 Found 32 hits of Enzyme Inhibition Constant Data
Found 32 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313664
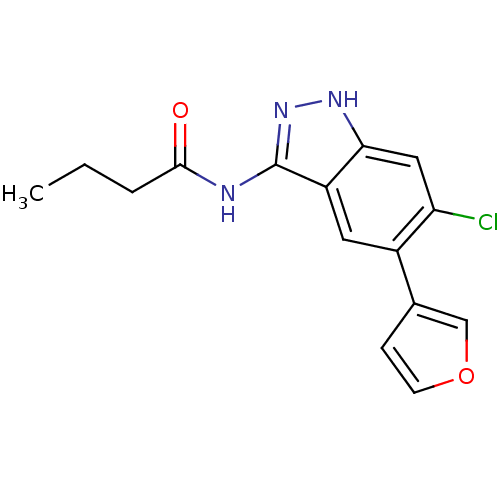
(CHEMBL1077218 | N-(6-chloro-5-(furan-3-yl)-1H-inda...)Show InChI InChI=1S/C15H14ClN3O2/c1-2-3-14(20)17-15-11-6-10(9-4-5-21-8-9)12(16)7-13(11)18-19-15/h4-8H,2-3H2,1H3,(H2,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta by kinetic assay |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313678

(CHEMBL1093747 | N-(5-bromo-6-(4-hydroxyphenyl)-1H-...)Show SMILES CCCC(=O)Nc1n[nH]c2cc(c(Br)cc12)-c1ccc(O)cc1 Show InChI InChI=1S/C17H16BrN3O2/c1-2-3-16(23)19-17-13-8-14(18)12(9-15(13)20-21-17)10-4-6-11(22)7-5-10/h4-9,22H,2-3H2,1H3,(H2,19,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta by kinetic assay |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313661
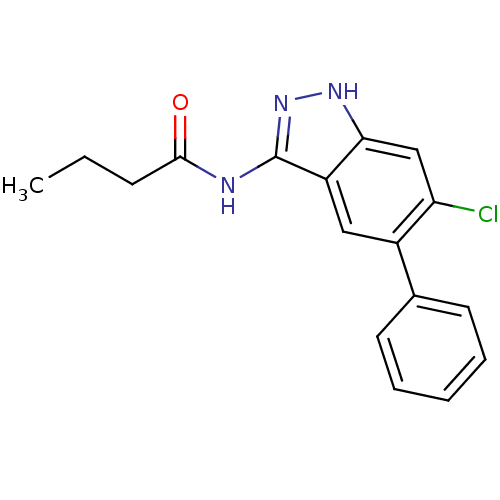
(CHEMBL1095040 | N-(6-chloro-5-phenyl-1H-indazol-3-...)Show InChI InChI=1S/C17H16ClN3O/c1-2-6-16(22)19-17-13-9-12(11-7-4-3-5-8-11)14(18)10-15(13)20-21-17/h3-5,7-10H,2,6H2,1H3,(H2,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta by kinetic assay |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313663
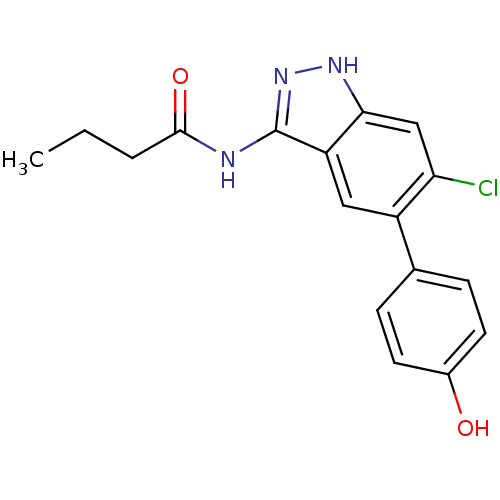
(CHEMBL1095366 | N-(6-chloro-5-(4-hydroxyphenyl)-1H...)Show SMILES CCCC(=O)Nc1n[nH]c2cc(Cl)c(cc12)-c1ccc(O)cc1 Show InChI InChI=1S/C17H16ClN3O2/c1-2-3-16(23)19-17-13-8-12(10-4-6-11(22)7-5-10)14(18)9-15(13)20-21-17/h4-9,22H,2-3H2,1H3,(H2,19,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta by kinetic assay |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313668
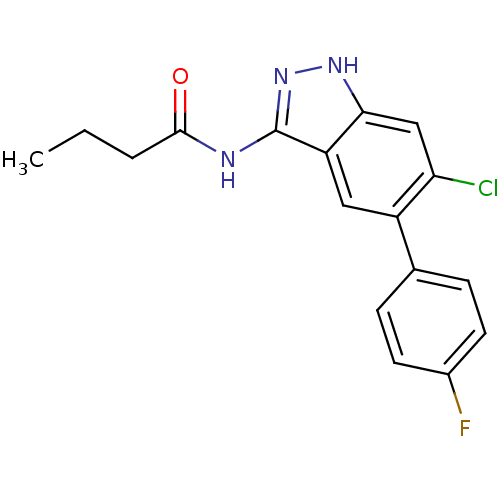
(CHEMBL1091082 | N-(6-chloro-5-(4-fluorophenyl)-1H-...)Show SMILES CCCC(=O)Nc1n[nH]c2cc(Cl)c(cc12)-c1ccc(F)cc1 Show InChI InChI=1S/C17H15ClFN3O/c1-2-3-16(23)20-17-13-8-12(10-4-6-11(19)7-5-10)14(18)9-15(13)21-22-17/h4-9H,2-3H2,1H3,(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta by kinetic assay |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50314525

