

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosylhomocysteinase (Mus musculus) | BDBM50280299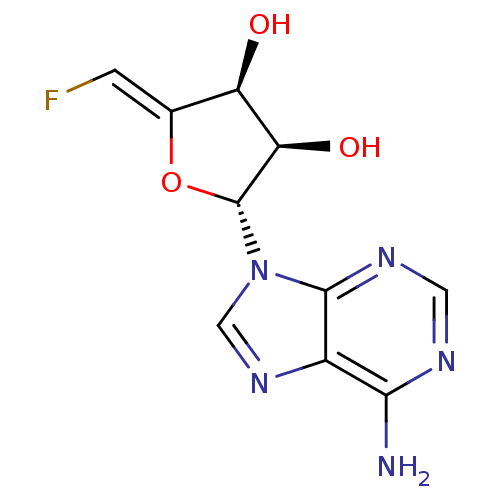 ((2R,3R,4S)-2-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in mouse liver S-adenosyl-L-homocysteine hydrolase and expressed as KI values. | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50280301 ((2R,3R,4S)-2-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in mouse liver S-adenosyl-L-homocysteine hydrolase and expressed as KI values. | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50368169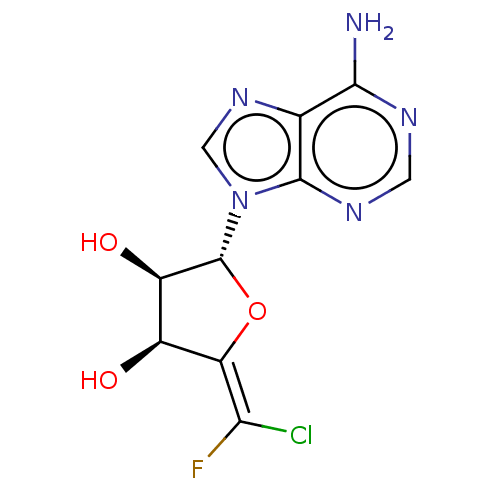 (CHEMBL2368687) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as Kinactivator values; NA= not applicable | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50368167 (CHEMBL3349334 | CHEMBL611905) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as Kinactivator values; NA= not applicable | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50280301 ((2R,3R,4S)-2-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as KI values. | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50368170 (CHEMBL2368677) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Competitive inhibitory activity against rat liver S-Adenosyl-homocysteine hydrolase | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50406477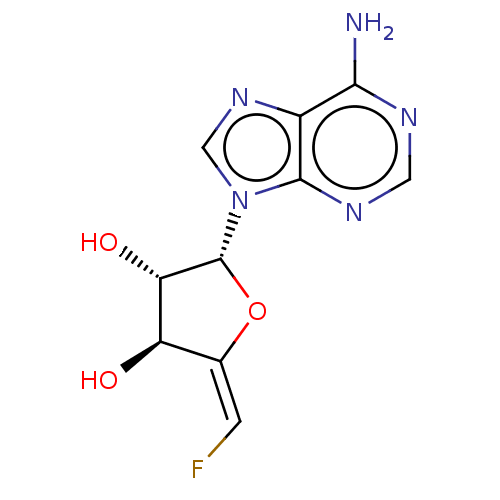 (CHEMBL2051968 | CHEMBL2069133) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Competitive inhibitory activity against rat liver S-Adenosyl-homocysteine hydrolase | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50280299 ((2R,3R,4S)-2-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as KI values. | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50368168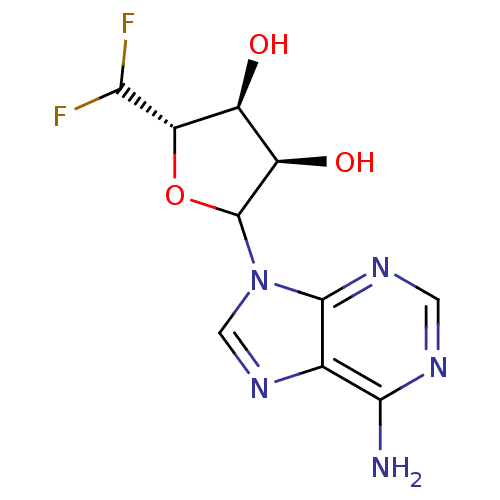 (CHEMBL609353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as Kinactivator values. | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50368168 (CHEMBL609353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Competitive inhibitory activity against rat liver S-adenosyl-L-homocysteine hydrolase | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50368168 (CHEMBL609353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in mouse liver S-adenosyl-L-homocysteine hydrolase and expressed as KI values. | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50229043 (CHEMBL2051969) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Competitive inhibitory activity against rat liver S-Adenosyl-homocysteine hydrolase | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50144936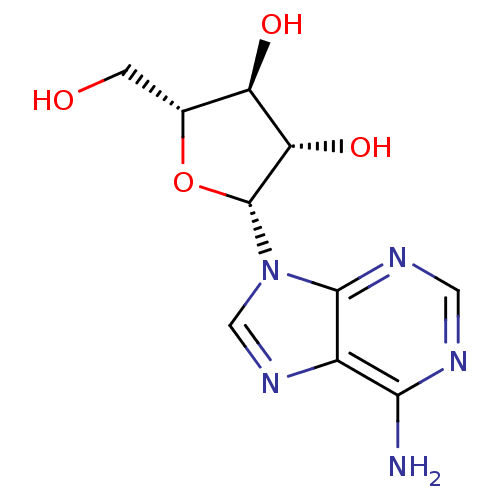 (CHEMBL1090 | VIDARABINE | adenine arabinoside) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in mouse liver S-adenosyl-L-homocysteine hydrolase and expressed as KI values. | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50011352 (5-(6-Amino-purin-9-yl)-2-fluoromethylene-tetrahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as Kinactivator values; NA= not applicable | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||