 Found 47 hits of Enzyme Inhibition Constant Data
Found 47 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin S
(Homo sapiens (Human)) | BDBM50314176

(4-(1-(3-(5-(methylsulfonyl)-3-(3-(2-(piperidin-1-y...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCC(CC1)N1C(=O)COc2ccccc12)-c1ccc(c(SCCN2CCCCC2)c1)C(F)(F)F Show InChI InChI=1S/C37H47F3N6O4S2/c1-52(48,49)44-21-14-31-29(25-44)36(27-10-11-30(37(38,39)40)34(24-27)51-23-22-42-15-5-2-6-16-42)41-45(31)18-7-17-43-19-12-28(13-20-43)46-32-8-3-4-9-33(32)50-26-35(46)47/h3-4,8-11,24,28H,2,5-7,12-23,25-26H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314168
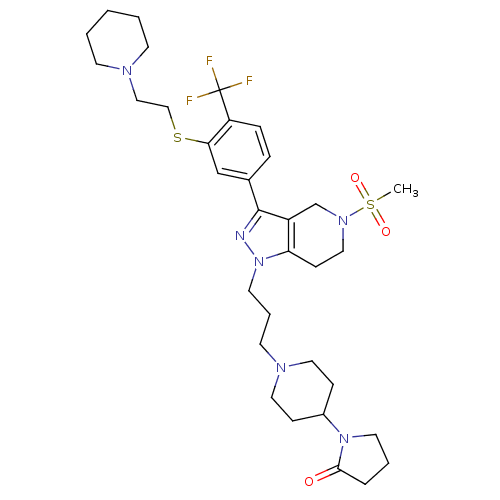
(1-(1-(3-(5-(methylsulfonyl)-3-(3-(2-(piperidin-1-y...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCC(CC1)N1CCCC1=O)-c1ccc(c(SCCN2CCCCC2)c1)C(F)(F)F Show InChI InChI=1S/C33H47F3N6O3S2/c1-47(44,45)40-20-12-29-27(24-40)32(37-42(29)17-6-15-39-18-10-26(11-19-39)41-16-5-7-31(41)43)25-8-9-28(33(34,35)36)30(23-25)46-22-21-38-13-3-2-4-14-38/h8-9,23,26H,2-7,10-22,24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S in human JY cells assessed as inhibition of invariant chain degradation |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314171
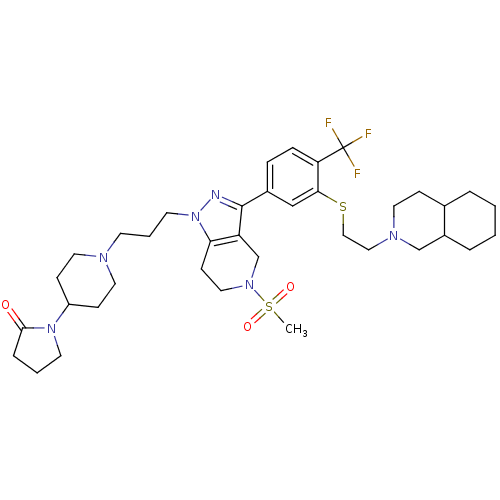
(1-(1-(3-(5-(methylsulfonyl)-3-(3-(2-(octahydroisoq...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCC(CC1)N1CCCC1=O)-c1ccc(c(SCCN2CCC3CCCCC3C2)c1)C(F)(F)F Show InChI InChI=1S/C37H53F3N6O3S2/c1-51(48,49)44-21-14-33-31(26-44)36(41-46(33)17-5-15-42-19-12-30(13-20-42)45-16-4-8-35(45)47)28-9-10-32(37(38,39)40)34(24-28)50-23-22-43-18-11-27-6-2-3-7-29(27)25-43/h9-10,24,27,29-30H,2-8,11-23,25-26H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314164
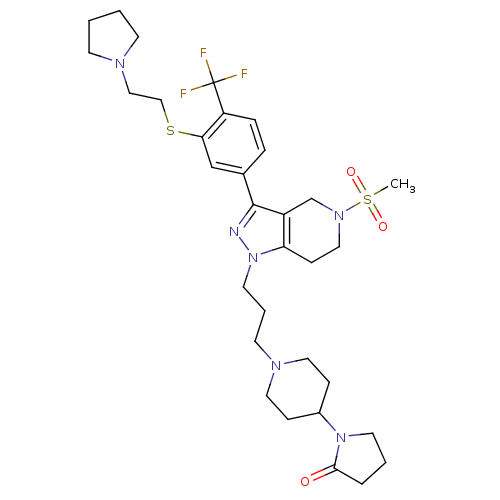
(1-(1-(3-(5-(methylsulfonyl)-3-(3-(2-(pyrrolidin-1-...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCC(CC1)N1CCCC1=O)-c1ccc(c(SCCN2CCCC2)c1)C(F)(F)F Show InChI InChI=1S/C32H45F3N6O3S2/c1-46(43,44)39-19-11-28-26(23-39)31(24-7-8-27(32(33,34)35)29(22-24)45-21-20-37-12-2-3-13-37)36-41(28)16-5-14-38-17-9-25(10-18-38)40-15-4-6-30(40)42/h7-8,22,25H,2-6,9-21,23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S in human JY cells assessed as inhibition of invariant chain degradation |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314170