(1-(6-chloro-5-phenyl-1H-indazol-3-yl)-3-phenylurea...)Show SMILES Clc1cc2[nH]nc(NC(=O)Nc3ccccc3)c2cc1-c1ccccc1 Show InChI InChI=1S/C20H15ClN4O/c21-17-12-18-16(11-15(17)13-7-3-1-4-8-13)19(25-24-18)23-20(26)22-14-9-5-2-6-10-14/h1-12H,(H3,22,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta assessed as tau phosphorylation |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313656

(CHEMBL1086174 | N-(6-phenyl-1H-indazol-3-yl)butyra...)Show InChI InChI=1S/C17H17N3O/c1-2-6-16(21)18-17-14-10-9-13(11-15(14)19-20-17)12-7-4-3-5-8-12/h3-5,7-11H,2,6H2,1H3,(H2,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta by kinetic assay |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50313700
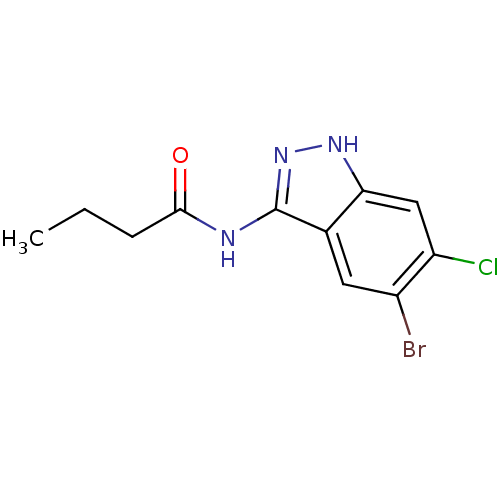
(CHEMBL1089722 | N-(5-bromo-6-chloro-1H-indazol-3-y...)Show InChI InChI=1S/C11H11BrClN3O/c1-2-3-10(17)14-11-6-4-7(12)8(13)5-9(6)15-16-11/h4-5H,2-3H2,1H3,(H2,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50313668

