

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM35275 (tripeptide-based inhibitor, 14r) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Dainippon Sumitomo Pharma Co | Assay Description To evaluate inhibitory effect of test compound, HNE was preincubated with each compound in the assay solution at 37 deg C for 4 min. The reaction was... | Bioorg Med Chem 17: 7477-86 (2009) Article DOI: 10.1016/j.bmc.2009.09.020 BindingDB Entry DOI: 10.7270/Q22Z13W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM35276 (tripeptide-based inhibitor, 14s) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Dainippon Sumitomo Pharma Co | Assay Description To evaluate inhibitory effect of test compound, HNE was preincubated with each compound in the assay solution at 37 deg C for 4 min. The reaction was... | Bioorg Med Chem 17: 7477-86 (2009) Article DOI: 10.1016/j.bmc.2009.09.020 BindingDB Entry DOI: 10.7270/Q22Z13W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM35257 (tripeptide-based inhibitor, 14a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Dainippon Sumitomo Pharma Co | Assay Description To evaluate inhibitory effect of test compound, HNE was preincubated with each compound in the assay solution at 37 deg C for 4 min. The reaction was... | Bioorg Med Chem 17: 7477-86 (2009) Article DOI: 10.1016/j.bmc.2009.09.020 BindingDB Entry DOI: 10.7270/Q22Z13W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM35261 (tripeptide-based inhibitor, 14d) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Dainippon Sumitomo Pharma Co | Assay Description To evaluate inhibitory effect of test compound, HNE was preincubated with each compound in the assay solution at 37 deg C for 4 min. The reaction was... | Bioorg Med Chem 17: 7477-86 (2009) Article DOI: 10.1016/j.bmc.2009.09.020 BindingDB Entry DOI: 10.7270/Q22Z13W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM35269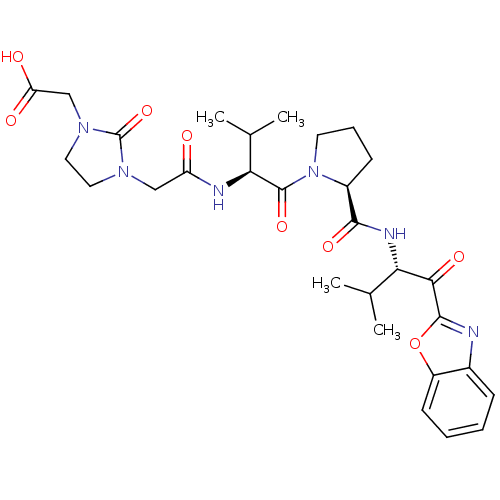 (tripeptide-based inhibitor, 14l) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Dainippon Sumitomo Pharma Co | Assay Description To evaluate inhibitory effect of test compound, HNE was preincubated with each compound in the assay solution at 37 deg C for 4 min. The reaction was... | Bioorg Med Chem 17: 7477-86 (2009) Article DOI: 10.1016/j.bmc.2009.09.020 BindingDB Entry DOI: 10.7270/Q22Z13W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM35274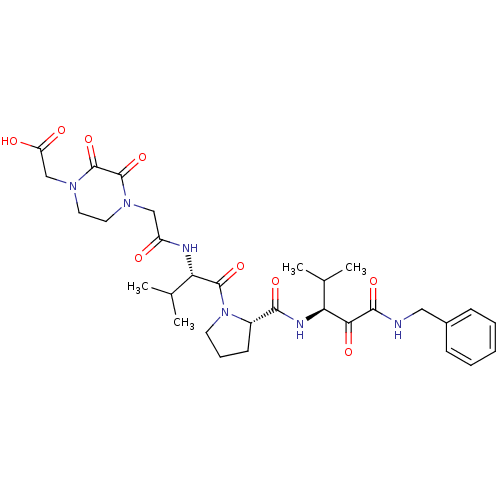 (tripeptide-based inhibitor, 14q) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Dainippon Sumitomo Pharma Co | Assay Description To evaluate inhibitory effect of test compound, HNE was preincubated with each compound in the assay solution at 37 deg C for 4 min. The reaction was... | Bioorg Med Chem 17: 