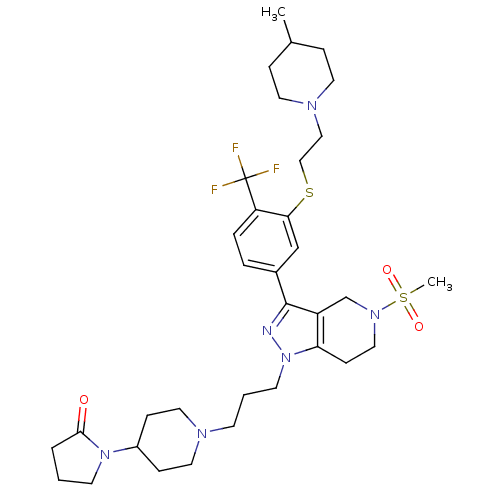
(1-(1-(3-(3-(3-(2-(4-methylpiperidin-1-yl)ethylthio...)Show SMILES CC1CCN(CCSc2cc(ccc2C(F)(F)F)-c2nn(CCCN3CCC(CC3)N3CCCC3=O)c3CCN(Cc23)S(C)(=O)=O)CC1 Show InChI InChI=1S/C34H49F3N6O3S2/c1-25-8-16-40(17-9-25)21-22-47-31-23-26(6-7-29(31)34(35,36)37)33-28-24-41(48(2,45)46)20-12-30(28)43(38-33)15-4-13-39-18-10-27(11-19-39)42-14-3-5-32(42)44/h6-7,23,25,27H,3-5,8-22,24H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314161
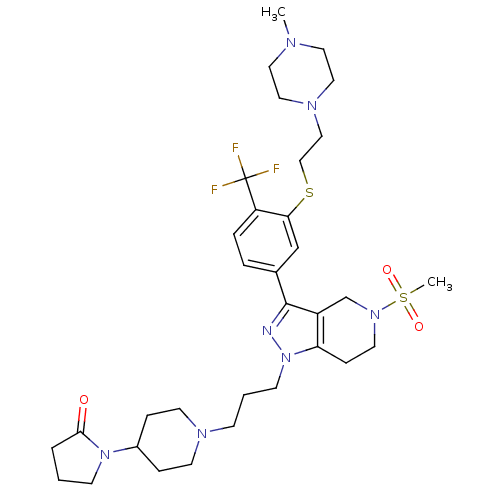
(1-(1-(3-(3-(3-(2-(4-methylpiperazin-1-yl)ethylthio...)Show SMILES CN1CCN(CCSc2cc(ccc2C(F)(F)F)-c2nn(CCCN3CCC(CC3)N3CCCC3=O)c3CCN(Cc23)S(C)(=O)=O)CC1 Show InChI InChI=1S/C33H48F3N7O3S2/c1-38-17-19-40(20-18-38)21-22-47-30-23-25(6-7-28(30)33(34,35)36)32-27-24-41(48(2,45)46)16-10-29(27)43(37-32)13-4-11-39-14-8-26(9-15-39)42-12-3-5-31(42)44/h6-7,23,26H,3-5,8-22,24H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S in human JY cells assessed as inhibition of invariant chain degradation |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314181
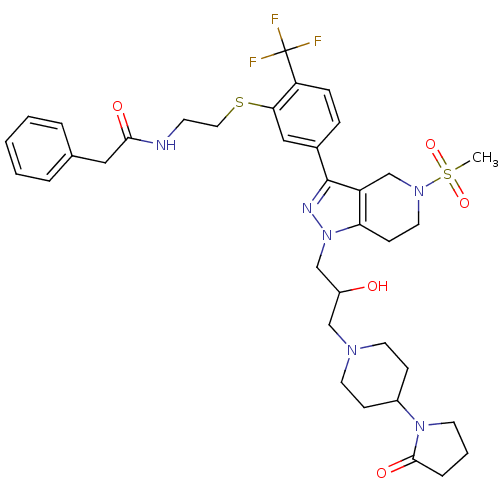
(CHEMBL1089278 | N-(2-(5-(1-(2-hydroxy-3-(4-(2-oxop...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CC(O)CN1CCC(CC1)N1CCCC1=O)-c1ccc(c(SCCNC(=O)Cc2ccccc2)c1)C(F)(F)F Show InChI InChI=1S/C36H45F3N6O5S2/c1-52(49,50)43-18-13-31-29(24-43)35(41-45(31)23-28(46)22-42-16-11-27(12-17-42)44-15-5-8-34(44)48)26-9-10-30(36(37,38)39)32(21-26)51-19-14-40-33(47)20-25-6-3-2-4-7-25/h2-4,6-7,9-10,21,27-28,46H,5,8,11-20,22-24H2,1H3,(H,40,47) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314169
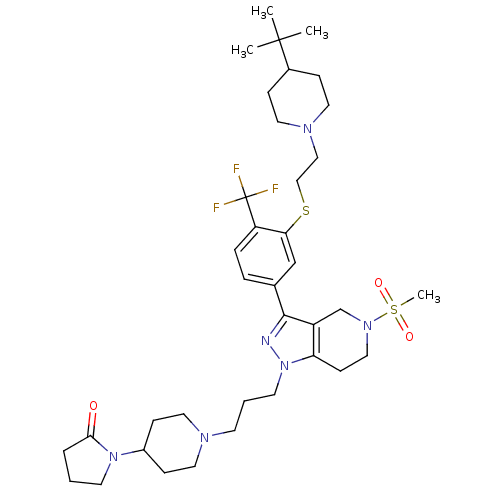
(1-(1-(3-(3-(3-(2-(4-tert-butylpiperidin-1-yl)ethyl...)Show SMILES CC(C)(C)C1CCN(CCSc2cc(ccc2C(F)(F)F)-c2nn(CCCN3CCC(CC3)N3CCCC3=O)c3CCN(Cc23)S(C)(=O)=O)CC1 Show InChI InChI=1S/C37H55F3N6O3S2/c1-36(2,3)28-10-18-43(19-11-28)23-24-50-33-25-27(8-9-31(33)37(38,39)40)35-30-26-44(51(4,48)49)22-14-32(30)46(41-35)17-6-15-42-20-12-29(13-21-42)45-16-5-7-34(45)47/h8-9,25,28-29H,5-7,10-24,26H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314168

