

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50415629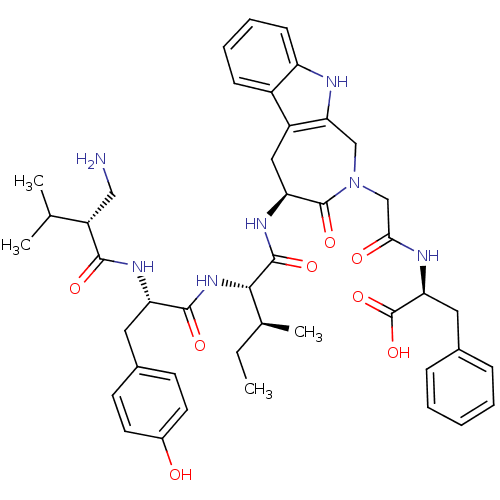 (CHEMBL1077583) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human aminopeptidase N transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50415628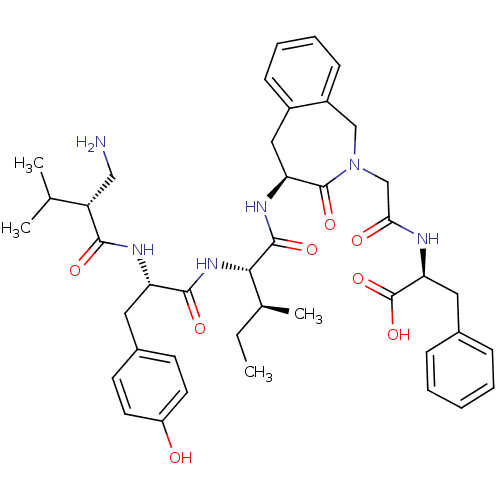 (CHEMBL1077582) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human aminopeptidase N transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50415633 (CHEMBL259019) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human aminopeptidase N transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50415627 (CHEMBL1077592) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human aminopeptidase N transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50415632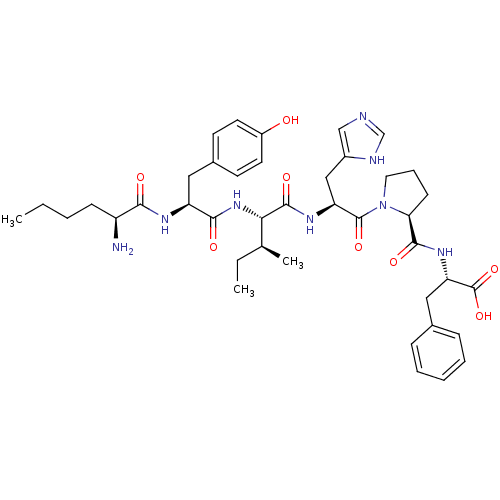 (CHEMBL1077586) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 52.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human aminopeptidase N transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50415631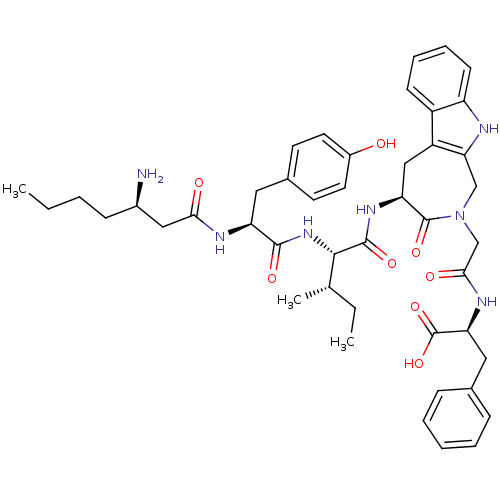 (CHEMBL1077585) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 55.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human aminopeptidase N transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM85550 (Ang IV | CAS_12676-15-2 | CHEMBL261120 | cid_12381...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 56.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human aminopeptidase N transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50415634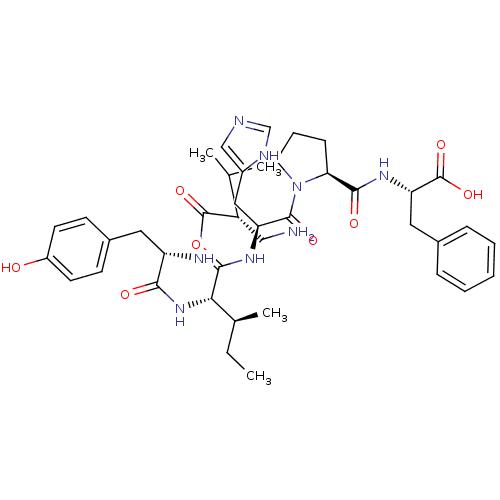 (CHEMBL260622) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human aminopeptidase N transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50415630 (CHEMBL1077584) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human aminopeptidase N transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50415628 (CHEMBL1077582) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]valsartan from human recombinant AT1 receptor expressed in CHO cells after 40 mins by liquid scintillation counting | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50415626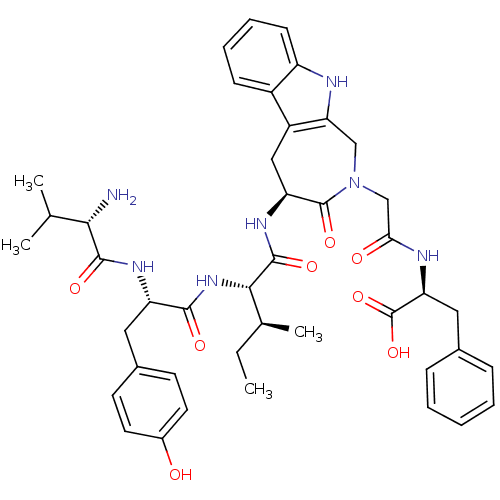 (CHEMBL1077591) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human aminopeptidase N transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50415636 (CHEMBL1077590) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 219 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human aminopeptidase N transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM85550 (Ang IV | CAS_12676-15-2 | CHEMBL261120 | cid_12381...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]valsartan from human recombinant AT1 receptor expressed in CHO cells after 40 mins by liquid scintillation counting | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50415635 (CHEMBL1077589) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human aminopeptidase N transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50415629 (CHEMBL1077583) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human IRAP transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM85550 (Ang IV | CAS_12676-15-2 | CHEMBL261120 | cid_12381...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 832 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human IRAP transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50415627 (CHEMBL1077592) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human IRAP transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50415636 (CHEMBL1077590) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human IRAP transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50415628 (CHEMBL1077582) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human IRAP transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50415635 (CHEMBL1077589) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human IRAP transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50415631 (CHEMBL1077585) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human IRAP transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50415634 (CHEMBL260622) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human IRAP transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50415630 (CHEMBL1077584) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human IRAP transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50415633 (CHEMBL259019) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human IRAP transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50415626 (CHEMBL1077591) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human IRAP transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50415632 (CHEMBL1077586) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human IRAP transfected in HEK293 cells assessed as formation of p-nitroaniline | J Med Chem 52: 5612-8 (2009) Article DOI: 10.1021/jm900651p BindingDB Entry DOI: 10.7270/Q2TB185F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||