

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50150766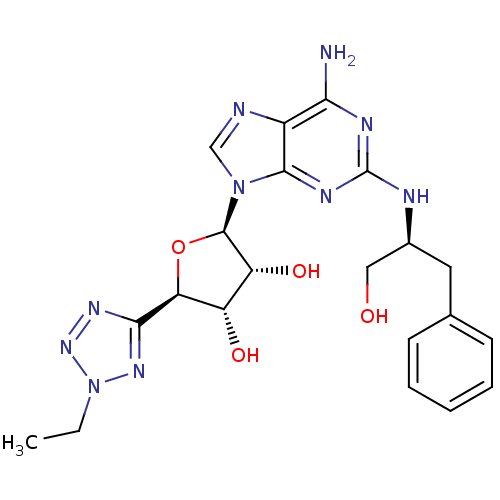 ((2R,3R,4S,5R)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309479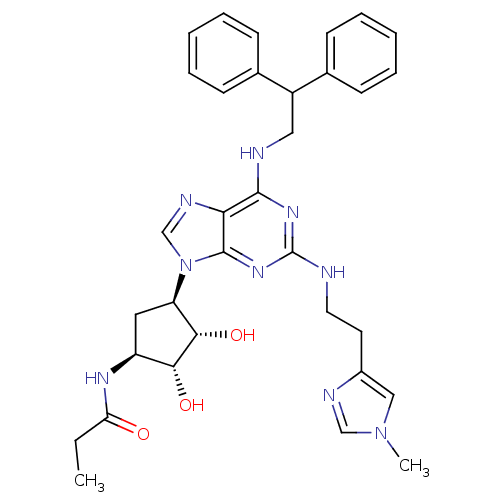 (CHEMBL591423 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309478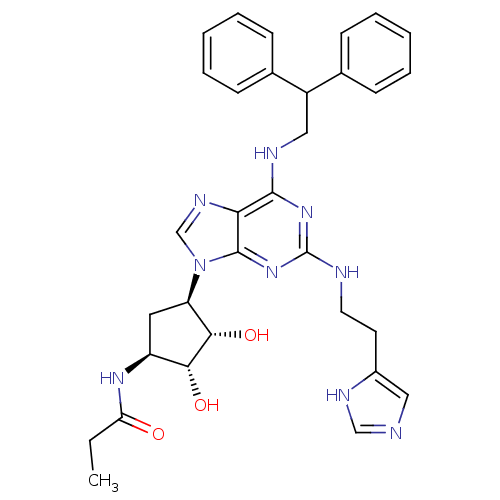 (CHEMBL601514 | N-((2S,3S,4R,5R)-5-(2-(2-(1H-imidaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309480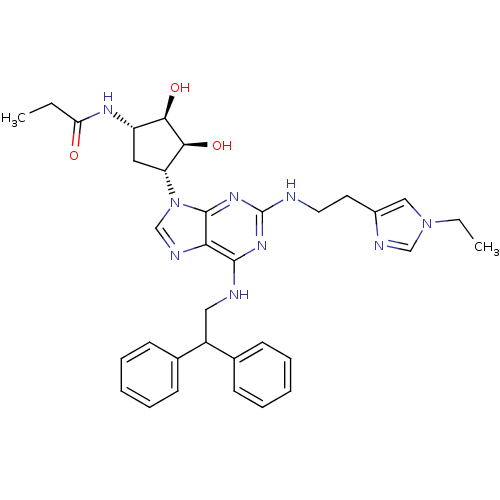 (CHEMBL591356 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309481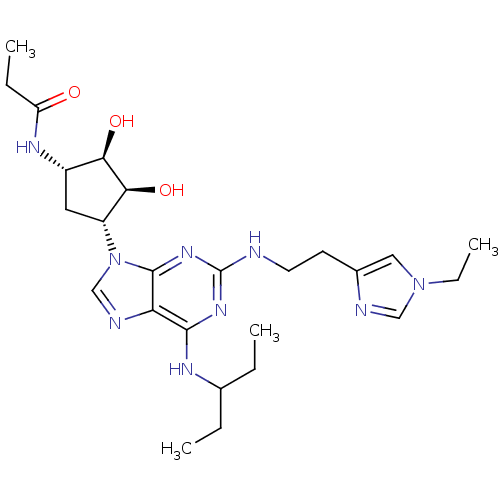 (CHEMBL592541 | N-((2S,3S,4R,5R)-5-(2-(2-(1-ethyl-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309494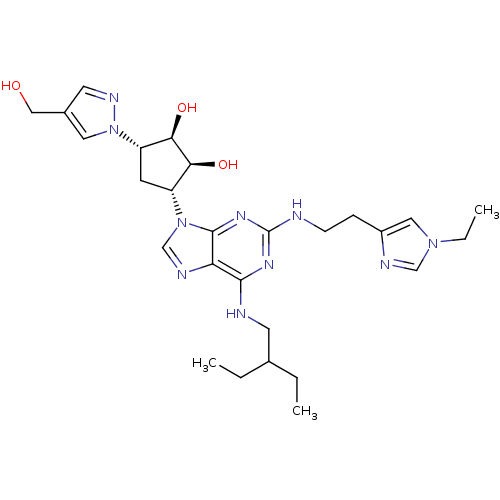 ((1R,2S,3R,5S)-3-(2-(2-(1-ethyl-1H-imidazol-4-yl)et...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309487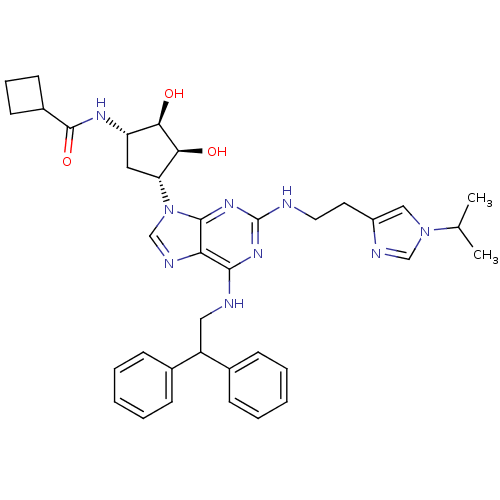 (CHEMBL601515 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309482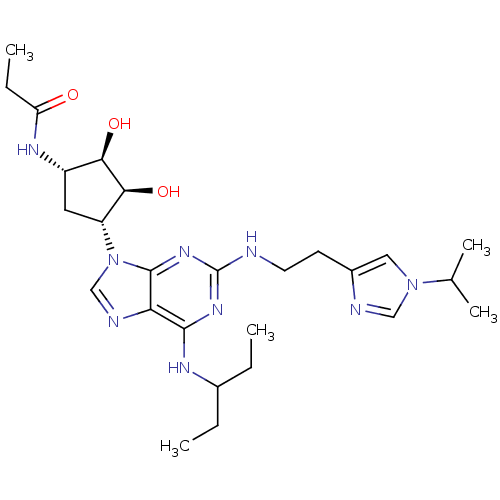 (CHEMBL592540 | N-((2S,3S,4R,5R)-3,4-dihydroxy-5-(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309486 (CHEMBL589348 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309496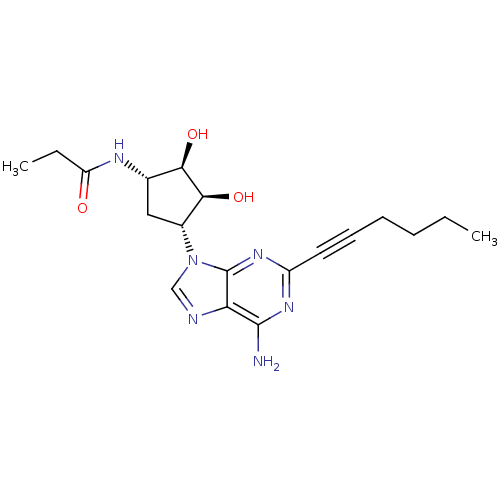 (CHEMBL591999 | N-((1S,2R,3S,4R)-4-(6-amino-2-(hex-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM35804 ((CGS21680) 3-(4-{2-[6-Amino-9-(5-ethylcarbamoylmet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309484 (CHEMBL590484 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309493 ((2R,3R,4S,5S)-2-(2-(2-(1-ethyl-1H-imidazol-4-yl)et...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309492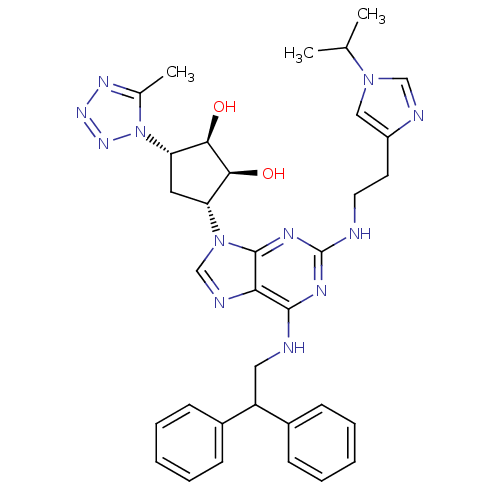 ((2R,3R,4S,5S)-2-(6-(2,2-diphenylethylamino)-2-(2-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309483 (CHEMBL590718 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309495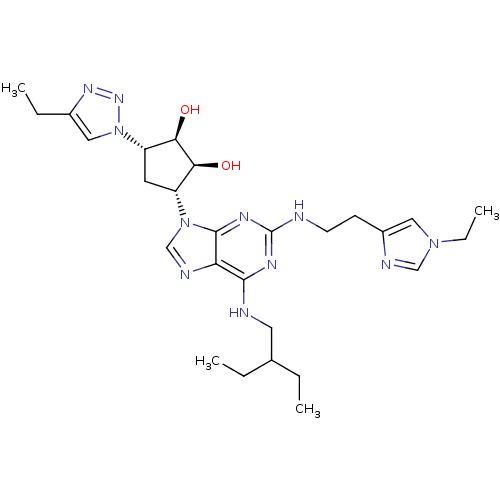 ((2S,3S,4R,5R)-2-(4-ethyl-1H-1,2,3-triazol-1-yl)-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309490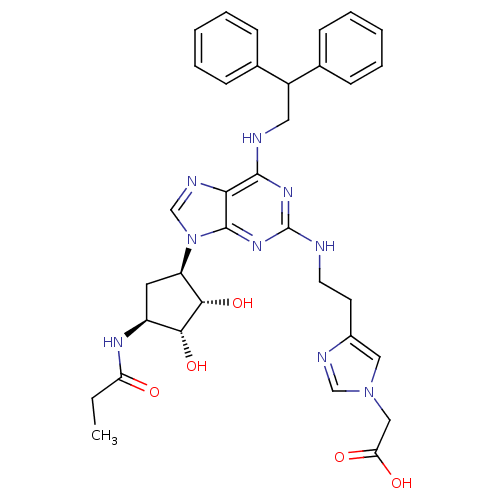 (2-(4-(2-(9-((2R,3R,4S,5S)-3,4-dihydroxy-5-propiona...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309491 ((2R,3R,4S,5S)-2-(6-(2,2-diphenylethylamino)-2-(2-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309474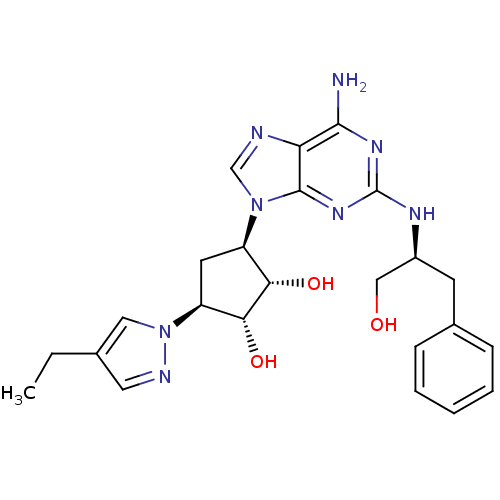 ((2R,3R,4S,5S)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309473 ((2R,3R,4S,5S)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 229 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309485 (CHEMBL601081 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 382 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309472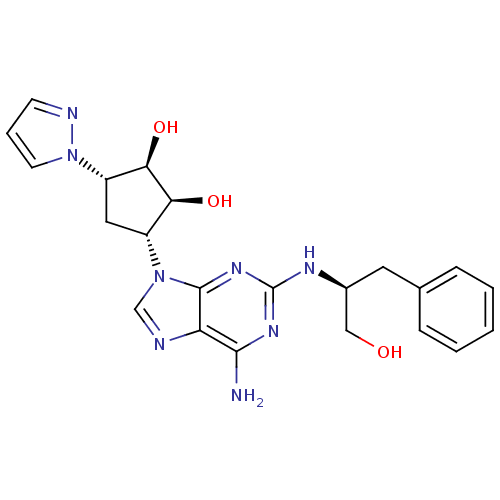 ((2R,3R,4S,5S)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 702 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309488 (CHEMBL591286 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309476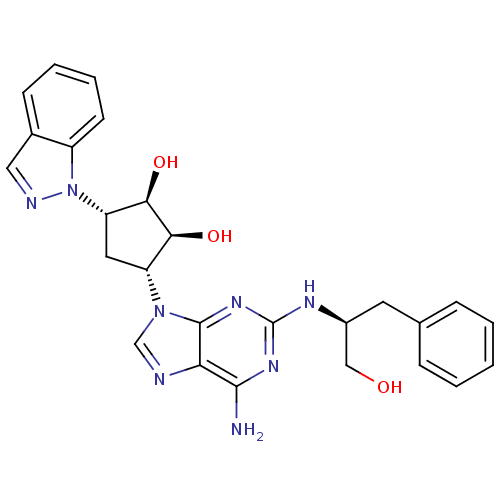 ((2R,3R,4S,5S)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309489 (CHEMBL601516 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309477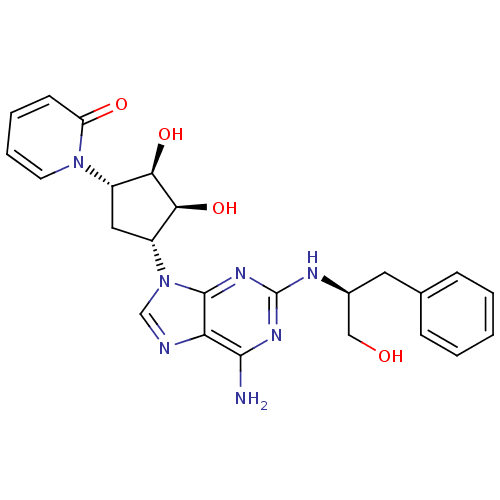 (1-((2S,3S,4R,5R)-5-(6-amino-2-((S)-1-hydroxy-3-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309475 ((2R,3R,4S,5S)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50150766 ((2R,3R,4S,5R)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in human neutrophils assessed as inhibition of fMLP-induced reactive oxygen species release by chemilumine... | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309481 (CHEMBL592541 | N-((2S,3S,4R,5R)-5-(2-(2-(1-ethyl-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in human neutrophils assessed as inhibition of fMLP-induced reactive oxygen species release by chemilumine... | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309479 (CHEMBL591423 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in human neutrophils assessed as inhibition of fMLP-induced reactive oxygen species release by chemilumine... | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309480 (CHEMBL591356 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in human neutrophils assessed as inhibition of fMLP-induced reactive oxygen species release by chemilumine... | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309494 ((1R,2S,3R,5S)-3-(2-(2-(1-ethyl-1H-imidazol-4-yl)et...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in human neutrophils assessed as inhibition of fMLP-induced reactive oxygen species release by chemilumine... | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309478 (CHEMBL601514 | N-((2S,3S,4R,5R)-5-(2-(2-(1H-imidaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in human neutrophils assessed as inhibition of fMLP-induced reactive oxygen species release by chemilumine... | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309482 (CHEMBL592540 | N-((2S,3S,4R,5R)-3,4-dihydroxy-5-(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in human neutrophils assessed as inhibition of fMLP-induced reactive oxygen species release by chemilumine... | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309496 (CHEMBL591999 | N-((1S,2R,3S,4R)-4-(6-amino-2-(hex-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in human neutrophils assessed as inhibition of fMLP-induced reactive oxygen species release by chemilumine... | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in human neutrophils assessed as inhibition of fMLP-induced reactive oxygen species release by chemilumine... | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM35804 ((CGS21680) 3-(4-{2-[6-Amino-9-(5-ethylcarbamoylmet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in human neutrophils assessed as inhibition of fMLP-induced reactive oxygen species release by chemilumine... | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309487 (CHEMBL601515 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in human neutrophils assessed as inhibition of fMLP-induced reactive oxygen species release by chemilumine... | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309473 ((2R,3R,4S,5S)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in human neutrophils assessed as inhibition of fMLP-induced reactive oxygen species release by chemilumine... | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309486 (CHEMBL589348 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in human neutrophils assessed as inhibition of fMLP-induced reactive oxygen species release by chemilumine... | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309474 ((2R,3R,4S,5S)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in human neutrophils assessed as inhibition of fMLP-induced reactive oxygen species release by chemilumine... | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309484 (CHEMBL590484 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in human neutrophils assessed as inhibition of fMLP-induced reactive oxygen species release by chemilumine... | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309492 ((2R,3R,4S,5S)-2-(6-(2,2-diphenylethylamino)-2-(2-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in human neutrophils assessed as inhibition of fMLP-induced reactive oxygen species release by chemilumine... | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309483 (CHEMBL590718 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in human neutrophils assessed as inhibition of fMLP-induced reactive oxygen species release by chemilumine... | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309493 ((2R,3R,4S,5S)-2-(2-(2-(1-ethyl-1H-imidazol-4-yl)et...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in human neutrophils assessed as inhibition of fMLP-induced reactive oxygen species release by chemilumine... | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309485 (CHEMBL601081 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 542 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in human neutrophils assessed as inhibition of fMLP-induced reactive oxygen species release by chemilumine... | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50309481 (CHEMBL592541 | N-((2S,3S,4R,5R)-5-(2-(2-(1-ethyl-1...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A3 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50309481 (CHEMBL592541 | N-((2S,3S,4R,5R)-5-(2-(2-(1-ethyl-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as effect on cAMP production by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50309493 ((2R,3R,4S,5S)-2-(2-(2-(1-ethyl-1H-imidazol-4-yl)et...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A3 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50309479 (CHEMBL591423 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A3 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50309494 ((1R,2S,3R,5S)-3-(2-(2-(1-ethyl-1H-imidazol-4-yl)et...