 Found 57 hits of Enzyme Inhibition Constant Data
Found 57 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM35283

(CHEMBL551991 | cyanoguanidine, 3)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of t-PA |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM35283

(CHEMBL551991 | cyanoguanidine, 3)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM35283

(CHEMBL551991 | cyanoguanidine, 3)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of u-PA |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM35283

(CHEMBL551991 | cyanoguanidine, 3)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of plasmin |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM35283

(CHEMBL551991 | cyanoguanidine, 3)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296293
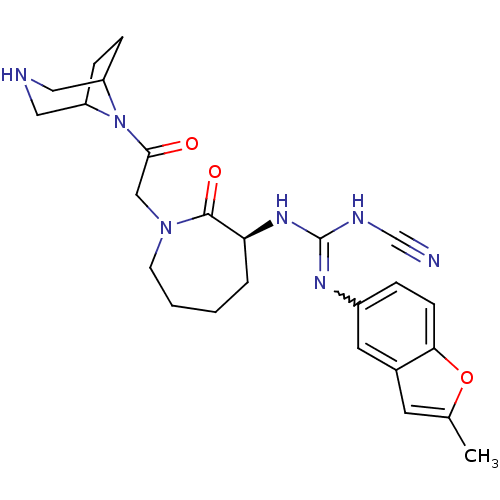
(1-((3S)-1-(2-(3,8-diazabicyclo[3.2.1]octan-8-yl)-2...)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2C3CCC2CNC3)C1=O |r,w:10.11,TLB:23:25:31.32.30:27.28| Show InChI InChI=1S/C25H31N7O3/c1-16-10-17-11-18(5-8-22(17)35-16)29-25(28-15-26)30-21-4-2-3-9-31(24(21)34)14-23(33)32-19-6-7-20(32)13-27-12-19/h5,8,10-11,19-21,27H,2-4,6-7,9,12-14H2,1H3,(H2,28,29,30)/t19?,20?,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM35283

(CHEMBL551991 | cyanoguanidine, 3)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296274

((S)-2-cyano-1-(7-fluoro-2-methylbenzofuran-5-yl)-3...)Show SMILES Cc1cc2cc(cc(F)c2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:11.12| Show InChI InChI=1S/C23H27FN6O3/c1-15-10-16-11-17(12-18(24)21(16)33-15)27-23(26-14-25)28-19-6-2-3-9-30(22(19)32)13-20(31)29-7-4-5-8-29/h10-12,19H,2-9,13H2,1H3,(H2,26,27,28)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296286

(1-((3S)-1-(2-(3-azabicyclo[3.1.0]hexan-3-yl)-2-oxo...)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CC3CC3C2)C1=O |r,w:10.11| Show InChI InChI=1S/C24H28N6O3/c1-15-8-16-10-19(5-6-21(16)33-15)27-24(26-14-25)28-20-4-2-3-7-29(23(20)32)13-22(31)30-11-17-9-18(17)12-30/h5-6,8,10,17-18,20H,2-4,7,9,11-13H2,1H3,(H2,26,27,28)/t17?,18?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296287
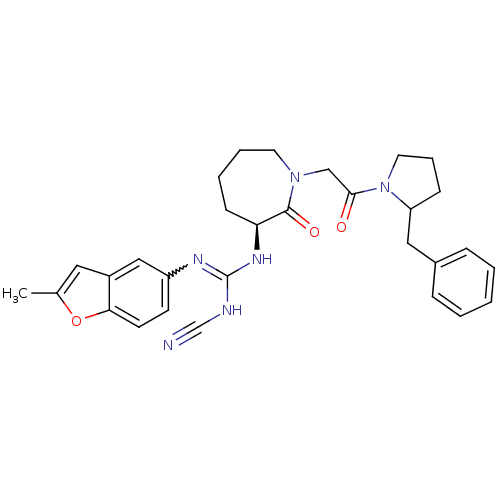
(1-((3S)-1-(2-(2-benzylpyrrolidin-1-yl)-2-oxoethyl)...)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2Cc2ccccc2)C1=O |r,w:10.11| Show InChI InChI=1S/C30H34N6O3/c1-21-16-23-18-24(12-13-27(23)39-21)33-30(32-20-31)34-26-11-5-6-14-35(29(26)38)19-28(37)36-15-7-10-25(36)17-22-8-3-2-4-9-22/h2-4,8-9,12-13,16,18,25-26H,5-7,10-11,14-15,17,19H2,1H3,(H2,32,33,34)/t25?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296278

