

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50258790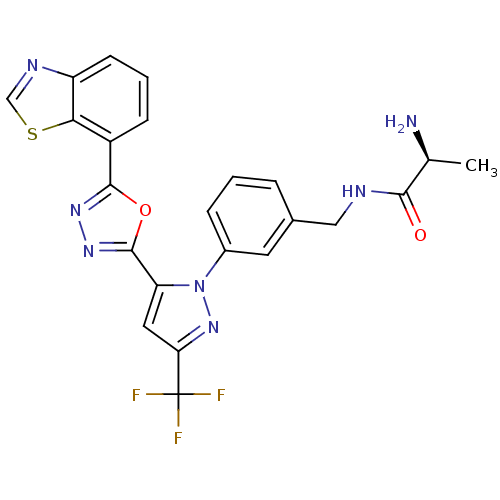 ((S)-2-amino-N-(3-(5-(5-(benzo[d]thiazol-7-yl)-1,3,...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CARM1 assessed as blockade of histone H3 methylation | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50258814 ((S)-2-amino-N-(3-(5-(5-(2-methoxyphenylamino)-1,3,...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CARM1 assessed as blockade of histone H3 methylation | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50258789 ((S)-2-amino-N-(3-(5-(5-(2-chlorophenyl)-1,3,4-oxad...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CARM1 assessed as blockade of histone H3 methylation | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50258787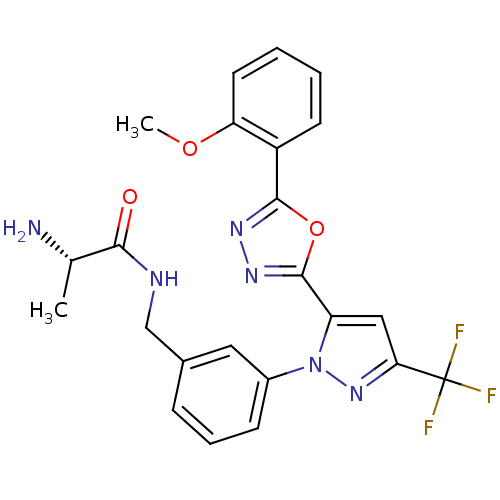 ((S)-2-amino-N-(3-(5-(5-(2-methoxyphenyl)-1,3,4-oxa...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CARM1 assessed as blockade of histone H3 methylation | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50258747 ((S)-2-amino-N-(3-(5-(5-phenyl-1,3,4-oxadiazol-2-yl...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CARM1 assessed as blockade of histone H3 methylation | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM27659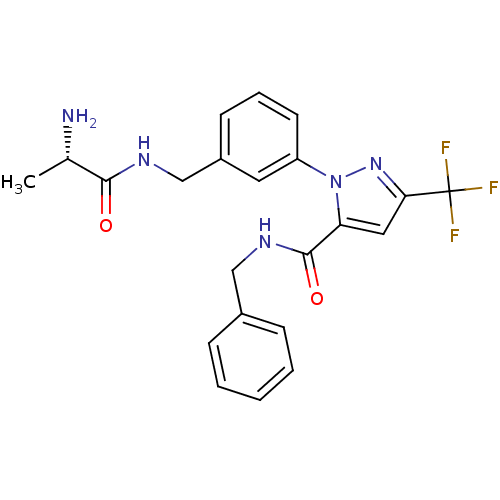 (1-(3-{[(2S)-2-aminopropanamido]methyl}phenyl)-N-be...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CARM1 assessed as blockade of histone H3 methylation | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50258815 ((S)-2-amino-N-(3-(3-methyl-5-(5-phenyl-1,3,4-oxadi...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CARM1 assessed as blockade of histone H3 methylation | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50258788 ((S)-2-amino-N-(3-(5-(5-(3-methoxyphenyl)-1,3,4-oxa...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CARM1 assessed as blockade of histone H3 methylation | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50258748 ((S)-2-amino-N-(3-(5-(3-phenyl-1,2,4-oxadiazol-5-yl...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CARM1 assessed as blockade of histone H3 methylation | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50258749 ((S)-2-amino-N-(3-(5-(5-benzyl-1,3,4-oxadiazol-2-yl...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CARM1 assessed as blockade of histone H3 methylation | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50258859 ((S)-2-amino-N-(3-(4-(5-phenyl-1,3,4-oxadiazol-2-yl...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CARM1 assessed as blockade of histone H3 methylation | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50258817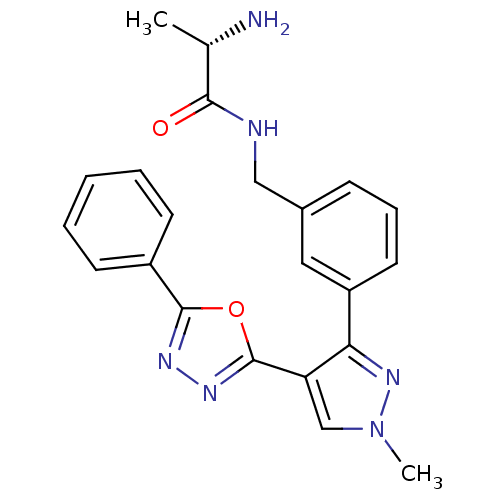 ((S)-2-amino-N-(3-(1-methyl-4-(5-phenyl-1,3,4-oxadi...