(1-(1-(3-(5-(methylsulfonyl)-3-(3-(2-(piperidin-1-y...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCC(CC1)N1CCCC1=O)-c1ccc(c(SCCN2CCCCC2)c1)C(F)(F)F Show InChI InChI=1S/C33H47F3N6O3S2/c1-47(44,45)40-20-12-29-27(24-40)32(37-42(29)17-6-15-39-18-10-26(11-19-39)41-16-5-7-31(41)43)25-8-9-28(33(34,35)36)30(23-25)46-22-21-38-13-3-2-4-14-38/h8-9,23,26H,2-7,10-22,24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314157
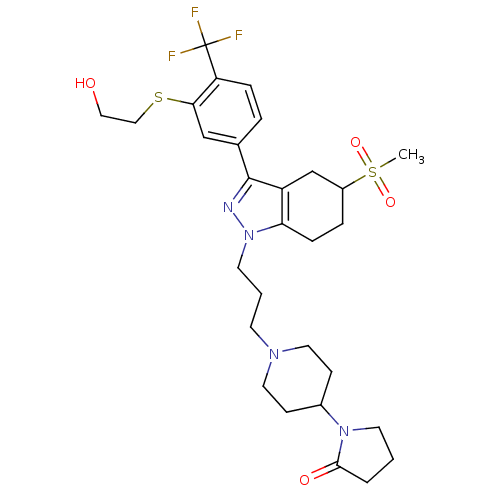
(1-(1-(3-(3-(3-(2-hydroxyethylthio)-4-(trifluoromet...)Show SMILES CS(=O)(=O)C1CCc2c(C1)c(nn2CCCN1CCC(CC1)N1CCCC1=O)-c1ccc(c(SCCO)c1)C(F)(F)F Show InChI InChI=1S/C29H39F3N4O4S2/c1-42(39,40)22-6-8-25-23(19-22)28(20-5-7-24(29(30,31)32)26(18-20)41-17-16-37)33-36(25)13-3-11-34-14-9-21(10-15-34)35-12-2-4-27(35)38/h5,7,18,21-22,37H,2-4,6,8-17,19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314167
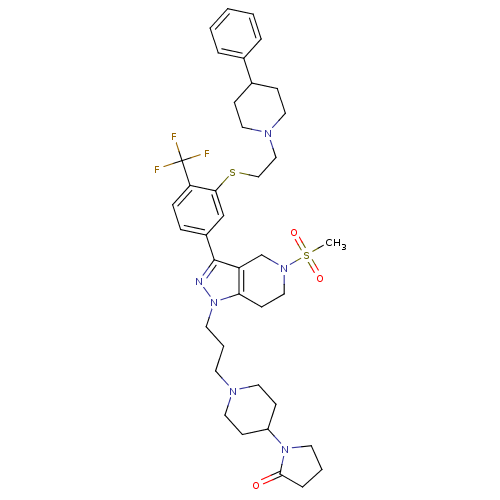
(1-(1-(3-(5-(methylsulfonyl)-3-(3-(2-(4-phenylpiper...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCC(CC1)N1CCCC1=O)-c1ccc(c(SCCN2CCC(CC2)c2ccccc2)c1)C(F)(F)F Show InChI InChI=1S/C39H51F3N6O3S2/c1-53(50,51)46-24-16-35-33(28-46)38(43-48(35)19-6-17-44-22-14-32(15-23-44)47-18-5-9-37(47)49)31-10-11-34(39(40,41)42)36(27-31)52-26-25-45-20-12-30(13-21-45)29-7-3-2-4-8-29/h2-4,7-8,10-11,27,30,32H,5-6,9,12-26,28H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314176

(4-(1-(3-(5-(methylsulfonyl)-3-(3-(2-(piperidin-1-y...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCC(CC1)N1C(=O)COc2ccccc12)-c1ccc(c(SCCN2CCCCC2)c1)C(F)(F)F Show InChI InChI=1S/C37H47F3N6O4S2/c1-52(48,49)44-21-14-31-29(25-44)36(27-10-11-30(37(38,39)40)34(24-27)51-23-22-42-15-5-2-6-16-42)41-45(31)18-7-17-43-19-12-28(13-20-43)46-32-8-3-4-9-33(32)50-26-35(46)47/h3-4,8-11,24,28H,2,5-7,12-23,25-26H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S in human JY cells assessed as inhibition of invariant chain degradation |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314156

(1-(1-(3-(5-(methylsulfonyl)-3-(3-(2-phenoxyethylth...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCC(CC1)N1CCCC1=O)-c1ccc(c(SCCOc2ccccc2)c1)C(F)(F)F Show InChI InChI=1S/C34H42F3N5O4S2/c1-48(44,45)40-20-14-30-28(24-40)33(38-42(30)17-6-15-39-18-12-26(13-19-39)41-16-5-9-32(41)43)25-10-11-29(34(35,36)37)31(23-25)47-22-21-46-27-7-3-2-4-8-27/h2-4,7-8,10-11,23,26H,5-6,9,12-22,24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314166

(1-(1-(3-(5-(methylsulfonyl)-3-(3-(2-morpholinoethy...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCC(CC1)N1CCCC1=O)-c1ccc(c(SCCN2CCOCC2)c1)C(F)(F)F Show InChI InChI=1S/C32H45F3N6O4S2/c1-47(43,44)39-15-9-28-26(23-39)31(36-41(28)12-3-10-37-13-7-25(8-14-37)40-11-2-4-30(40)42)24-5-6-27(32(33,34)35)29(22-24)46-21-18-38-16-19-45-20-17-38/h5-6,22,25H,2-4,7-21,23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314173

