

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50011704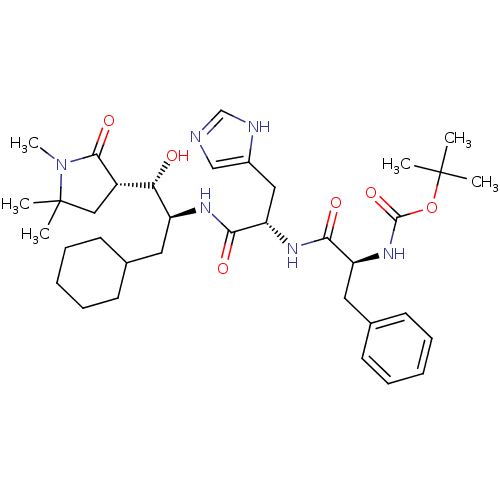 (CHEMBL170504 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using purified human kidney renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011704 (CHEMBL170504 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011708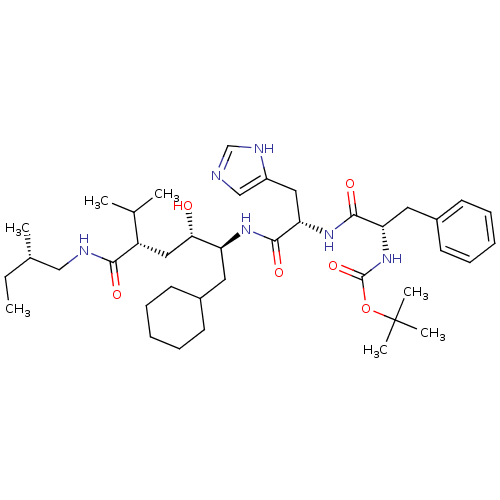 (CHEMBL171319 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011713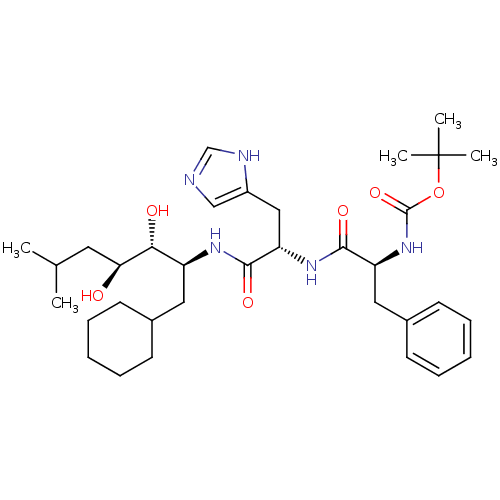 (CHEMBL430933 | {1-[1-(1-Cyclohexylmethyl-2,3-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011702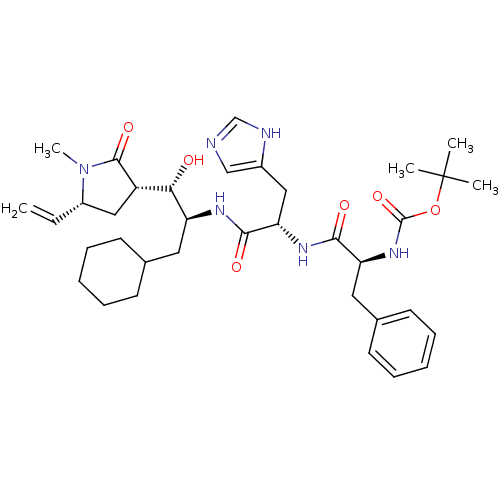 (CHEMBL169870 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using purified human kidney renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011706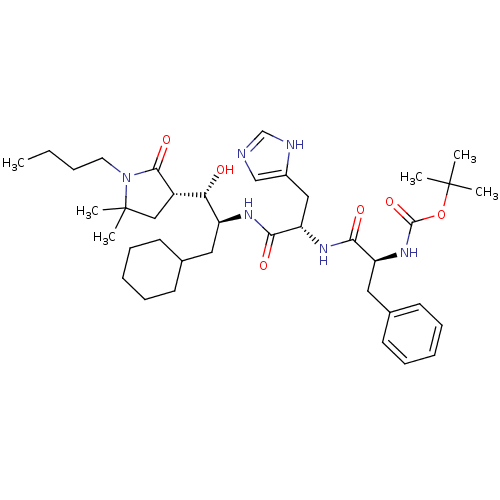 (CHEMBL353944 | {1-[1-[2-(1-Butyl-5,5-dimethyl-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using purified human kidney renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011706 (CHEMBL353944 | {1-[1-[2-(1-Butyl-5,5-dimethyl-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011702 (CHEMBL169870 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011710 (CHEMBL355458 | {1-[1-[1-Cyclohexylmethyl-2-(5-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using purified human kidney renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011710 (CHEMBL355458 | {1-[1-[1-Cyclohexylmethyl-2-(5-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011703 (CHEMBL355128 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using purified human kidney renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011693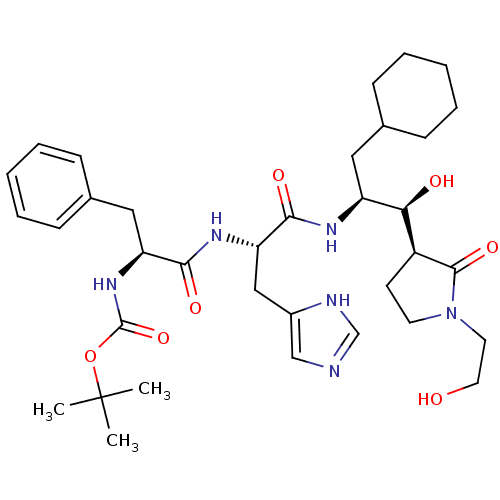 (CHEMBL352839 | {1-[1-{1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011698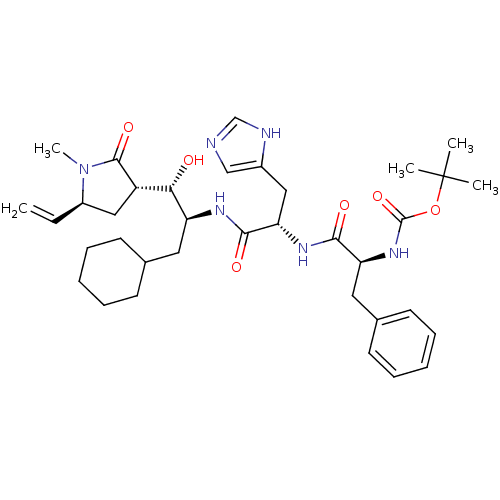 (CHEMBL354505 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011695 (CHEMBL170287 | {1-[1-[1-Cyclohexylmethyl-2-(5-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011698 (CHEMBL354505 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Gastricsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011703 (CHEMBL355128 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011707 (CHEMBL405692 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011696 (CHEMBL170772 | {1-[1-[1-Cyclohexylmethyl-2-(1-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011695 (CHEMBL170287 | {1-[1-[1-Cyclohexylmethyl-2-(5-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against cathepsin D | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011712 (CHEMBL171185 | {1-[1-{2-[1-(3-Benzylamino-propyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011707 (CHEMBL405692 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011714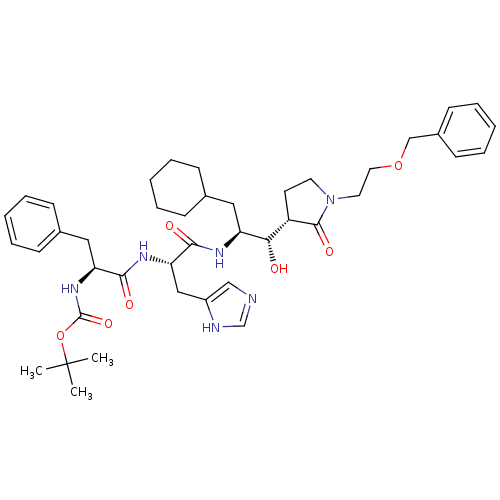 (CHEMBL435274 | {1-[1-{2-[1-(2-Benzyloxy-ethyl)-2-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011699 (CHEMBL171236 