

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50306617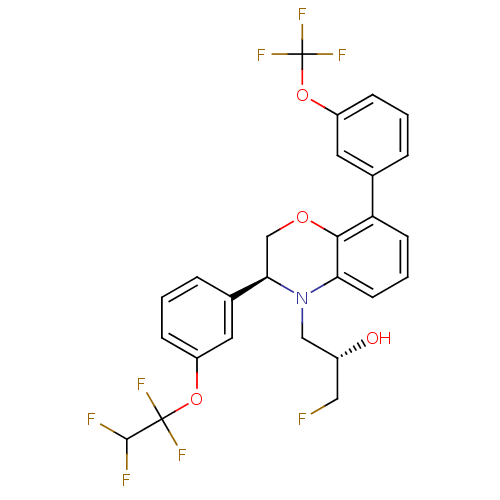 ((S)-1-fluoro-3-((S)-3-(3-(1,1,2,2-tetrafluoroethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human cholesteryl ester transfer protein by scintillation proximity assay | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50306605 ((S)-1,1,1-trifluoro-3-((S)-3-(3-(1,1,2,2-tetrafluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human cholesteryl ester transfer protein by scintillation proximity assay | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50306608 ((S)-1,1,1-trifluoro-3-((S)-3-(3-(1,1,2,2-tetrafluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human cholesteryl ester transfer protein by scintillation proximity assay | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50306605 ((S)-1,1,1-trifluoro-3-((S)-3-(3-(1,1,2,2-tetrafluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human cholesteryl ester transfer protein by scintillation proximity assay | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50306607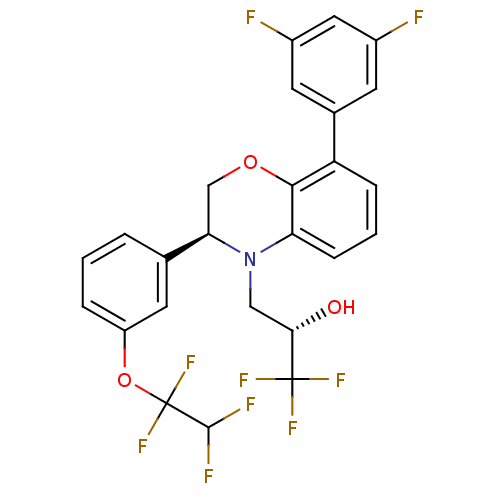 ((S)-3-((S)-8-(3,5-difluorophenyl)-3-(3-(1,1,2,2-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human cholesteryl ester transfer protein by scintillation proximity assay | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50306619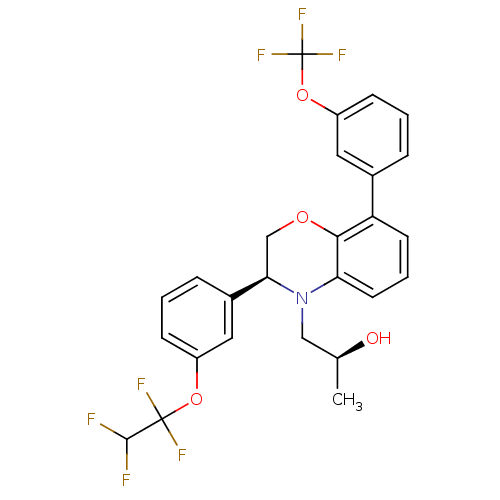 ((S)-1-((S)-3-(3-(1,1,2,2-tetrafluoroethoxy)phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human cholesteryl ester transfer protein by scintillation proximity assay | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50306611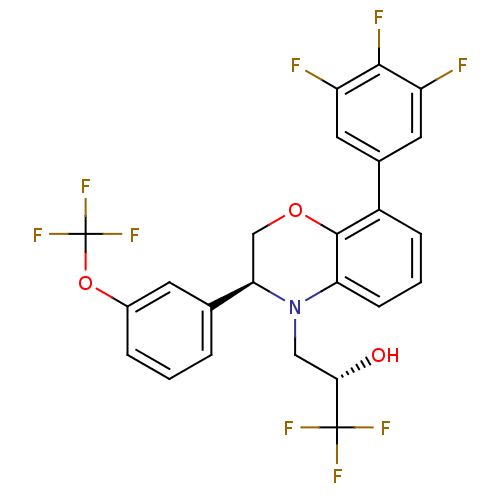 ((S)-1,1,1-trifluoro-3-((S)-3-(3-(trifluoromethoxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human cholesteryl ester transfer protein by scintillation proximity assay | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50306612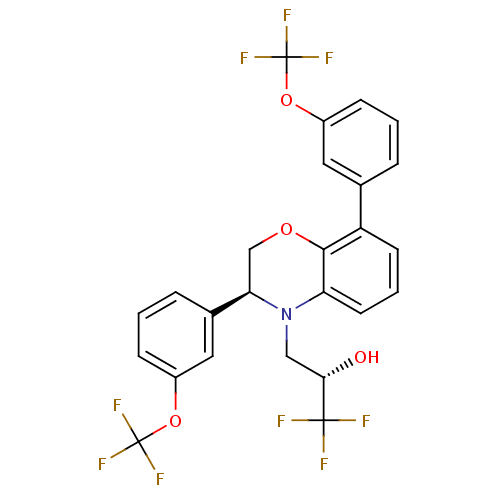 ((S)-3-((S)-3,8-bis(3-(trifluoromethoxy)phenyl)-2H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human cholesteryl ester transfer protein by scintillation proximity assay | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50306602 (3-methyl-1-(3-(3-(1,1,2,2-tetrafluoroethoxy)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human cholesteryl ester transfer protein by scintillation proximity assay | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50306612 ((S)-3-((S)-3,8-bis(3-(trifluoromethoxy)phenyl)-2H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human cholesteryl ester transfer protein by scintillation proximity assay | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50306618 ((R)-1-fluoro-3-((S)-3-(3-(1,1,2,2-tetrafluoroethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human cholesteryl ester transfer protein by scintillation proximity assay | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50306604 (4-propyl-3-(3-(1,1,2,2-tetrafluoroethoxy)phenyl)-8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human cholesteryl ester transfer protein by scintillation proximity assay | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50306610 ((S)-3-((S)-8-(3,5-difluorophenyl)-3-(3-(trifluorom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 193 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human cholesteryl ester transfer protein by