7477-86 (2009) Article DOI: 10.1016/j.bmc.2009.09.020 BindingDB Entry DOI: 10.7270/Q22Z13W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM35273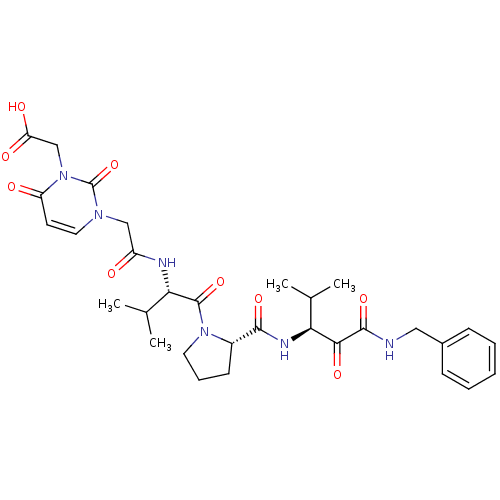 (tripeptide-based inhibitor, 14p) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Dainippon Sumitomo Pharma Co | Assay Description To evaluate inhibitory effect of test compound, HNE was preincubated with each compound in the assay solution at 37 deg C for 4 min. The reaction was... | Bioorg Med Chem 17: 7477-86 (2009) Article DOI: 10.1016/j.bmc.2009.09.020 BindingDB Entry DOI: 10.7270/Q22Z13W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM35268 (tripeptide-based inhibitor, 14k) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Dainippon Sumitomo Pharma Co | Assay Description To evaluate inhibitory effect of test compound, HNE was preincubated with each compound in the assay solution at 37 deg C for 4 min. The reaction was... | Bioorg Med Chem 17: 7477-86 (2009) Article DOI: 10.1016/j.bmc.2009.09.020 BindingDB Entry DOI: 10.7270/Q22Z13W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM35259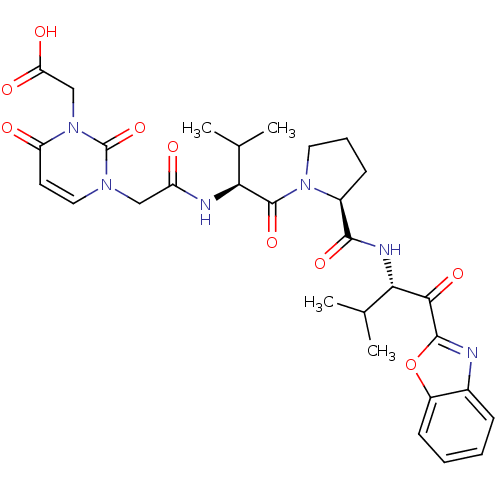 (tripeptide-based inhibitor, 14b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Dainippon Sumitomo Pharma Co | Assay Description To evaluate inhibitory effect of test compound, HNE was preincubated with each compound in the assay solution at 37 deg C for 4 min. The reaction was... | Bioorg Med Chem 17: 7477-86 (2009) Article DOI: 10.1016/j.bmc.2009.09.020 BindingDB Entry DOI: 10.7270/Q22Z13W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM35263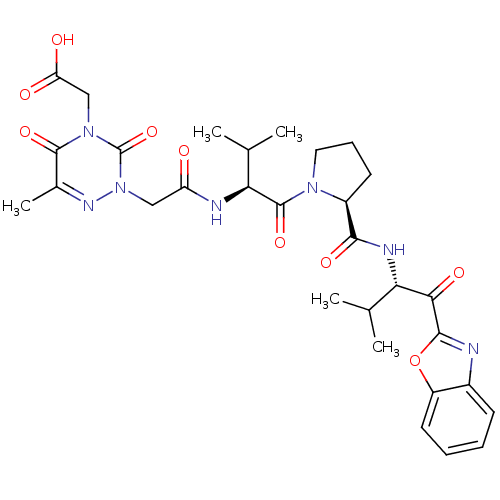 (tripeptide-based inhibitor, 14f) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Dainippon Sumitomo Pharma Co | Assay Description To evaluate inhibitory effect of test compound, HNE was preincubated with each compound in the assay solution at 37 deg C for 4 min. The reaction was... | Bioorg Med Chem 17: 7477-86 (2009) Article DOI: 10.1016/j.bmc.2009.09.020 BindingDB Entry DOI: 10.7270/Q22Z13W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM35260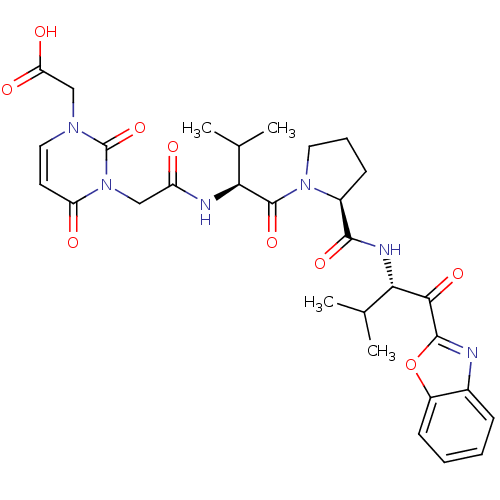 (tripeptide-based inhibitor, 14c) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Dainippon Sumitomo Pharma Co | Assay Description To evaluate inhibitory effect of test compound, HNE was preincubated with each compound in the assay solution at 37 deg C for 4 min. The reaction was... | Bioorg Med Chem 17: 7477-86 (2009) Article DOI: 10.1016/j.bmc.2009.09.020 BindingDB Entry DOI: 10.7270/Q22Z13W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM35277 (tripeptide-based inhibitor, 14t) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Dainippon Sumitomo Pharma Co | Assay Description To