((S)-2-cyano-1-(2,7-dimethylbenzofuran-5-yl)-3-(2-o...)Show SMILES Cc1cc2cc(cc(C)c2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:11.12| Show InChI InChI=1S/C24H30N6O3/c1-16-11-19(13-18-12-17(2)33-22(16)18)27-24(26-15-25)28-20-7-3-4-10-30(23(20)32)14-21(31)29-8-5-6-9-29/h11-13,20H,3-10,14H2,1-2H3,(H2,26,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296290
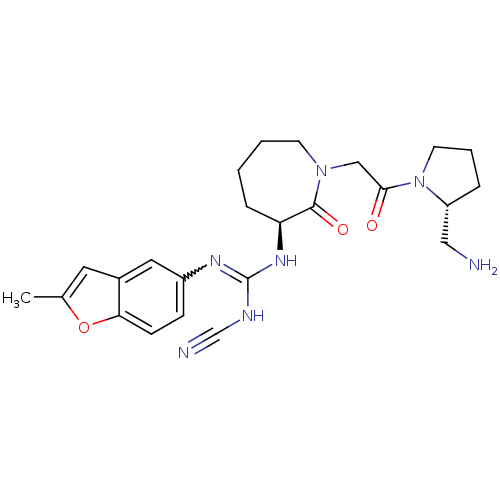
(1-((S)-1-(2-((R)-2-(aminomethyl)pyrrolidin-1-yl)-2...)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCC[C@@H]2CN)C1=O |r,w:10.11| Show InChI InChI=1S/C24H31N7O3/c1-16-11-17-12-18(7-8-21(17)34-16)28-24(27-15-26)29-20-6-2-3-9-30(23(20)33)14-22(32)31-10-4-5-19(31)13-25/h7-8,11-12,19-20H,2-6,9-10,13-14,25H2,1H3,(H2,27,28,29)/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296277
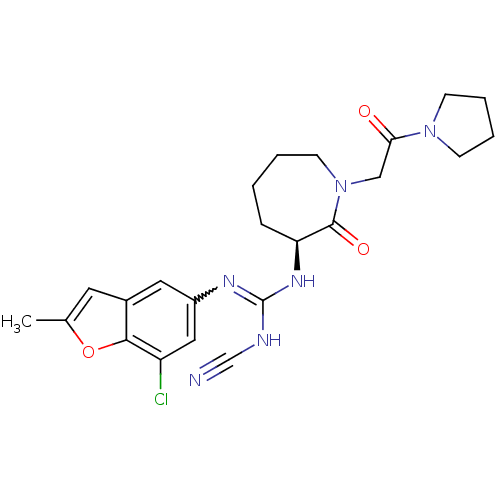
((S)-1-(7-chloro-2-methylbenzofuran-5-yl)-2-cyano-3...)Show SMILES Cc1cc2cc(cc(Cl)c2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:11.12| Show InChI InChI=1S/C23H27ClN6O3/c1-15-10-16-11-17(12-18(24)21(16)33-15)27-23(26-14-25)28-19-6-2-3-9-30(22(19)32)13-20(31)29-7-4-5-8-29/h10-12,19H,2-9,13H2,1H3,(H2,26,27,28)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296292
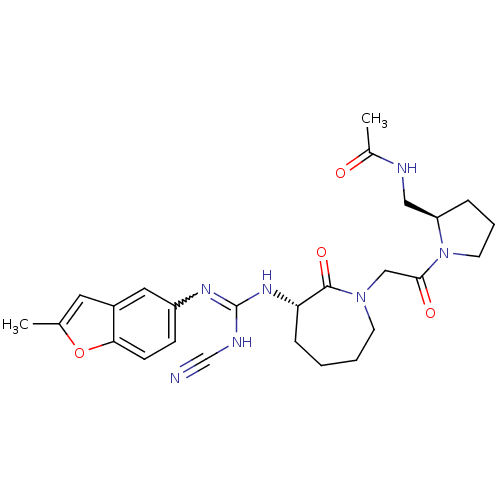
(CHEMBL550171 | N-(((R)-1-(2-((S)-3-((Z)-2-cyano-3-...)Show SMILES CC(=O)NC[C@H]1CCCN1C(=O)CN1CCCC[C@H](NC(NC#N)=Nc2ccc3oc(C)cc3c2)C1=O |r,w:24.25| Show InChI InChI=1S/C26H33N7O4/c1-17-12-19-13-20(8-9-23(19)37-17)30-26(29-16-27)31-22-7-3-4-10-32(25(22)36)15-24(35)33-11-5-6-21(33)14-28-18(2)34/h8-9,12-13,21-22H,3-7,10-11,14-15H2,1-2H3,(H,28,34)(H2,29,30,31)/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM26351

(2-methylbenzofuran compound, 2 | 2-{[(2-methyl-1-b...)Show SMILES Cc1cc2cc(ccc2o1)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)C#N |r,w:10.11| Show InChI InChI=1S/C25H28N6O3/c1-17-12-18-13-20(7-8-22(18)34-17)28-24(19(14-26)15-27)29-21-6-2-3-11-31(25(21)33)16-23(32)30-9-4-5-10-30/h7-8,12-13,19,21H,2-6,9-11,16H2,1H3,(H,28,29)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296288

(1-((3S)-1-(2-(3-benzylpyrrolidin-1-yl)-2-oxoethyl)...)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCC(Cc3ccccc3)C2)C1=O |r,w:10.11| Show InChI InChI=1S/C30H34N6O3/c1-21-15-24-17-25(10-11-27(24)39-21)33-30(32-20-31)34-26-9-5-6-13-36(29(26)38)19-28(37)35-14-12-23(18-35)16-22-7-3-2-4-8-22/h2-4,7-8,10-11,15,17,23,26H,5-6,9,12-14,16,18-19H2,1H3,(H2,32,33,34)/t23?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296291

