CCc1cc(O)ccc-21 Show InChI InChI=1S/C21H18N2O5/c24-13-6-8-14-12(11-13)5-7-15-18(23-28-20(14)15)9-10-19(25)22-17-4-2-1-3-16(17)21(26)27/h1-4,6,8,11,24H,5,7,9-10H2,(H,22,25)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277675

(2-(3-(6-hydroxy-1-methyl-1,4-dihydroindeno[1,2-c]p...)Show SMILES Cn1nc(CCC(=O)Nc2ccccc2C(O)=O)c2Cc3cc(O)ccc3-c12 Show InChI InChI=1S/C21H19N3O4/c1-24-20-14-7-6-13(25)10-12(14)11-16(20)18(23-24)8-9-19(26)22-17-5-3-2-4-15(17)21(27)28/h2-7,10,25H,8-9,11H2,1H3,(H,22,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277677

(2-(3-(3H-benzo[e]indazol-7-yl)propanamido)benzoic ...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1ccc2c3c[nH]nc3ccc2c1 Show InChI InChI=1S/C21H17N3O3/c25-20(23-18-4-2-1-3-16(18)21(26)27)10-6-13-5-8-15-14(11-13)7-9-19-17(15)12-22-24-19/h1-5,7-9,11-12H,6,10H2,(H,22,24)(H,23,25)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277753

(2-[3-(7-Hydroxy-benzo[d]imidazo[2,1-b]thiazol-2-yl...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1cn2c(n1)sc1cc(O)ccc21 |t:3| Show InChI InChI=1S/C19H19N3O4S/c23-12-6-7-15-16(9-12)27-19-20-11(10-22(15)19)5-8-17(24)21-14-4-2-1-3-13(14)18(25)26/h6-7,9-10,23H,1-5,8H2,(H,21,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 76 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277579

(2-(3-(7-hydroxy-2H-benzo[g]indazol-3-yl)propanamid...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1[nH]nc2c1ccc1cc(O)ccc21 Show InChI InChI=1S/C21H17N3O4/c25-13-6-8-14-12(11-13)5-7-15-18(23-24-20(14)15)9-10-19(26)22-17-4-2-1-3-16(17)21(27)28/h1-8,11,25H,9-10H2,(H,22,26)(H,23,24)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 34 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277543

(2-(3-(7-hydroxy-4,5-dihydro-2H-benzo[g]indazol-3-y...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1[nH]nc-2c1CCc1cc(O)ccc-21 Show InChI InChI=1S/C21H19N3O4/c25-13-6-8-14-12(11-13)5-7-15-18(23-24-20(14)15)9-10-19(26)22-17-4-2-1-3-16(17)21(27)28/h1-4,6,8,11,25H,5,7,9-10H2,(H,22,26)(H,23,24)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 74 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data