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Pepsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011697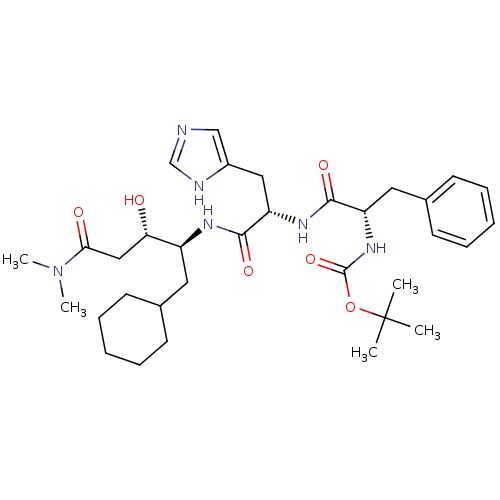 (CHEMBL355726 | {1-[1-(1-Cyclohexylmethyl-3-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011715 (CHEMBL434110 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011699 (CHEMBL171236 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50011706 (CHEMBL353944 | {1-[1-[2-(1-Butyl-5,5-dimethyl-2-ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against cathepsin D | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50011694 (CHEMBL354360 | {1-[1-[2-(5-Benzyloxymethyl-1-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against cathepsin D | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50011706 (CHEMBL353944 | {1-[1-[2-(1-Butyl-5,5-dimethyl-2-ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against cathepsin E | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50011705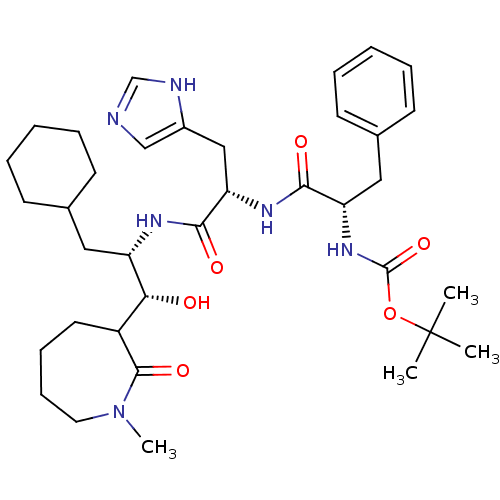 (CHEMBL173204 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against cathepsin D | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50011704 (CHEMBL170504 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against cathepsin D | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011700 (Acetic acid 4-{2-[2-(2-tert-butoxycarbonylamino-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 155 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using purified human kidney renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011700 (Acetic acid 4-{2-[2-(2-tert-butoxycarbonylamino-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 157 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50011702 (CHEMBL169870 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against cathepsin E | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50011705 (CHEMBL173204 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against cathepsin E | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50011698 (CHEMBL354505 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against cathepsin D | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011705 (CHEMBL173204 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50011696 (CHEMBL170772 | {1-[1-[1-Cyclohexylmethyl-2-(1-cycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against cathepsin E | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50011702 (CHEMBL169870 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Cathepsin D | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011709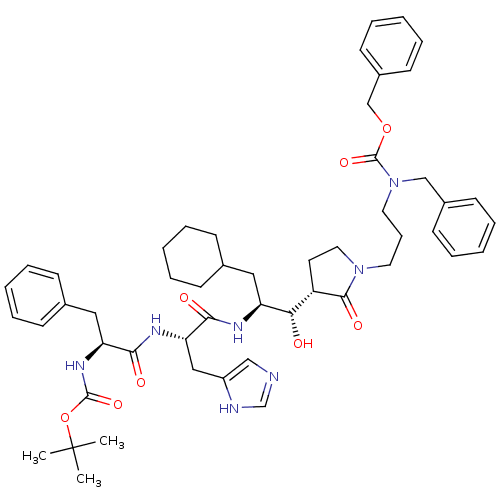 (CHEMBL354056 | {1-[1-(2-{1-[3-(Benzyl-benzyloxycar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 275 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50011699 (CHEMBL171236 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against cathepsin D | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50011698 (CHEMBL354505 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against cathepsin E | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50368239 (CHEMBL1169539) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50368239 (CHEMBL1169539) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50011696 (CHEMBL170772 | {1-[1-[1-Cyclohexylmethyl-2-(1-cycl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against cathepsin D | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50011703 (CHEMBL355128 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against cathepsin D | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50368239 (CHEMBL1169539) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Pepsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50011698 (CHEMBL354505 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Pepsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastricsin (Homo sapiens (Human)) | BDBM50011698 (CHEMBL354505 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Gastricsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50011699 (CHEMBL171236 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Pepsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastricsin (Homo sapiens (Human)) | BDBM50011696 (CHEMBL170772 | {1-[1-[1-Cyclohexylmethyl-2-(1-cycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Gastricsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50011703 (CHEMBL355128 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Pepsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50368239 (CHEMBL1169539) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Pepsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastricsin (Homo sapiens (Human)) | BDBM50011703 (CHEMBL355128 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Gastricsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastricsin (Homo sapiens (Human)) | BDBM50011699 (CHEMBL171236 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Gastricsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50011704 (CHEMBL170504 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Pepsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50011694 (CHEMBL354360 | {1-[1-[2-(5-Benzyloxymethyl-1-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Pepsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50011696 (CHEMBL170772 | {1-[1-[1-Cyclohexylmethyl-2-(1-cycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Pepsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastricsin (Homo sapiens (Human)) | BDBM50011704 (CHEMBL170504 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Gastricsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastricsin (Homo sapiens (Human)) | BDBM50011707 (CHEMBL405692 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Gastricsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastricsin (Homo sapiens (Human)) | BDBM50011702 (CHEMBL169870 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Gastricsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastricsin (Homo sapiens (Human)) | BDBM50011706 (CHEMBL353944 | {1-[1-[2-(1-Butyl-5,5-dimethyl-2-ox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Gastricsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastricsin (Homo sapiens (Human)) | BDBM50011705 (CHEMBL173204 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Gastricsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50368240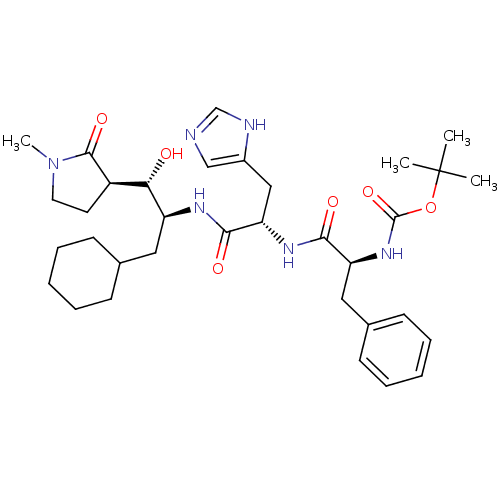 (CHEMBL1169538) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Pepsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastricsin (Homo sapiens (Human)) | BDBM50368240 (CHEMBL1169538) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Gastricsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50011705 (CHEMBL173204 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Pepsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50011707 (CHEMBL405692 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Pepsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastricsin (Homo sapiens (Human)) | BDBM50368239 (CHEMBL1169539) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Gastricsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastricsin (Homo sapiens (Human)) | BDBM50011694 (CHEMBL354360 | {1-[1-[2-(5-Benzyloxymethyl-1-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Gastricsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50011702 (CHEMBL169870 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Pepsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastricsin (Homo sapiens (Human)) | BDBM50368239 (CHEMBL1169539) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Gastricsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50011706 (CHEMBL353944 | {1-[1-[2-(1-Butyl-5,5-dimethyl-2-ox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against Pepsin | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011694 (CHEMBL354360 | {1-[1-[2-(5-Benzyloxymethyl-1-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50368240 (CHEMBL1169538) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against cathepsin D | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50011703 (CHEMBL355128 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against cathepsin E | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50368240 (CHEMBL1169538) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against cathepsin E | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50368239 (CHEMBL1169539) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against cathepsin D | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50368239 (CHEMBL1169539) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against cathepsin D | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50011711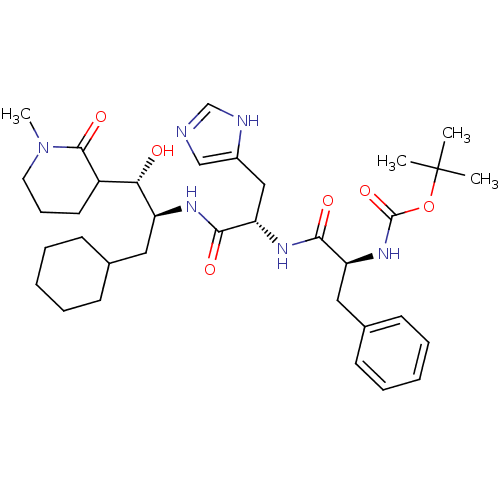 (CHEMBL170187 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50368240 (CHEMBL1169538) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay | J Med Chem 34: 887-900 (1991) BindingDB Entry DOI: 10.7270/Q2MK6DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||