(CHEMBL549972 | N-(((S)-1-(2-((S)-3-((Z)-2-cyano-3-...)Show SMILES CC(=O)NC[C@@H]1CCCN1C(=O)CN1CCCC[C@H](NC(NC#N)=Nc2ccc3oc(C)cc3c2)C1=O |r,w:24.25| Show InChI InChI=1S/C26H33N7O4/c1-17-12-19-13-20(8-9-23(19)37-17)30-26(29-16-27)31-22-7-3-4-10-32(25(22)36)15-24(35)33-11-5-6-21(33)14-28-18(2)34/h8-9,12-13,21-22H,3-7,10-11,14-15H2,1-2H3,(H,28,34)(H2,29,30,31)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296276
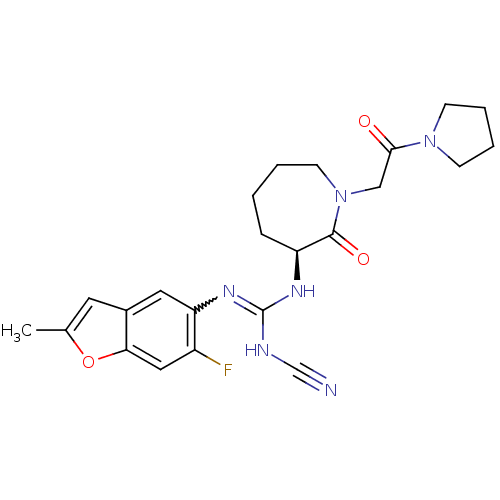
((S)-2-cyano-1-(6-fluoro-2-methylbenzofuran-5-yl)-3...)Show SMILES Cc1cc2cc(N=C(NC#N)N[C@H]3CCCCN(CC(=O)N4CCCC4)C3=O)c(F)cc2o1 |r,w:6.5| Show InChI InChI=1S/C23H27FN6O3/c1-15-10-16-11-19(17(24)12-20(16)33-15)28-23(26-14-25)27-18-6-2-3-9-30(22(18)32)13-21(31)29-7-4-5-8-29/h10-12,18H,2-9,13H2,1H3,(H2,26,27,28)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296269
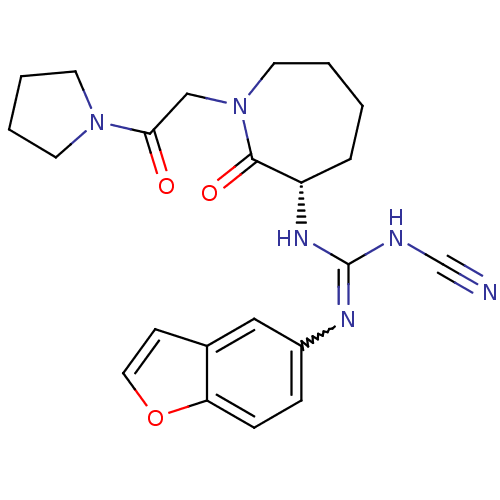
((S)-1-(benzofuran-5-yl)-2-cyano-3-(2-oxo-1-(2-oxo-...)Show SMILES O=C(CN1CCCC[C@H](NC(NC#N)=Nc2ccc3occc3c2)C1=O)N1CCCC1 |r,w:14.14| Show InChI InChI=1S/C22H26N6O3/c23-15-24-22(25-17-6-7-19-16(13-17)8-12-31-19)26-18-5-1-2-11-28(21(18)30)14-20(29)27-9-3-4-10-27/h6-8,12-13,18H,1-5,9-11,14H2,(H2,24,25,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM26350
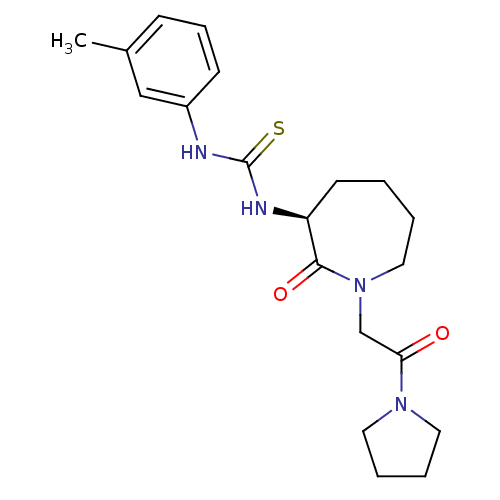
((S)-1-(2-oxo-1-(2-oxo-2-(pyrrolidin-1-yl)ethyl)aze...)Show SMILES Cc1cccc(NC(=S)N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)c1 |r| Show InChI InChI=1S/C20H28N4O2S/c1-15-7-6-8-16(13-15)21-20(27)22-17-9-2-3-12-24(19(17)26)14-18(25)23-10-4-5-11-23/h6-8,13,17H,2-5,9-12,14H2,1H3,(H2,21,22,27)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296279
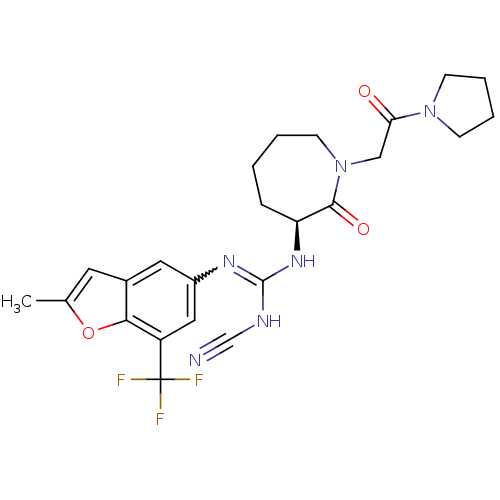
((S)-2-cyano-1-(2-methyl-7-(trifluoromethyl)benzofu...)Show SMILES Cc1cc2cc(cc(c2o1)C(F)(F)F)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:14.15| Show InChI InChI=1S/C24H27F3N6O3/c1-15-10-16-11-17(12-18(21(16)36-15)24(25,26)27)30-23(29-14-28)31-19-6-2-3-9-33(22(19)35)13-20(34)32-7-4-5-8-32/h10-12,19H,2-9,13H2,1H3,(H2,29,30,31)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296285
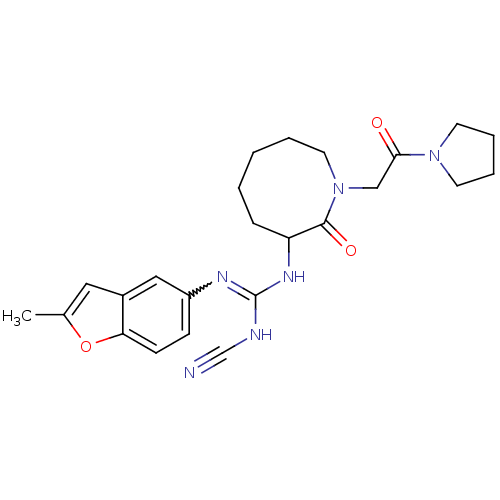
((+/-)-2-cyano-1-(2-methylbenzofuran-5-yl)-3-(2-oxo...)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)NC1CCCCCN(CC(=O)N2CCCC2)C1=O |w:10.11| Show InChI InChI=1S/C24H30N6O3/c1-17-13-18-14-19(8-9-21(18)33-17)27-24(26-16-25)28-20-7-3-2-4-12-30(23(20)32)15-22(31)29-10-5-6-11-29/h8-9,13-14,20H,2-7,10-12,15H2,1H3,(H2,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296273
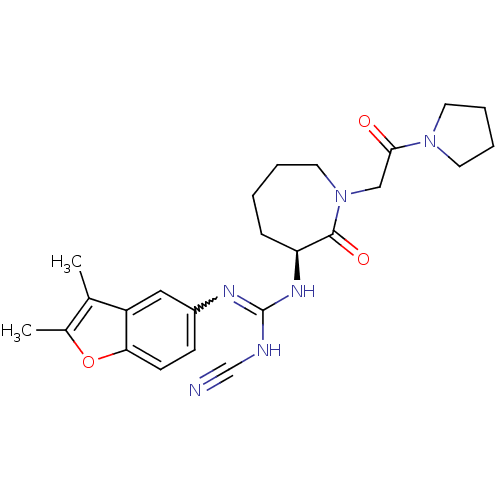
((S)-2-cyano-1-(2,3-dimethylbenzofuran-5-yl)-3-(2-o...)Show SMILES Cc1oc2ccc(cc2c1C)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:11.12| Show InChI InChI=1S/C24H30N6O3/c1-16-17(2)33-21-9-8-18(13-19(16)21)27-24(26-15-25)28-20-7-3-4-12-30(23(20)32)14-22(31)29-10-5-6-11-29/h8-9,13,20H,3-7,10-12,14H2,1-2H3,(H2,26,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 349 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296289
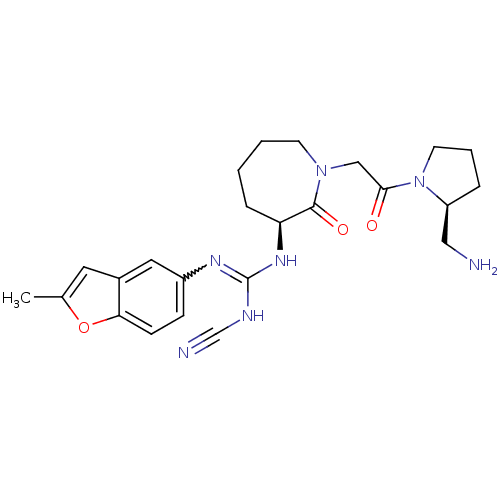
(1-((S)-1-(2-((S)-2-(aminomethyl)pyrrolidin-1-yl)-2...)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCC[C@H]2CN)C1=O |r,w:10.11| Show InChI InChI=1S/C24H31N7O3/c1-16-11-17-12-18(7-8-21(17)34-16)28-24(27-15-26)29-20-6-2-3-9-30(23(20)33)14-22(32)31-10-4-5-19(31)13-25/h7-8,11-12,19-20H,2-6,9-10,13-14,25H2,1H3,(H2,27,28,29)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 386 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296259
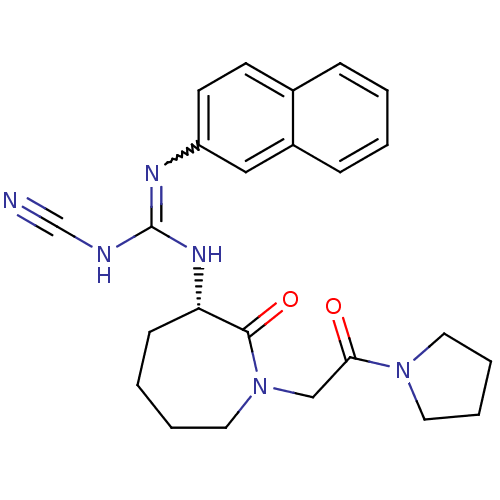
((S)-2-cyano-1-(naphthalen-2-yl)-3-(2-oxo-1-(2-oxo-...)Show SMILES O=C(CN1CCCC[C@H](NC(NC#N)=Nc2ccc3ccccc3c2)C1=O)N1CCCC1 |r,w:14.14| Show InChI InChI=1S/C24H28N6O2/c25-17-26-24(27-20-11-10-18-7-1-2-8-19(18)15-20)28-21-9-3-4-14-30(23(21)32)16-22(31)29-12-5-6-13-29/h1-2,7-8,10-11,15,21H,3-6,9,12-14,16H2,(H2,26,27,28)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296283
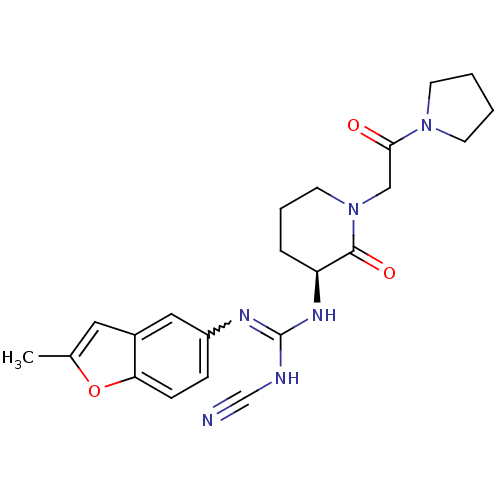
((S)-2-cyano-1-(2-methylbenzofuran-5-yl)-3-(2-oxo-1...)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C22H26N6O3/c1-15-11-16-12-17(6-7-19(16)31-15)25-22(24-14-23)26-18-5-4-10-28(21(18)30)13-20(29)27-8-2-3-9-27/h6-7,11-12,18H,2-5,8-10,13H2,1H3,(H2,24,25,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 928 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
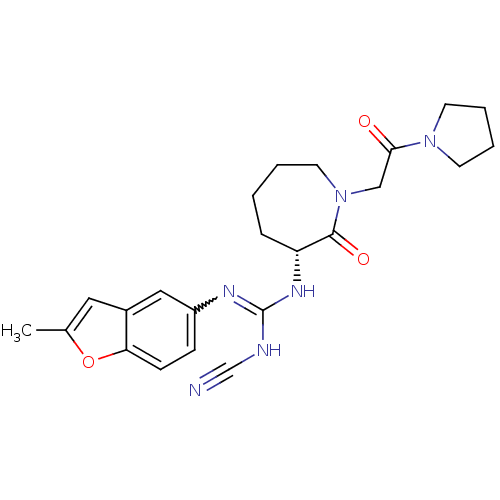
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296284
((R)-2-cyano-1-(2-methylbenzofuran-5-yl)-3-(2-oxo-1...)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296267
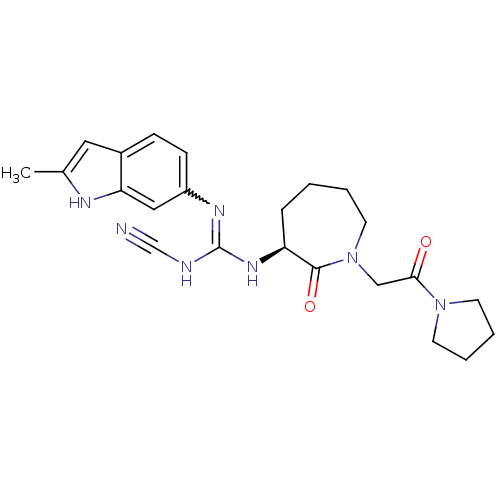
((S)-2-cyano-1-(2-methyl-1H-indol-6-yl)-3-(2-oxo-1-...)Show SMILES Cc1cc2ccc(cc2[nH]1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H29N7O2/c1-16-12-17-7-8-18(13-20(17)26-16)27-23(25-15-24)28-19-6-2-3-11-30(22(19)32)14-21(31)29-9-4-5-10-29/h7-8,12-13,19,26H,2-6,9-11,14H2,1H3,(H2,25,27,28)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296268
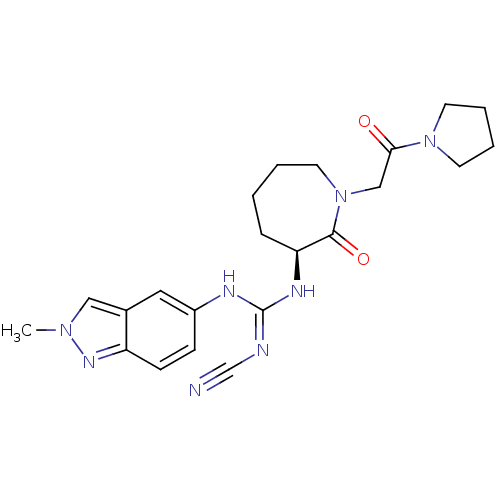
((S)-2-cyano-1-(2-methyl-2H-indazol-5-yl)-3-(2-oxo-...)Show SMILES Cn1cc2cc(N\C(N[C@H]3CCCCN(CC(=O)N4CCCC4)C3=O)=N/C#N)ccc2n1 |r| Show InChI InChI=1S/C22H28N8O2/c1-28-13-16-12-17(7-8-18(16)27-28)25-22(24-15-23)26-19-6-2-3-11-30(21(19)32)14-20(31)29-9-4-5-10-29/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,24,25,26)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296271
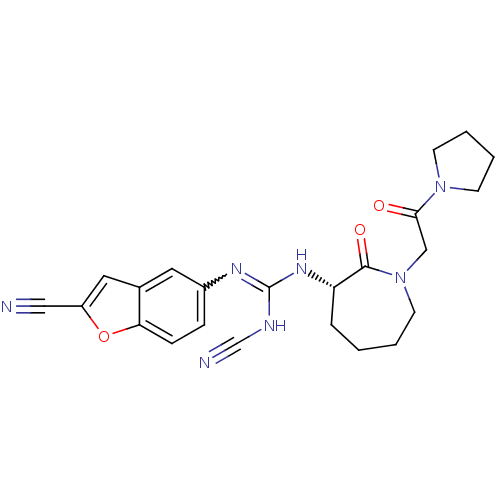
((S)-2-cyano-1-(2-cyanobenzofuran-5-yl)-3-(2-oxo-1-...)Show SMILES O=C(CN1CCCC[C@H](NC(NC#N)=Nc2ccc3oc(cc3c2)C#N)C1=O)N1CCCC1 |r,w:14.14| Show InChI InChI=1S/C23H25N7O3/c24-13-18-12-16-11-17(6-7-20(16)33-18)27-23(26-15-25)28-19-5-1-2-10-30(22(19)32)14-21(31)29-8-3-4-9-29/h6-7,11-12,19H,1-5,8-10,14H2,(H2,26,27,28)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296262