evaluate inhibitory effect of test compound, HNE was preincubated with each compound in the assay solution at 37 deg C for 4 min. The reaction was... | Bioorg Med Chem 17: 7477-86 (2009) Article DOI: 10.1016/j.bmc.2009.09.020 BindingDB Entry DOI: 10.7270/Q22Z13W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM35279 (AE-3763 | tripeptide-based inhibitor, 14v) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Dainippon Sumitomo Pharma Co | Assay Description To evaluate inhibitory effect of test compound, HNE was preincubated with each compound in the assay solution at 37 deg C for 4 min. The reaction was... | Bioorg Med Chem 17: 7477-86 (2009) Article DOI: 10.1016/j.bmc.2009.09.020 BindingDB Entry DOI: 10.7270/Q22Z13W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM35271 (tripeptide-based inhibitor, 14n) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Dainippon Sumitomo Pharma Co | Assay Description To evaluate inhibitory effect of test compound, HNE was preincubated with each compound in the assay solution at 37 deg C for 4 min. The reaction was... | Bioorg Med Chem 17: 7477-86 (2009) Article DOI: 10.1016/j.bmc.2009.09.020 BindingDB Entry DOI: 10.7270/Q22Z13W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM35262 (tripeptide-based inhibitor, 14e) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Dainippon Sumitomo Pharma Co | Assay Description To evaluate inhibitory effect of test compound, HNE was preincubated with each compound in the assay solution at 37 deg C for 4 min. The reaction was... | Bioorg Med Chem 17: 7477-86 (2009) Article DOI: 10.1016/j.bmc.2009.09.020 BindingDB Entry DOI: 10.7270/Q22Z13W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM35266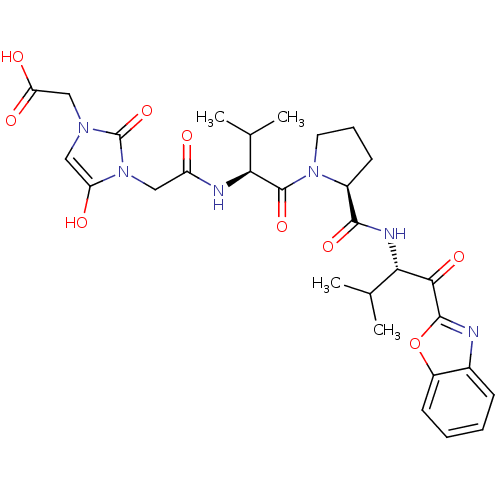 (tripeptide-based inhibitor, 14i) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Dainippon Sumitomo Pharma Co | Assay Description To evaluate inhibitory effect of test compound, HNE was preincubated with each compound in the assay solution at 37 deg C for 4 min. The reaction was... | Bioorg Med Chem 17: 7477-86 (2009) Article DOI: 10.1016/j.bmc.2009.09.020 BindingDB Entry DOI: 10.7270/Q22Z13W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM35265 (tripeptide-based inhibitor, 14h) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Dainippon Sumitomo Pharma Co | Assay Description To evaluate inhibitory effect of test compound, HNE was preincubated with each compound in the assay solution at 37 deg C for 4 min. The reaction was... | Bioorg Med Chem 17: 7477-86 (2009) Article DOI: 10.1016/j.bmc.2009.09.020 BindingDB Entry DOI: 10.7270/Q22Z13W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM35264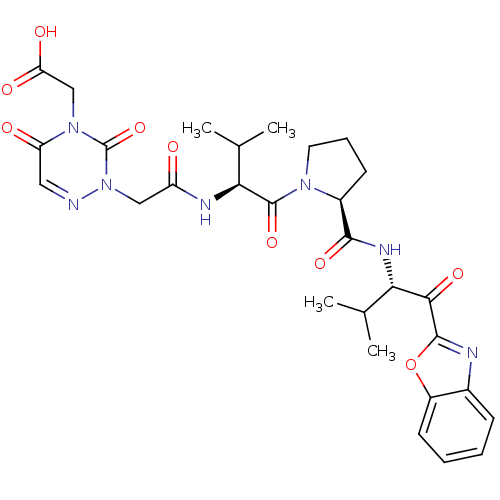 (tripeptide-based inhibitor, 