(5-(methylsulfonyl)-3-(3-(2-(piperidin-1-yl)ethylth...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCC(CC1)N1CCCC1)-c1ccc(c(SCCN2CCCCC2)c1)C(F)(F)F Show InChI InChI=1S/C33H49F3N6O2S2/c1-46(43,44)41-21-12-30-28(25-41)32(37-42(30)18-7-15-39-19-10-27(11-20-39)40-16-5-6-17-40)26-8-9-29(33(34,35)36)31(24-26)45-23-22-38-13-3-2-4-14-38/h8-9,24,27H,2-7,10-23,25H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S in human JY cells assessed as inhibition of invariant chain degradation |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314169

(1-(1-(3-(3-(3-(2-(4-tert-butylpiperidin-1-yl)ethyl...)Show SMILES CC(C)(C)C1CCN(CCSc2cc(ccc2C(F)(F)F)-c2nn(CCCN3CCC(CC3)N3CCCC3=O)c3CCN(Cc23)S(C)(=O)=O)CC1 Show InChI InChI=1S/C37H55F3N6O3S2/c1-36(2,3)28-10-18-43(19-11-28)23-24-50-33-25-27(8-9-31(33)37(38,39)40)35-30-26-44(51(4,48)49)22-14-32(30)46(41-35)17-6-15-42-20-12-29(13-21-42)45-16-5-7-34(45)47/h8-9,25,28-29H,5-7,10-24,26H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S in human JY cells assessed as inhibition of invariant chain degradation |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314165
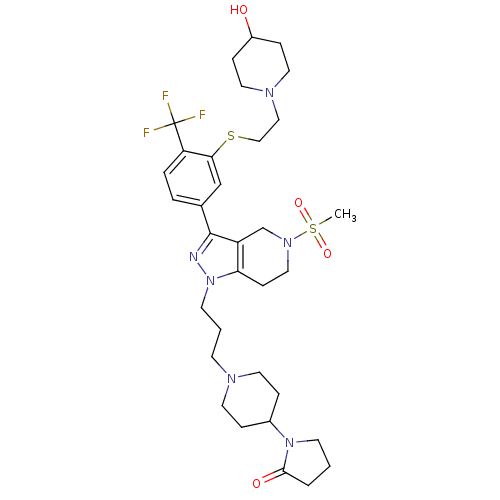
(1-(1-(3-(3-(3-(2-(4-hydroxypiperidin-1-yl)ethylthi...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCC(CC1)N1CCCC1=O)-c1ccc(c(SCCN2CCC(O)CC2)c1)C(F)(F)F Show InChI InChI=1S/C33H47F3N6O4S2/c1-48(45,46)40-19-11-29-27(23-40)32(37-42(29)14-3-12-38-15-7-25(8-16-38)41-13-2-4-31(41)44)24-5-6-28(33(34,35)36)30(22-24)47-21-20-39-17-9-26(43)10-18-39/h5-6,22,25-26,43H,2-4,7-21,23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314165

(1-(1-(3-(3-(3-(2-(4-hydroxypiperidin-1-yl)ethylthi...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCC(CC1)N1CCCC1=O)-c1ccc(c(SCCN2CCC(O)CC2)c1)C(F)(F)F Show InChI InChI=1S/C33H47F3N6O4S2/c1-48(45,46)40-19-11-29-27(23-40)32(37-42(29)14-3-12-38-15-7-25(8-16-38)41-13-2-4-31(41)44)24-5-6-28(33(34,35)36)30(22-24)47-21-20-39-17-9-26(43)10-18-39/h5-6,22,25-26,43H,2-4,7-21,23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S in human JY cells assessed as inhibition of invariant chain degradation |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314179

(CHEMBL1089277 | rac-4-fluoro-N-(2-(5-(1-(2-hydroxy...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CC(O)CN1CCC(CC1)N1CCCC1=O)-c1ccc(c(SCCNC(=O)c2ccc(F)cc2)c1)C(F)(F)F Show InChI InChI=1S/C35H42F4N6O5S2/c1-52(49,50)43-17-12-30-28(22-43)33(41-45(30)21-27(46)20-42-15-10-26(11-16-42)44-14-2-3-32(44)47)24-6-9-29(35(37,38)39)31(19-24)51-18-13-40-34(48)23-4-7-25(36)8-5-23/h4-9,19,26-27,46H,2-3,10-18,20-22H2,1H3,(H,40,48) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314175

(5-(methylsulfonyl)-3-(3-(2-(piperidin-1-yl)ethylth...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCCCC1)-c1ccc(c(SCCN2CCCCC2)c1)C(F)(F)F Show InChI InChI=1S/C29H42F3N5O2S2/c1-41(38,39)36-18-11-26-24(22-36)28(33-37(26)17-8-16-34-12-4-2-5-13-34)23-9-10-25(29(30,31)32)27(21-23)40-20-19-35-14-6-3-7-15-35/h9-10,21H,2-8,11-20,22H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S in human JY cells assessed as inhibition of invariant chain degradation |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314177

(CHEMBL1089275 | rac-N-(2-(5-(1-(2-hydroxy-3-(4-(2-...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CC(O)CN1CCC(CC1)N1CCCC1=O)-c1ccc(c(SCCNC(=O)c2ccccc2)c1)C(F)(F)F Show InChI InChI=1S/C35H43F3N6O5S2/c1-51(48,49)42-18-13-30-28(23-42)33(40-44(30)22-27(45)21-41-16-11-26(12-17-41)43-15-5-8-32(43)46)25-9-10-29(35(36,37)38)31(20-25)50-19-14-39-34(47)24-6-3-2-4-7-24/h2-4,6-7,9-10,20,26-27,45H,5,8,11-19,21-23H2,1H3,(H,39,47) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314175