((S)-2-cyano-1-(2-methylbenzo[d]oxazol-5-yl)-3-(2-o...)Show SMILES Cc1nc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C22H27N7O3/c1-15-25-18-12-16(7-8-19(18)32-15)26-22(24-14-23)27-17-6-2-3-11-29(21(17)31)13-20(30)28-9-4-5-10-28/h7-8,12,17H,2-6,9-11,13H2,1H3,(H2,24,26,27)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296275
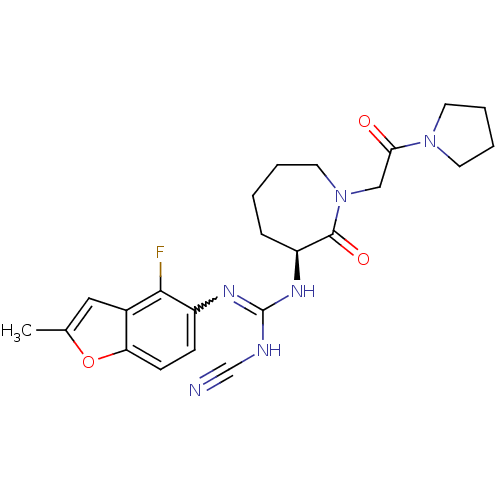
((S)-2-cyano-1-(4-fluoro-2-methylbenzofuran-5-yl)-3...)Show SMILES Cc1cc2c(F)c(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:11.12| Show InChI InChI=1S/C23H27FN6O3/c1-15-12-16-19(33-15)8-7-17(21(16)24)27-23(26-14-25)28-18-6-2-3-11-30(22(18)32)13-20(31)29-9-4-5-10-29/h7-8,12,18H,2-6,9-11,13H2,1H3,(H2,26,27,28)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296260
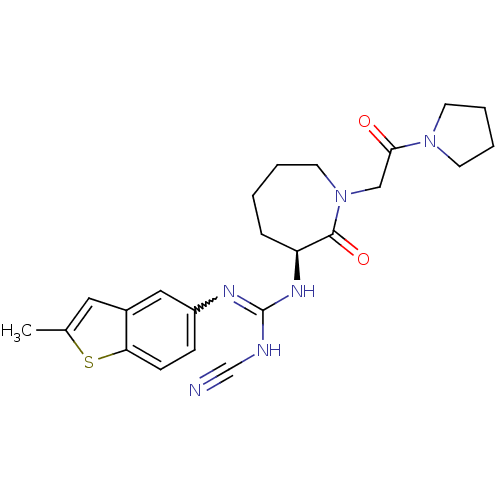
((S)-2-cyano-1-(2-methylbenzo[b]thiophen-5-yl)-3-(2...)Show SMILES Cc1cc2cc(ccc2s1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O2S/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296272