14g) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Dainippon Sumitomo Pharma Co | Assay Description To evaluate inhibitory effect of test compound, HNE was preincubated with each compound in the assay solution at 37 deg C for 4 min. The reaction was... | Bioorg Med Chem 17: 7477-86 (2009) Article DOI: 10.1016/j.bmc.2009.09.020 BindingDB Entry DOI: 10.7270/Q22Z13W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM35267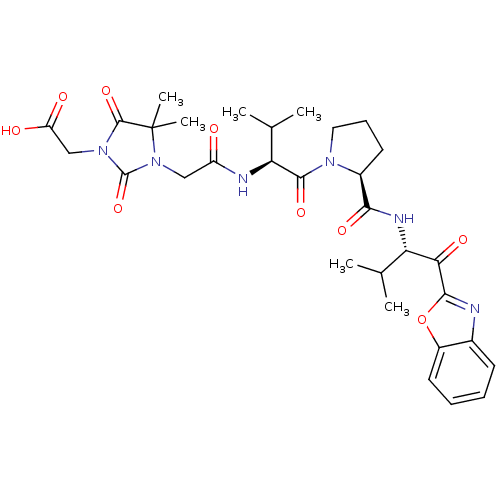 (tripeptide-based inhibitor, 14j) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Dainippon Sumitomo Pharma Co | Assay Description To evaluate inhibitory effect of test compound, HNE was preincubated with each compound in the assay solution at 37 deg C for 4 min. The reaction was... | Bioorg Med Chem 17: 7477-86 (2009) Article DOI: 10.1016/j.bmc.2009.09.020 BindingDB Entry DOI: 10.7270/Q22Z13W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM35272 (tripeptide-based inhibitor, 14o) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Dainippon Sumitomo Pharma Co | Assay Description To evaluate inhibitory effect of test compound, HNE was preincubated with each compound in the assay solution at 37 deg C for 4 min. The reaction was... | Bioorg Med Chem 17: 7477-86 (2009) Article DOI: 10.1016/j.bmc.2009.09.020 BindingDB Entry DOI: 10.7270/Q22Z13W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM35270 (tripeptide-based inhibitor, 14m) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Dainippon Sumitomo Pharma Co | Assay Description To evaluate inhibitory effect of test compound, HNE was preincubated with each compound in the assay solution at 37 deg C for 4 min. The reaction was... | Bioorg Med Chem 17: 7477-86 (2009) Article DOI: 10.1016/j.bmc.2009.09.020 BindingDB Entry DOI: 10.7270/Q22Z13W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM35278 (tripeptide-based inhibitor, 14u) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Dainippon Sumitomo Pharma Co | Assay Description To evaluate inhibitory effect of test compound, HNE was preincubated with each compound in the assay solution at 37 deg C for 4 min. The reaction was... | Bioorg Med Chem 17: 7477-86 (2009) Article DOI: 10.1016/j.bmc.2009.09.020 BindingDB Entry DOI: 10.7270/Q22Z13W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM35280 (tripeptide-based inhibitor, 14w) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Dainippon Sumitomo Pharma Co | Assay Description To evaluate inhibitory effect of test compound, HNE was preincubated with each compound in the assay solution at 37 deg C for 4 min. The reaction was... | Bioorg Med Chem 17: 7477-86 (2009) Article DOI: 10.1016/j.bmc.2009.09.020 BindingDB Entry DOI: 10.7270/Q22Z13W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||