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CARM1 assessed as blockade of histone H3 methylation | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50258750 ((S)-2-amino-N-(3-(5-(5-(pyridin-4-yl)-1,3,4-oxadia...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CARM1 assessed as blockade of histone H3 methylation | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50258860 ((S)-2-amino-N-(3-(5-(5-phenyl-1,3,4-oxadiazol-2-yl...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CARM1 assessed as blockade of histone H3 methylation | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50258816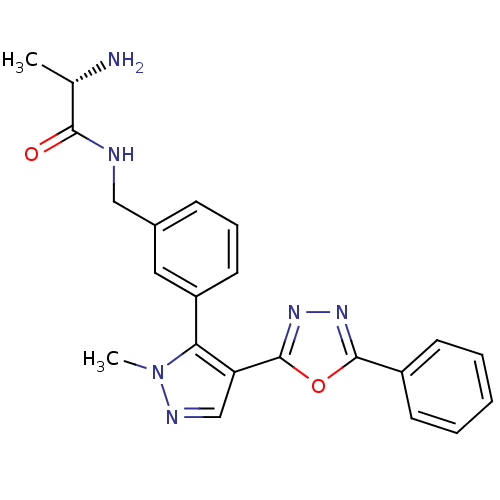 ((S)-2-amino-N-(3-(1-methyl-4-(5-phenyl-1,3,4-oxadi...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CARM1 assessed as blockade of histone H3 methylation | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50258862 ((S)-N1-(3-(5-(5-phenyl-1,3,4-oxadiazol-2-yl)-3-(tr...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CARM1 assessed as blockade of histone H3 methylation | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50258861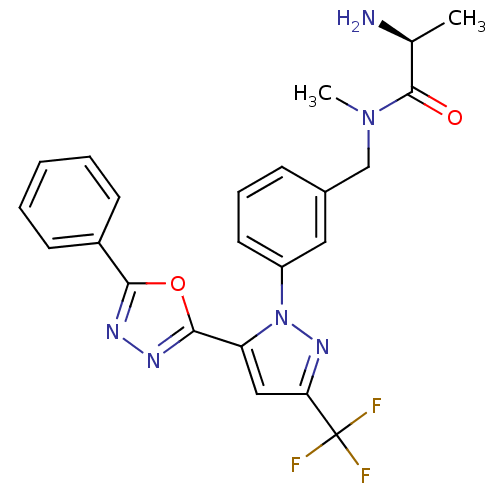 ((S)-2-amino-N-methyl-N-(3-(5-(5-phenyl-1,3,4-oxadi...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CARM1 assessed as blockade of histone H3 methylation | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM50258790 ((S)-2-amino-N-(3-(5-(5-(benzo[d]thiazol-7-yl)-1,3,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of PRMT1 by methylation assay | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 3 [N508S] (Homo sapiens (Human)) | BDBM50258790 ((S)-2-amino-N-(3-(5-(5-(benzo[d]thiazol-7-yl)-1,3,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of PRMT3 by methylation assay | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50258790 ((S)-2-amino-N-(3-(5-(5-(benzo[d]thiazol-7-yl)-1,3,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 expressed in human hepatocytes | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50258790 ((S)-2-amino-N-(3-(5-(5-(benzo[d]thiazol-7-yl)-1,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C9 expressed in human hepatocytes | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50258790 ((S)-2-amino-N-(3-(5-(5-(benzo[d]thiazol-7-yl)-1,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP3A4 expressed in human hepatocytes | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50258790 ((S)-2-amino-N-(3-(5-(5-(benzo[d]thiazol-7-yl)-1,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C19 expressed in human hepatocytes | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50258790 ((S)-2-amino-N-(3-(5-(5-(benzo[d]thiazol-7-yl)-1,3,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human PXR | Bioorg Med Chem Lett 19: 2924-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.075 BindingDB Entry DOI: 10.7270/Q24B3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||