(5-(methylsulfonyl)-3-(3-(2-(piperidin-1-yl)ethylth...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCCCC1)-c1ccc(c(SCCN2CCCCC2)c1)C(F)(F)F Show InChI InChI=1S/C29H42F3N5O2S2/c1-41(38,39)36-18-11-26-24(22-36)28(33-37(26)17-8-16-34-12-4-2-5-13-34)23-9-10-25(29(30,31)32)27(21-23)40-20-19-35-14-6-3-7-15-35/h9-10,21H,2-8,11-20,22H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314178
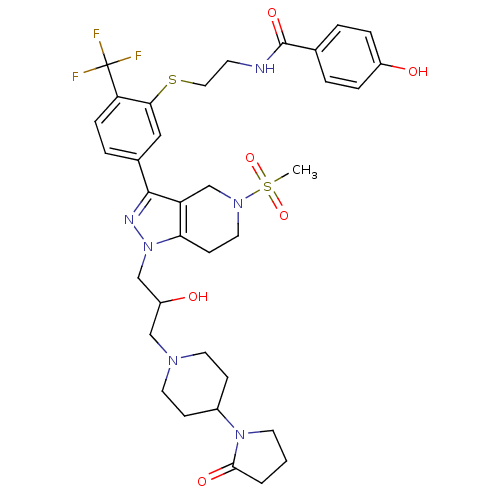
(CHEMBL1089276 | rac-4-hydroxy-N-(2-(5-(1-(2-hydrox...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CC(O)CN1CCC(CC1)N1CCCC1=O)-c1ccc(c(SCCNC(=O)c2ccc(O)cc2)c1)C(F)(F)F Show InChI InChI=1S/C35H43F3N6O6S2/c1-52(49,50)42-17-12-30-28(22-42)33(40-44(30)21-27(46)20-41-15-10-25(11-16-41)43-14-2-3-32(43)47)24-6-9-29(35(36,37)38)31(19-24)51-18-13-39-34(48)23-4-7-26(45)8-5-23/h4-9,19,25,27,45-46H,2-3,10-18,20-22H2,1H3,(H,39,48) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314164

(1-(1-(3-(5-(methylsulfonyl)-3-(3-(2-(pyrrolidin-1-...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCC(CC1)N1CCCC1=O)-c1ccc(c(SCCN2CCCC2)c1)C(F)(F)F Show InChI InChI=1S/C32H45F3N6O3S2/c1-46(43,44)39-19-11-28-26(23-39)31(24-7-8-27(32(33,34)35)29(22-24)45-21-20-37-12-2-3-13-37)36-41(28)16-5-14-38-17-9-25(10-18-38)40-15-4-6-30(40)42/h7-8,22,25H,2-6,9-21,23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314182

(CHEMBL1089279 | rac-N-(2-(5-(1-(2-hydroxy-3-(4-(2-...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CC(O)CN1CCC(CC1)N1CCCC1=O)-c1ccc(c(SCCNC(=O)C2CCCNC2)c1)C(F)(F)F Show InChI InChI=1S/C34H48F3N7O5S2/c1-51(48,49)42-16-10-29-27(22-42)32(40-44(29)21-26(45)20-41-14-8-25(9-15-41)43-13-3-5-31(43)46)23-6-7-28(34(35,36)37)30(18-23)50-17-12-39-33(47)24-4-2-11-38-19-24/h6-7,18,24-26,38,45H,2-5,8-17,19-22H2,1H3,(H,39,47) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314171

(1-(1-(3-(5-(methylsulfonyl)-3-(3-(2-(octahydroisoq...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCC(CC1)N1CCCC1=O)-c1ccc(c(SCCN2CCC3CCCCC3C2)c1)C(F)(F)F Show InChI InChI=1S/C37H53F3N6O3S2/c1-51(48,49)44-21-14-33-31(26-44)36(41-46(33)17-5-15-42-19-12-30(13-20-42)45-16-4-8-35(45)47)28-9-10-32(37(38,39)40)34(24-28)50-23-22-43-18-11-27-6-2-3-7-29(27)25-43/h9-10,24,27,29-30H,2-8,11-23,25-26H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S in human JY cells assessed as inhibition of invariant chain degradation |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314163

(1-(1-(3-(3-(3-(2-(benzylamino)ethylthio)-4-(triflu...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCC(CC1)N1CCCC1=O)-c1ccc(c(SCCNCc2ccccc2)c1)C(F)(F)F Show InChI InChI=1S/C35H45F3N6O3S2/c1-49(46,47)42-21-14-31-29(25-42)34(40-44(31)18-6-16-41-19-12-28(13-20-41)43-17-5-9-33(43)45)27-10-11-30(35(36,37)38)32(23-27)48-22-15-39-24-26-7-3-2-4-8-26/h2-4,7-8,10-11,23,28,39H,5-6,9,12-22,24-25H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S in human JY cells assessed as inhibition of invariant chain degradation |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314174
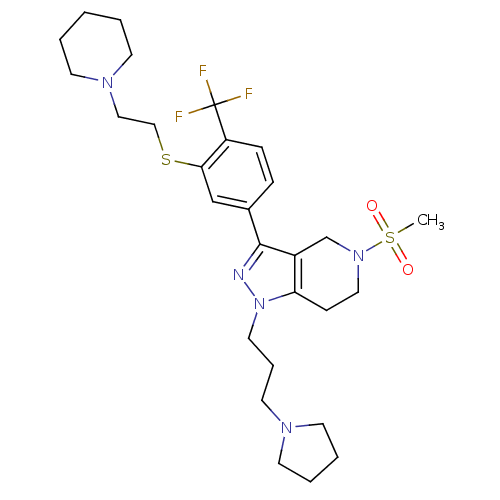
(5-(methylsulfonyl)-3-(3-(2-(piperidin-1-yl)ethylth...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCCC1)-c1ccc(c(SCCN2CCCCC2)c1)C(F)(F)F Show InChI InChI=1S/C28H40F3N5O2S2/c1-40(37,38)35-17-10-25-23(21-35)27(32-36(25)16-7-15-33-13-5-6-14-33)22-8-9-24(28(29,30)31)26(20-22)39-19-18-34-11-3-2-4-12-34/h8-9,20H,2-7,10-19,21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S in human JY cells assessed as inhibition of invariant chain degradation |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314174