((S)-2-cyano-1-(3-methylbenzofuran-5-yl)-3-(2-oxo-1...)Show SMILES Cc1coc2ccc(cc12)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-14-32-20-8-7-17(12-18(16)20)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)13-21(30)28-9-4-5-10-28/h7-8,12,14,19H,2-6,9-11,13H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296265
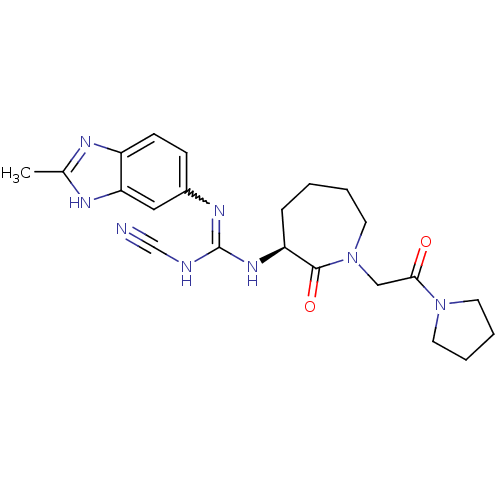
((S)-2-cyano-1-(2-methyl-1H-benzo[d]imidazol-5-yl)-...)Show SMILES Cc1nc2ccc(cc2[nH]1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C22H28N8O2/c1-15-25-17-8-7-16(12-19(17)26-15)27-22(24-14-23)28-18-6-2-3-11-30(21(18)32)13-20(31)29-9-4-5-10-29/h7-8,12,18H,2-6,9-11,13H2,1H3,(H,25,26)(H2,24,27,28)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296280