(CHEMBL1091082 | N-(6-chloro-5-(4-fluorophenyl)-1H-...)Show SMILES CCCC(=O)Nc1n[nH]c2cc(Cl)c(cc12)-c1ccc(F)cc1 Show InChI InChI=1S/C17H15ClFN3O/c1-2-3-16(23)20-17-13-8-12(10-4-6-11(19)7-5-10)14(18)9-15(13)21-22-17/h4-9H,2-3H2,1H3,(H2,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50313662
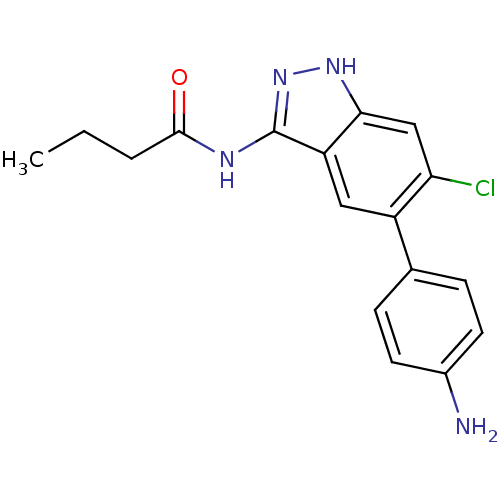
(CHEMBL1095041 | N-(5-(4-aminophenyl)-6-chloro-1H-i...)Show SMILES CCCC(=O)Nc1n[nH]c2cc(Cl)c(cc12)-c1ccc(N)cc1 Show InChI InChI=1S/C17H17ClN4O/c1-2-3-16(23)20-17-13-8-12(10-4-6-11(19)7-5-10)14(18)9-15(13)21-22-17/h4-9H,2-3,19H2,1H3,(H2,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50313686

(CHEMBL1085918 | N-(6-(pyridin-3-yl)-1H-indazol-3-y...)Show InChI InChI=1S/C16H16N4O/c1-2-4-15(21)18-16-13-7-6-11(9-14(13)19-20-16)12-5-3-8-17-10-12/h3,5-10H,2,4H2,1H3,(H2,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50313666
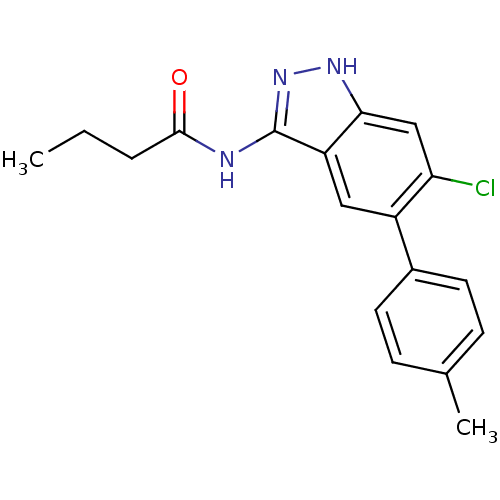
(CHEMBL1097694 | N-(6-chloro-5-p-tolyl-1H-indazol-3...)Show SMILES CCCC(=O)Nc1n[nH]c2cc(Cl)c(cc12)-c1ccc(C)cc1 Show InChI InChI=1S/C18H18ClN3O/c1-3-4-17(23)20-18-14-9-13(12-7-5-11(2)6-8-12)15(19)10-16(14)21-22-18/h5-10H,3-4H2,1-2H3,(H2,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50313653

(CHEMBL1083208 | N-(6-(thiophen-3-yl)-1H-indazol-3-...)Show InChI InChI=1S/C15H15N3OS/c1-2-3-14(19)16-15-12-5-4-10(8-13(12)17-18-15)11-6-7-20-9-11/h4-9H,2-3H2,1H3,(H2,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313659

(CHEMBL1095038 | N-(6-(3,5-difluorophenyl)-1H-indaz...)Show InChI InChI=1S/C17H15F2N3O/c1-2-3-16(23)20-17-14-5-4-10(8-15(14)21-22-17)11-6-12(18)9-13(19)7-11/h4-9H,2-3H2,1H3,(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta by kinetic assay |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50313667

(CHEMBL1091081 | N-(6-chloro-5-(4-nitrophenyl)-1H-i...)Show SMILES CCCC(=O)Nc1n[nH]c2cc(Cl)c(cc12)-c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C17H15ClN4O3/c1-2-3-16(23)19-17-13-8-12(14(18)9-15(13)20-21-17)10-4-6-11(7-5-10)22(24)25/h4-9H,2-3H2,1H3,(H2,19,20,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313686