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as effect on cAMP production by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50309473 ((2R,3R,4S,5S)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A1 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50309478 (CHEMBL601514 | N-((2S,3S,4R,5R)-5-(2-(2-(1H-imidaz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as effect on cAMP production by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50309480 (CHEMBL591356 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A3 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50309496 (CHEMBL591999 | N-((1S,2R,3S,4R)-4-(6-amino-2-(hex-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 105 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A3 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50309473 ((2R,3R,4S,5S)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 449 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A3 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50309474 ((2R,3R,4S,5S)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 776 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A3 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 38 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as effect on cAMP production by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50309496 (CHEMBL591999 | N-((1S,2R,3S,4R)-4-(6-amino-2-(hex-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A1 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50309494 ((1R,2S,3R,5S)-3-(2-(2-(1-ethyl-1H-imidazol-4-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A1 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50150766 ((2R,3R,4S,5R)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A3 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50309494 ((1R,2S,3R,5S)-3-(2-(2-(1-ethyl-1H-imidazol-4-yl)et...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 789 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A3 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A3 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM35804 ((CGS21680) 3-(4-{2-[6-Amino-9-(5-ethylcarbamoylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A1 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM35804 ((CGS21680) 3-(4-{2-[6-Amino-9-(5-ethylcarbamoylmet...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as effect on cAMP production by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50309479 (CHEMBL591423 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A1 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50150766 ((2R,3R,4S,5R)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 51 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as effect on cAMP production by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50309486 (CHEMBL589348 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 357 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A3 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50309482 (CHEMBL592540 | N-((2S,3S,4R,5R)-3,4-dihydroxy-5-(2...