(5-(methylsulfonyl)-3-(3-(2-(piperidin-1-yl)ethylth...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCCC1)-c1ccc(c(SCCN2CCCCC2)c1)C(F)(F)F Show InChI InChI=1S/C28H40F3N5O2S2/c1-40(37,38)35-17-10-25-23(21-35)27(32-36(25)16-7-15-33-13-5-6-14-33)22-8-9-24(28(29,30)31)26(20-22)39-19-18-34-11-3-2-4-12-34/h8-9,20H,2-7,10-19,21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314180
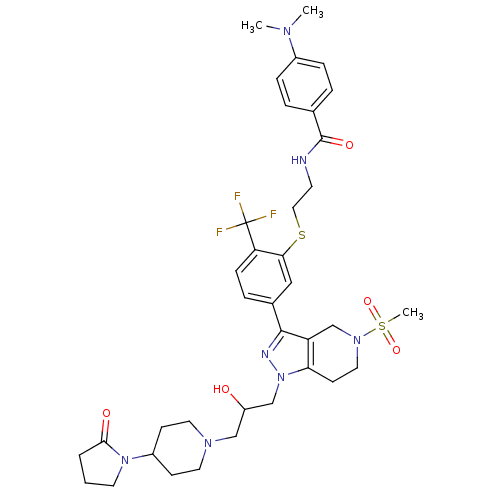
(CHEMBL1076796 | rac-4-(dimethylamino)-N-(2-(5-(1-(...)Show SMILES CN(C)c1ccc(cc1)C(=O)NCCSc1cc(ccc1C(F)(F)F)-c1nn(CC(O)CN2CCC(CC2)N2CCCC2=O)c2CCN(Cc12)S(C)(=O)=O Show InChI InChI=1S/C37H48F3N7O5S2/c1-43(2)27-9-6-25(7-10-27)36(50)41-15-20-53-33-21-26(8-11-31(33)37(38,39)40)35-30-24-45(54(3,51)52)19-14-32(30)47(42-35)23-29(48)22-44-17-12-28(13-18-44)46-16-4-5-34(46)49/h6-11,21,28-29,48H,4-5,12-20,22-24H2,1-3H3,(H,41,50) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314173

(5-(methylsulfonyl)-3-(3-(2-(piperidin-1-yl)ethylth...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCC(CC1)N1CCCC1)-c1ccc(c(SCCN2CCCCC2)c1)C(F)(F)F Show InChI InChI=1S/C33H49F3N6O2S2/c1-46(43,44)41-21-12-30-28(25-41)32(37-42(30)18-7-15-39-19-10-27(11-20-39)40-16-5-6-17-40)26-8-9-29(33(34,35)36)31(24-26)45-23-22-38-13-3-2-4-14-38/h8-9,24,27H,2-7,10-23,25H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314154

(4-(3-(3-(4-chloro-3-(2-phenoxyethylthio)phenyl)-5-...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCOCC1)-c1ccc(Cl)c(SCCOc2ccccc2)c1 Show InChI InChI=1S/C28H35ClN4O4S2/c1-39(34,35)32-13-10-26-24(21-32)28(30-33(26)12-5-11-31-14-16-36-17-15-31)22-8-9-25(29)27(20-22)38-19-18-37-23-6-3-2-4-7-23/h2-4,6-9,20H,5,10-19,21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314166

(1-(1-(3-(5-(methylsulfonyl)-3-(3-(2-morpholinoethy...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCC(CC1)N1CCCC1=O)-c1ccc(c(SCCN2CCOCC2)c1)C(F)(F)F Show InChI InChI=1S/C32H45F3N6O4S2/c1-47(43,44)39-15-9-28-26(23-39)31(36-41(28)12-3-10-37-13-7-25(8-14-37)40-11-2-4-30(40)42)24-5-6-27(32(33,34)35)29(22-24)46-21-18-38-16-19-45-20-17-38/h5-6,22,25H,2-4,7-21,23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S in human JY cells assessed as inhibition of invariant chain degradation |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314163