(CHEMBL1085918 | N-(6-(pyridin-3-yl)-1H-indazol-3-y...)Show InChI InChI=1S/C16H16N4O/c1-2-4-15(21)18-16-13-7-6-11(9-14(13)19-20-16)12-5-3-8-17-10-12/h3,5-10H,2,4H2,1H3,(H2,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta by kinetic assay |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50313661

(CHEMBL1095040 | N-(6-chloro-5-phenyl-1H-indazol-3-...)Show InChI InChI=1S/C17H16ClN3O/c1-2-6-16(22)19-17-13-9-12(11-7-4-3-5-8-11)14(18)10-15(13)20-21-17/h3-5,7-10H,2,6H2,1H3,(H2,19,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50313678

(CHEMBL1093747 | N-(5-bromo-6-(4-hydroxyphenyl)-1H-...)Show SMILES CCCC(=O)Nc1n[nH]c2cc(c(Br)cc12)-c1ccc(O)cc1 Show InChI InChI=1S/C17H16BrN3O2/c1-2-3-16(23)19-17-13-8-14(18)12(9-15(13)20-21-17)10-4-6-11(22)7-5-10/h4-9,22H,2-3H2,1H3,(H2,19,20,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50313669

(CHEMBL1091083 | N-(5-(4-(benzyloxy)phenyl)-6-chlor...)Show SMILES CCCC(=O)Nc1n[nH]c2cc(Cl)c(cc12)-c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C24H22ClN3O2/c1-2-6-23(29)26-24-20-13-19(21(25)14-22(20)27-28-24)17-9-11-18(12-10-17)30-15-16-7-4-3-5-8-16/h3-5,7-14H,2,6,15H2,1H3,(H2,26,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50313661

(CHEMBL1095040 | N-(6-chloro-5-phenyl-1H-indazol-3-...)Show InChI InChI=1S/C17H16ClN3O/c1-2-6-16(22)19-17-13-9-12(11-7-4-3-5-8-11)14(18)10-15(13)20-21-17/h3-5,7-10H,2,6H2,1H3,(H2,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50313678

(CHEMBL1093747 | N-(5-bromo-6-(4-hydroxyphenyl)-1H-...)Show SMILES CCCC(=O)Nc1n[nH]c2cc(c(Br)cc12)-c1ccc(O)cc1 Show InChI InChI=1S/C17H16BrN3O2/c1-2-3-16(23)19-17-13-8-14(18)12(9-15(13)20-21-17)10-4-6-11(22)7-5-10/h4-9,22H,2-3H2,1H3,(H2,19,20,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50313656

(CHEMBL1086174 | N-(6-phenyl-1H-indazol-3-yl)butyra...)Show InChI InChI=1S/C17H17N3O/c1-2-6-16(21)18-17-14-10-9-13(11-15(14)19-20-17)12-7-4-3-5-8-12/h3-5,7-11H,2,6H2,1H3,(H2,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50313678

(CHEMBL1093747 | N-(5-bromo-6-(4-hydroxyphenyl)-1H-...)Show SMILES CCCC(=O)Nc1n[nH]c2cc(c(Br)cc12)-c1ccc(O)cc1 Show InChI InChI=1S/C17H16BrN3O2/c1-2-3-16(23)19-17-13-8-14(18)12(9-15(13)20-21-17)10-4-6-11(22)7-5-10/h4-9,22H,2-3H2,1H3,(H2,19,20,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50313661

(CHEMBL1095040 | N-(6-chloro-5-phenyl-1H-indazol-3-...)Show InChI InChI=1S/C17H16ClN3O/c1-2-6-16(22)19-17-13-9-12(11-7-4-3-5-8-11)14(18)10-15(13)20-21-17/h3-5,7-10H,2,6H2,1H3,(H2,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50313661