scintillation proximity assay | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50306613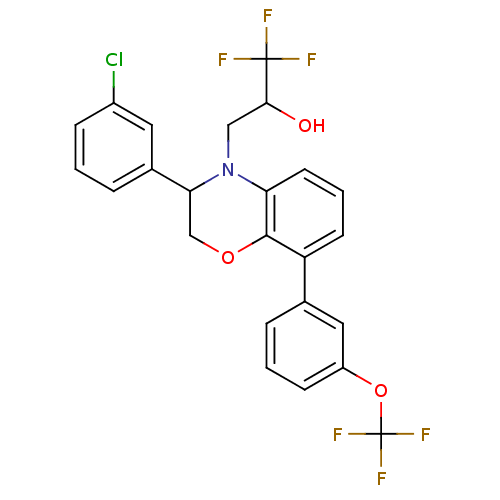 (3-(3-(3-chlorophenyl)-8-(3-(trifluoromethoxy)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 214 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human cholesteryl ester transfer protein by scintillation proximity assay | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50306616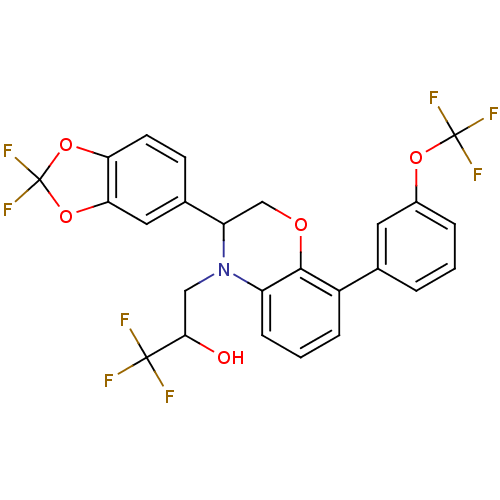 (3-(3-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)-8-(3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 271 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human cholesteryl ester transfer protein by scintillation proximity assay | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50306606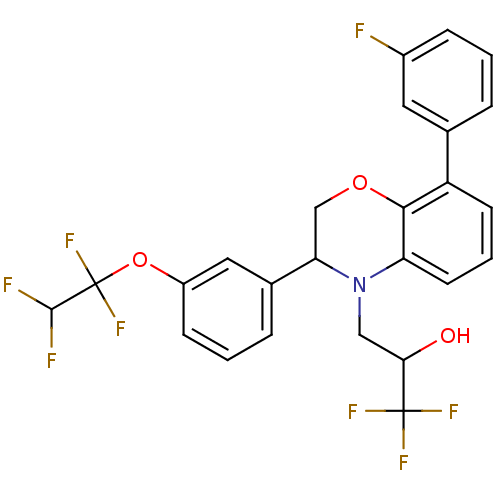 (1,1,1-trifluoro-3-(8-(3-fluorophenyl)-3-(3-(1,1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 322 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human cholesteryl ester transfer protein by scintillation proximity assay | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50306615 (1,1,1-trifluoro-3-(8-(3-(trifluoromethoxy)phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human cholesteryl ester transfer protein by scintillation proximity assay | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50306614 (3-(3-(3-ethoxyphenyl)-8-(3-(trifluoromethoxy)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human cholesteryl ester transfer protein by scintillation proximity assay | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50306603 (2-methyl-1-(3-(3-(1,1,2,2-tetrafluoroethoxy)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human cholesteryl ester transfer protein by scintillation proximity assay | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50306609 ((S)-3-((S)-8-(3,5-bis(trifluoromethyl)phenyl)-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human cholesteryl ester transfer protein by scintillation proximity assay | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50306605 ((S)-1,1,1-trifluoro-3-((S)-3-(3-(1,1,2,2-tetrafluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of cholesteryl ester transfer protein in human plasma by [3H]CE HDL assay | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50306605 ((S)-1,1,1-trifluoro-3-((S)-3-(3-(1,1,2,2-tetrafluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50306605 ((S)-1,1,1-trifluoro-3-((S)-3-(3-(1,1,2,2-tetrafluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50306605 ((S)-1,1,1-trifluoro-3-((S)-3-(3-(1,1,2,2-tetrafluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP3A4 tissue isoform in human liver microsomes | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50306605 ((S)-1,1,1-trifluoro-3-((S)-3-(3-(1,1,2,2-tetrafluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50306605 ((S)-1,1,1-trifluoro-3-((S)-3-(3-(1,1,2,2-tetrafluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP3A4 muscle isoform in human liver microsomes | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50306605 ((S)-1,1,1-trifluoro-3-((S)-3-(3-(1,1,2,2-tetrafluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes | Bioorg Med Chem Lett 20: 1432-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.096 BindingDB Entry DOI: 10.7270/Q2WW7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||