(1-(1-(3-(3-(3-(2-(benzylamino)ethylthio)-4-(triflu...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCC(CC1)N1CCCC1=O)-c1ccc(c(SCCNCc2ccccc2)c1)C(F)(F)F Show InChI InChI=1S/C35H45F3N6O3S2/c1-49(46,47)42-21-14-31-29(25-42)34(40-44(31)18-6-16-41-19-12-28(13-20-41)43-17-5-9-33(43)45)27-10-11-30(35(36,37)38)32(23-27)48-22-15-39-24-26-7-3-2-4-8-26/h2-4,7-8,10-11,23,28,39H,5-6,9,12-22,24-25H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314155
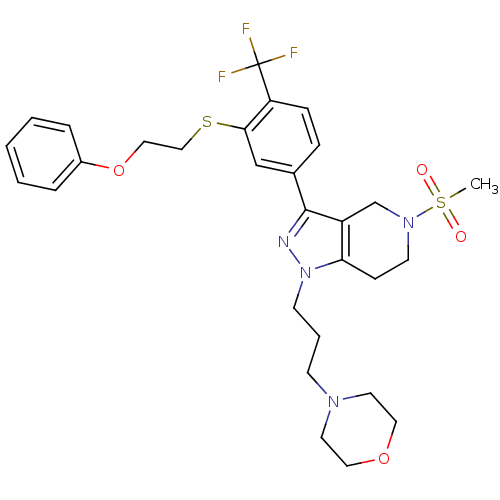
(4-(3-(5-(methylsulfonyl)-3-(3-(2-phenoxyethylthio)...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCOCC1)-c1ccc(c(SCCOc2ccccc2)c1)C(F)(F)F Show InChI InChI=1S/C29H35F3N4O4S2/c1-42(37,38)35-13-10-26-24(21-35)28(33-36(26)12-5-11-34-14-16-39-17-15-34)22-8-9-25(29(30,31)32)27(20-22)41-19-18-40-23-6-3-2-4-7-23/h2-4,6-9,20H,5,10-19,21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314162
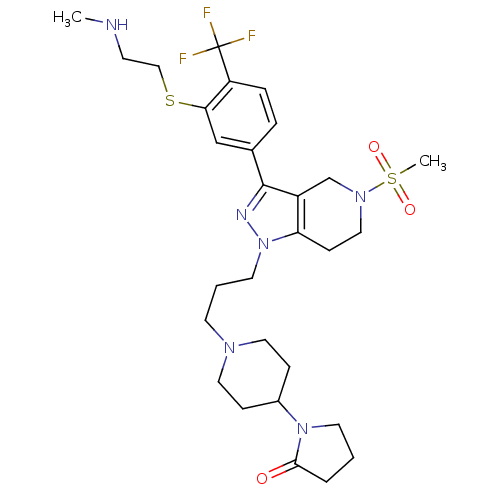
(1-(1-(3-(3-(3-(2-(methylamino)ethylthio)-4-(triflu...)Show SMILES CNCCSc1cc(ccc1C(F)(F)F)-c1nn(CCCN2CCC(CC2)N2CCCC2=O)c2CCN(Cc12)S(C)(=O)=O Show InChI InChI=1S/C29H41F3N6O3S2/c1-33-11-18-42-26-19-21(6-7-24(26)29(30,31)32)28-23-20-36(43(2,40)41)17-10-25(23)38(34-28)14-4-12-35-15-8-22(9-16-35)37-13-3-5-27(37)39/h6-7,19,22,33H,3-5,8-18,20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314172
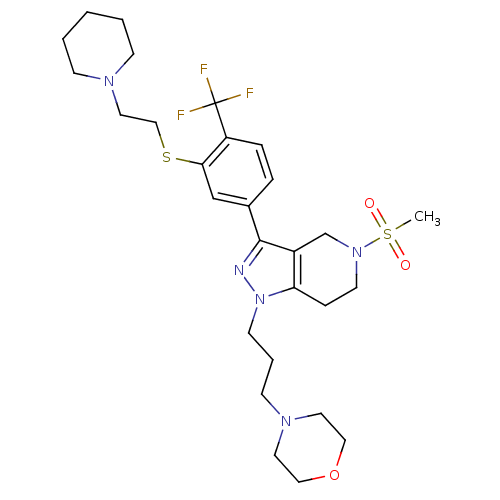
(4-(3-(5-(methylsulfonyl)-3-(3-(2-(piperidin-1-yl)e...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCOCC1)-c1ccc(c(SCCN2CCCCC2)c1)C(F)(F)F Show InChI InChI=1S/C28H40F3N5O3S2/c1-41(37,38)35-13-8-25-23(21-35)27(32-36(25)12-5-11-34-14-17-39-18-15-34)22-6-7-24(28(29,30)31)26(20-22)40-19-16-33-9-3-2-4-10-33/h6-7,20H,2-5,8-19,21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314170

(1-(1-(3-(3-(3-(2-(4-methylpiperidin-1-yl)ethylthio...)Show SMILES CC1CCN(CCSc2cc(ccc2C(F)(F)F)-c2nn(CCCN3CCC(CC3)N3CCCC3=O)c3CCN(Cc23)S(C)(=O)=O)CC1 Show InChI InChI=1S/C34H49F3N6O3S2/c1-25-8-16-40(17-9-25)21-22-47-31-23-26(6-7-29(31)34(35,36)37)33-28-24-41(48(2,45)46)20-12-30(28)43(38-33)15-4-13-39-18-10-27(11-19-39)42-14-3-5-32(42)44/h6-7,23,25,27H,3-5,8-22,24H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S in human JY cells assessed as inhibition of invariant chain degradation |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314167

(1-(1-(3-(5-(methylsulfonyl)-3-(3-(2-(4-phenylpiper...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCC(CC1)N1CCCC1=O)-c1ccc(c(SCCN2CCC(CC2)c2ccccc2)c1)C(F)(F)F Show InChI InChI=1S/C39H51F3N6O3S2/c1-53(50,51)46-24-16-35-33(28-46)38(43-48(35)19-6-17-44-22-14-32(15-23-44)47-18-5-9-37(47)49)31-10-11-34(39(40,41)42)36(27-31)52-26-25-45-20-12-30(13-21-45)29-7-3-2-4-8-29/h2-4,7-8,10-11,27,30,32H,5-6,9,12-26,28H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S in human JY cells assessed as inhibition of invariant chain degradation |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314158

(2-(2-chloro-5-(5-(methylsulfonyl)-1-(3-morpholinop...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCOCC1)-c1ccc(Cl)c(SCCO)c1 Show InChI InChI=1S/C22H31ClN4O4S2/c1-33(29,30)26-8-5-20-18(16-26)22(17-3-4-19(23)21(15-17)32-14-11-28)24-27(20)7-2-6-25-9-12-31-13-10-25/h3-4,15,28H,2,5-14,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314161

(1-(1-(3-(3-(3-(2-(4-methylpiperazin-1-yl)ethylthio...)Show SMILES CN1CCN(CCSc2cc(ccc2C(F)(F)F)-c2nn(CCCN3CCC(CC3)N3CCCC3=O)c3CCN(Cc23)S(C)(=O)=O)CC1 Show InChI InChI=1S/C33H48F3N7O3S2/c1-38-17-19-40(20-18-38)21-22-47-30-23-25(6-7-28(30)33(34,35)36)32-27-24-41(48(2,45)46)16-10-29(27)43(37-32)13-4-11-39-14-8-26(9-15-39)42-12-3-5-31(42)44/h6-7,23,26H,3-5,8-22,24H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314160