((S)-2-cyano-1-(7-methoxy-2-methylbenzofuran-5-yl)-...)Show SMILES COc1cc(cc2cc(C)oc12)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:12.13| Show InChI InChI=1S/C24H30N6O4/c1-16-11-17-12-18(13-20(33-2)22(17)34-16)27-24(26-15-25)28-19-7-3-4-10-30(23(19)32)14-21(31)29-8-5-6-9-29/h11-13,19H,3-10,14H2,1-2H3,(H2,26,27,28)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296270
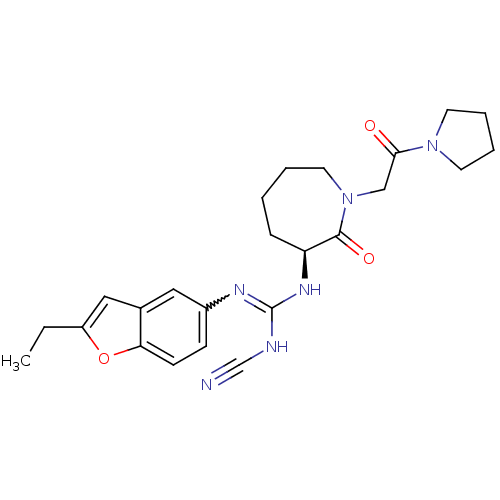
((S)-2-cyano-1-(2-ethylbenzofuran-5-yl)-3-(2-oxo-1-...)Show SMILES CCc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:11.12| Show InChI InChI=1S/C24H30N6O3/c1-2-19-14-17-13-18(8-9-21(17)33-19)27-24(26-16-25)28-20-7-3-4-12-30(23(20)32)15-22(31)29-10-5-6-11-29/h8-9,13-14,20H,2-7,10-12,15H2,1H3,(H2,26,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM35283