(CHEMBL1095040 | N-(6-chloro-5-phenyl-1H-indazol-3-...)Show InChI InChI=1S/C17H16ClN3O/c1-2-6-16(22)19-17-13-9-12(11-7-4-3-5-8-11)14(18)10-15(13)20-21-17/h3-5,7-10H,2,6H2,1H3,(H2,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50314525

(1-(6-chloro-5-phenyl-1H-indazol-3-yl)-3-phenylurea...)Show SMILES Clc1cc2[nH]nc(NC(=O)Nc3ccccc3)c2cc1-c1ccccc1 Show InChI InChI=1S/C20H15ClN4O/c21-17-12-18-16(11-15(17)13-7-3-1-4-8-13)19(25-24-18)23-20(26)22-14-9-5-2-6-10-14/h1-12H,(H3,22,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50313678

(CHEMBL1093747 | N-(5-bromo-6-(4-hydroxyphenyl)-1H-...)Show SMILES CCCC(=O)Nc1n[nH]c2cc(c(Br)cc12)-c1ccc(O)cc1 Show InChI InChI=1S/C17H16BrN3O2/c1-2-3-16(23)19-17-13-8-14(18)12(9-15(13)20-21-17)10-4-6-11(22)7-5-10/h4-9,22H,2-3H2,1H3,(H2,19,20,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50313678

(CHEMBL1093747 | N-(5-bromo-6-(4-hydroxyphenyl)-1H-...)Show SMILES CCCC(=O)Nc1n[nH]c2cc(c(Br)cc12)-c1ccc(O)cc1 Show InChI InChI=1S/C17H16BrN3O2/c1-2-3-16(23)19-17-13-8-14(18)12(9-15(13)20-21-17)10-4-6-11(22)7-5-10/h4-9,22H,2-3H2,1H3,(H2,19,20,21,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50313659

(CHEMBL1095038 | N-(6-(3,5-difluorophenyl)-1H-indaz...)Show InChI InChI=1S/C17H15F2N3O/c1-2-3-16(23)20-17-14-5-4-10(8-15(14)21-22-17)11-6-12(18)9-13(19)7-11/h4-9H,2-3H2,1H3,(H2,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313698

(CHEMBL1082237 | N-(6-(4-hydroxyphenyl)-1H-indazol-...)Show InChI InChI=1S/C17H17N3O2/c1-2-3-16(22)18-17-14-9-6-12(10-15(14)19-20-17)11-4-7-13(21)8-5-11/h4-10,21H,2-3H2,1H3,(H2,18,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta by kinetic assay |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50313701
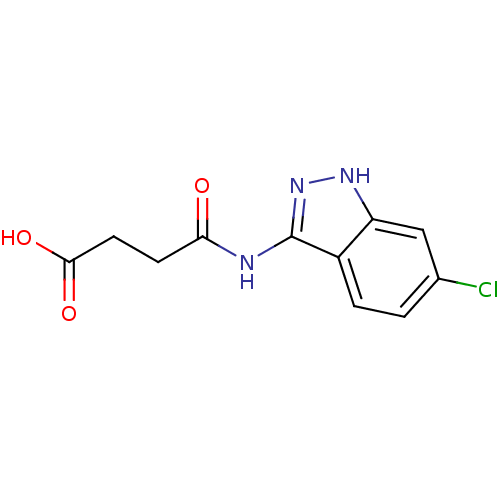
(4-(6-chloro-1H-indazol-3-ylamino)-4-oxobutanoic ac...)Show InChI InChI=1S/C11H10ClN3O3/c12-6-1-2-7-8(5-6)14-15-11(7)13-9(16)3-4-10(17)18/h1-2,5H,3-4H2,(H,17,18)(H2,13,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50313698

(CHEMBL1082237 | N-(6-(4-hydroxyphenyl)-1H-indazol-...)Show InChI InChI=1S/C17H17N3O2/c1-2-3-16(22)18-17-14-9-6-12(10-15(14)19-20-17)11-4-7-13(21)8-5-11/h4-10,21H,2-3H2,1H3,(H2,18,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data