(1-(1-(3-(3-(3-(2-(dimethylamino)ethylthio)-4-(trif...)Show SMILES CN(C)CCSc1cc(ccc1C(F)(F)F)-c1nn(CCCN2CCC(CC2)N2CCCC2=O)c2CCN(Cc12)S(C)(=O)=O Show InChI InChI=1S/C30H43F3N6O3S2/c1-35(2)18-19-43-27-20-22(7-8-25(27)30(31,32)33)29-24-21-37(44(3,41)42)17-11-26(24)39(34-29)14-5-12-36-15-9-23(10-16-36)38-13-4-6-28(38)40/h7-8,20,23H,4-6,9-19,21H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314152

(4-(3-(3-(4-chloro-3-(phenethylthio)phenyl)-5-(meth...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCOCC1)-c1ccc(Cl)c(SCCc2ccccc2)c1 Show InChI InChI=1S/C28H35ClN4O3S2/c1-38(34,35)32-14-10-26-24(21-32)28(30-33(26)13-5-12-31-15-17-36-18-16-31)23-8-9-25(29)27(20-23)37-19-11-22-6-3-2-4-7-22/h2-4,6-9,20H,5,10-19,21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314151
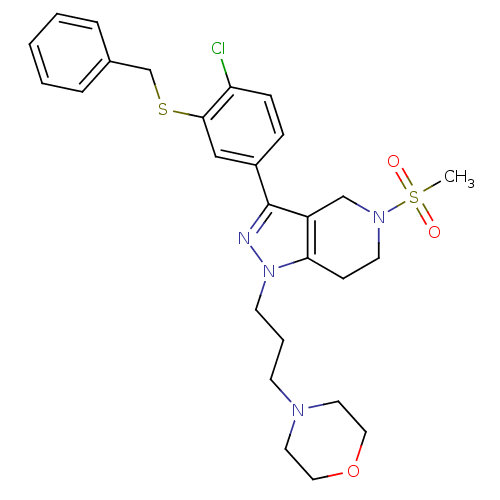
(4-(3-(3-(3-(benzylthio)-4-chlorophenyl)-5-(methyls...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCOCC1)-c1ccc(Cl)c(SCc2ccccc2)c1 Show InChI InChI=1S/C27H33ClN4O3S2/c1-37(33,34)31-13-10-25-23(19-31)27(29-32(25)12-5-11-30-14-16-35-17-15-30)22-8-9-24(28)26(18-22)36-20-21-6-3-2-4-7-21/h2-4,6-9,18H,5,10-17,19-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314159

(1-(1-(3-(3-(3-(2-(benzyl(methyl)amino)ethylthio)-4...)Show SMILES CN(CCSc1cc(ccc1C(F)(F)F)-c1nn(CCCN2CCC(CC2)N2CCCC2=O)c2CCN(Cc12)S(C)(=O)=O)Cc1ccccc1 Show InChI InChI=1S/C36H47F3N6O3S2/c1-41(25-27-8-4-3-5-9-27)22-23-49-33-24-28(11-12-31(33)36(37,38)39)35-30-26-43(50(2,47)48)21-15-32(30)45(40-35)18-7-16-42-19-13-29(14-20-42)44-17-6-10-34(44)46/h3-5,8-9,11-12,24,29H,6-7,10,13-23,25-26H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314172

(4-(3-(5-(methylsulfonyl)-3-(3-(2-(piperidin-1-yl)e...)Show SMILES CS(=O)(=O)N1CCc2c(C1)c(nn2CCCN1CCOCC1)-c1ccc(c(SCCN2CCCCC2)c1)C(F)(F)F Show InChI InChI=1S/C28H40F3N5O3S2/c1-41(37,38)35-13-8-25-23(21-35)27(32-36(25)12-5-11-34-14-17-39-18-15-34)22-6-7-24(28(29,30)31)26(20-22)40-19-16-33-9-3-2-4-10-33/h6-7,20H,2-5,8-19,21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S in human JY cells assessed as inhibition of invariant chain degradation |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50314153
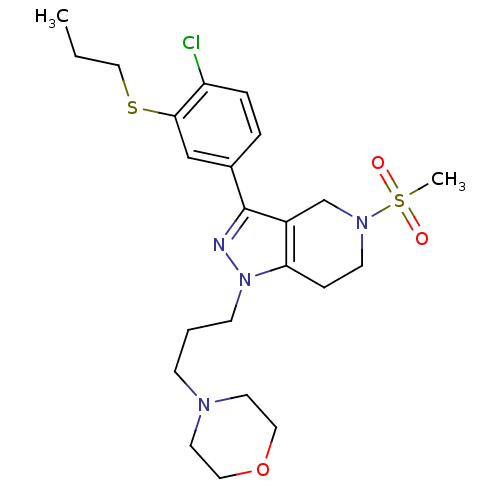
(4-(3-(3-(4-chloro-3-(propylthio)phenyl)-5-(methyls...)Show SMILES CCCSc1cc(ccc1Cl)-c1nn(CCCN2CCOCC2)c2CCN(Cc12)S(C)(=O)=O Show InChI InChI=1S/C23H33ClN4O3S2/c1-3-15-32-22-16-18(5-6-20(22)24)23-19-17-27(33(2,29)30)10-7-21(19)28(25-23)9-4-8-26-11-13-31-14-12-26/h5-6,16H,3-4,7-15,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 20: 2370-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.108
BindingDB Entry DOI: 10.7270/Q2G160Z4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data