(CHEMBL551991 | cyanoguanidine, 3)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using BFC as substrate |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296263
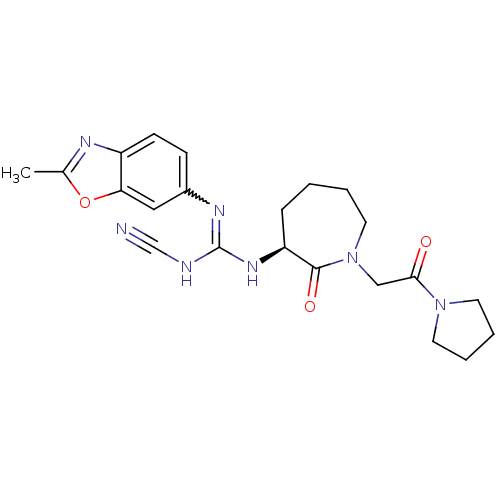
((S)-2-cyano-1-(2-methylbenzo[d]oxazol-6-yl)-3-(2-o...)Show SMILES Cc1nc2ccc(cc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C22H27N7O3/c1-15-25-17-8-7-16(12-19(17)32-15)26-22(24-14-23)27-18-6-2-3-11-29(21(18)31)13-20(30)28-9-4-5-10-28/h7-8,12,18H,2-6,9-11,13H2,1H3,(H2,24,26,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM35283

(CHEMBL551991 | cyanoguanidine, 3)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296282