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.52E+3 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A3 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50309478 (CHEMBL601514 | N-((2S,3S,4R,5R)-5-(2-(2-(1H-imidaz...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 139 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A3 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50309474 ((2R,3R,4S,5S)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as effect on cAMP production by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50309474 ((2R,3R,4S,5S)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A1 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50309473 ((2R,3R,4S,5S)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as effect on cAMP production by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM35804 ((CGS21680) 3-(4-{2-[6-Amino-9-(5-ethylcarbamoylmet...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 613 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A3 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50309493 ((2R,3R,4S,5S)-2-(2-(2-(1-ethyl-1H-imidazol-4-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A1 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50309482 (CHEMBL592540 | N-((2S,3S,4R,5R)-3,4-dihydroxy-5-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A1 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50309478 (CHEMBL601514 | N-((2S,3S,4R,5R)-5-(2-(2-(1H-imidaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A1 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50309487 (CHEMBL601515 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A1 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50150766 ((2R,3R,4S,5R)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 882 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A1 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A1 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50309487 (CHEMBL601515 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 480 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A3 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50309486 (CHEMBL589348 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A1 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50309496 (CHEMBL591999 | N-((1S,2R,3S,4R)-4-(6-amino-2-(hex-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as effect on cAMP production by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50309481 (CHEMBL592541 | N-((2S,3S,4R,5R)-5-(2-(2-(1-ethyl-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A1 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||