((R)-2-cyano-1-(2-methylbenzofuran-5-yl)-3-(2-oxo-1...)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@@H]1CCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C21H24N6O3/c1-14-10-15-11-16(4-5-18(15)30-14)24-21(23-13-22)25-17-6-9-27(20(17)29)12-19(28)26-7-2-3-8-26/h4-5,10-11,17H,2-3,6-9,12H2,1H3,(H2,23,24,25)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296281
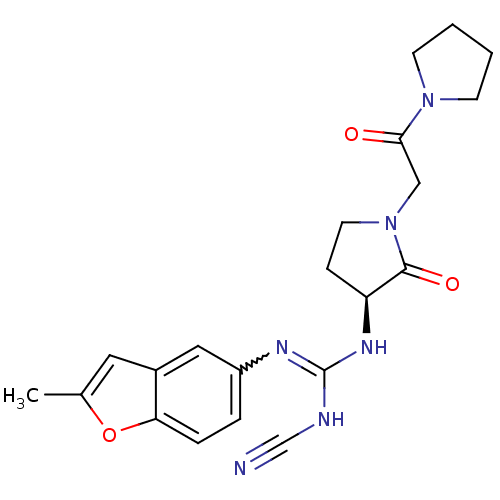
((S)-2-cyano-1-(2-methylbenzofuran-5-yl)-3-(2-oxo-1...)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C21H24N6O3/c1-14-10-15-11-16(4-5-18(15)30-14)24-21(23-13-22)25-17-6-9-27(20(17)29)12-19(28)26-7-2-3-8-26/h4-5,10-11,17H,2-3,6-9,12H2,1H3,(H2,23,24,25)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296266
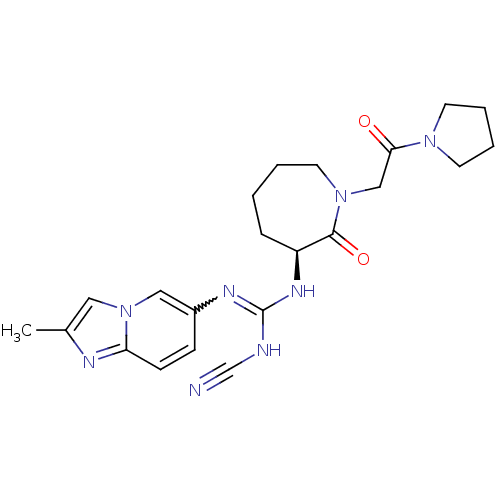
((S)-2-cyano-1-(2-methylimidazo[1,2-a]pyridin-6-yl)...)Show SMILES Cc1cn2cc(ccc2n1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C22H28N8O2/c1-16-12-30-13-17(7-8-19(30)25-16)26-22(24-15-23)27-18-6-2-3-11-29(21(18)32)14-20(31)28-9-4-5-10-28/h7-8,12-13,18H,2-6,9-11,14H2,1H3,(H2,24,26,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50296264
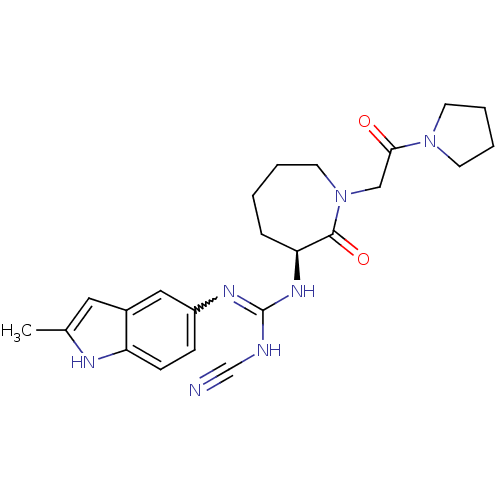
((S)-2-cyano-1-(2-methyl-1H-indol-5-yl)-3-(2-oxo-1-...)Show SMILES Cc1cc2cc(ccc2[nH]1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H29N7O2/c1-16-12-17-13-18(7-8-19(17)26-16)27-23(25-15-24)28-20-6-2-3-11-30(22(20)32)14-21(31)29-9-4-5-10-29/h7-8,12-13,20,26H,2-6,9-11,14H2,1H3,(H2,25,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
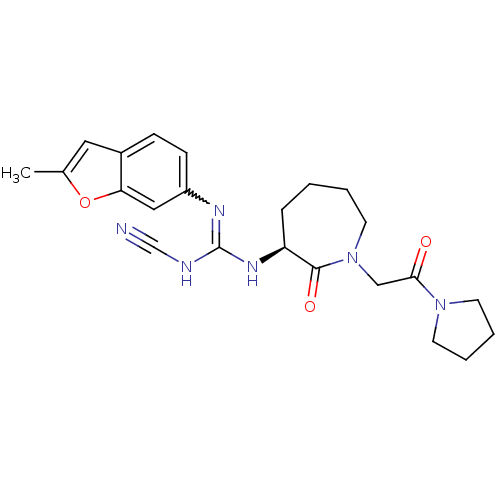
(Homo sapiens (Human)) | BDBM50296261
((S)-2-cyano-1-(2-methylbenzofuran-6-yl)-3-(2-oxo-1...)Show SMILES Cc1cc2ccc(cc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-7-8-18(13-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM35283

(CHEMBL551991 | cyanoguanidine, 3)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM35283

(CHEMBL551991 | cyanoguanidine, 3)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM35283

(CHEMBL551991 | cyanoguanidine, 3)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using BZR as substrate |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM35283

(CHEMBL551991 | cyanoguanidine, 3)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM35283

(CHEMBL551991 | cyanoguanidine, 3)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP2B6 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM35283

(CHEMBL551991 | cyanoguanidine, 3)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM35283

(CHEMBL551991 | cyanoguanidine, 3)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM35283

(CHEMBL551991 | cyanoguanidine, 3)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human TC5 cells |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM35283

(CHEMBL551991 | cyanoguanidine, 3)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human TC5 cells |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM35283

(CHEMBL551991 | cyanoguanidine, 3)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human TC5 cells |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM35283

(CHEMBL551991 | cyanoguanidine, 3)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human TC5 cells |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM35283

(CHEMBL551991 | cyanoguanidine, 3)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Activity at PXR |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data