 Found 68 hits of Enzyme Inhibition Constant Data
Found 68 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50072775
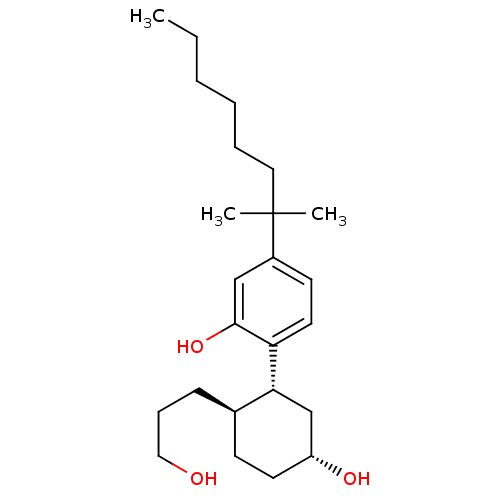
(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to cannabinoid CB1 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to cannabinoid CB2 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP4 subtype
(Mus musculus (Mouse)) | BDBM50101858
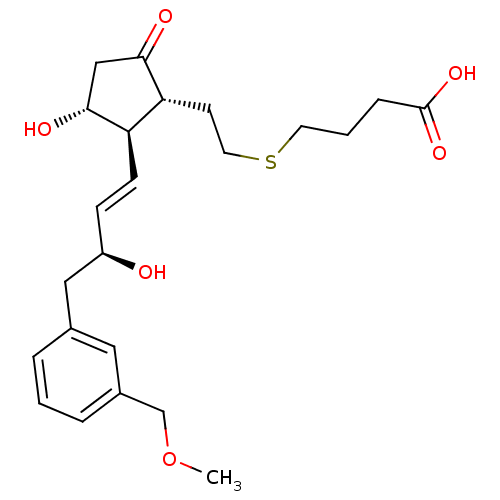
(4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...)Show SMILES COCc1cccc(C[C@H](O)\C=C\[C@H]2[C@H](O)CC(=O)[C@@H]2CCSCCCC(O)=O)c1 Show InChI InChI=1S/C23H32O6S/c1-29-15-17-5-2-4-16(12-17)13-18(24)7-8-19-20(22(26)14-21(19)25)9-11-30-10-3-6-23(27)28/h2,4-5,7-8,12,18-21,24-25H,3,6,9-11,13-15H2,1H3,(H,27,28)/b8-7+/t18-,19-,20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to mouse EP4 receptor by competitive binding assay |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50318881

(CHEMBL1086008 | N-(1-((2S,3S)-2-ethyl-4-oxotetrahy...)Show SMILES CC[C@@H]1OCC(=O)[C@H]1NC(=O)C1(CCCCC1)NC(=O)c1ccc(cc1)-c1csc(n1)N1CCN(C)CC1 |r| Show InChI InChI=1S/C28H37N5O4S/c1-3-23-24(22(34)17-37-23)30-26(36)28(11-5-4-6-12-28)31-25(35)20-9-7-19(8-10-20)21-18-38-27(29-21)33-15-13-32(2)14-16-33/h7-10,18,23-24H,3-6,11-17H2,1-2H3,(H,30,36)(H,31,35)/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50180036
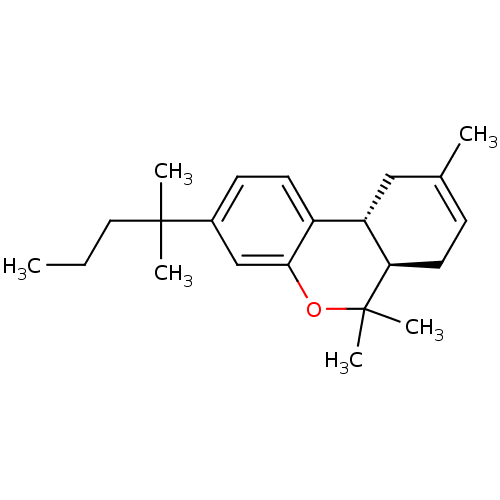
((6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetra...)Show SMILES CCCC(C)(C)c1ccc2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |r,c:13| Show InChI InChI=1S/C22H32O/c1-7-12-21(3,4)16-9-10-17-18-13-15(2)8-11-19(18)22(5,6)23-20(17)14-16/h8-10,14,18-19H,7,11-13H2,1-6H3/t18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to cannabinoid CB2 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50318882
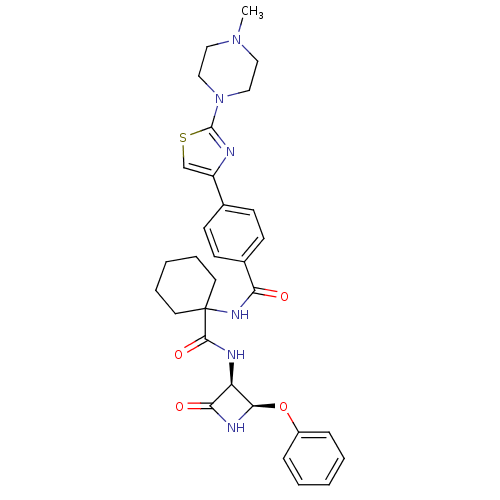
(4-(2-(4-methylpiperazin-1-yl)thiazol-4-yl)-N-(1-((...)Show SMILES CN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)N[C@H]1[C@H](NC1=O)Oc1ccccc1 |r| Show InChI InChI=1S/C31H36N6O4S/c1-36-16-18-37(19-17-36)30-32-24(20-42-30)21-10-12-22(13-11-21)26(38)35-31(14-6-3-7-15-31)29(40)33-25-27(39)34-28(25)41-23-8-4-2-5-9-23/h2,4-5,8-13,20,25,28H,3,6-7,14-19H2,1H3,(H,33,40)(H,34,39)(H,35,38)/t25-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21279
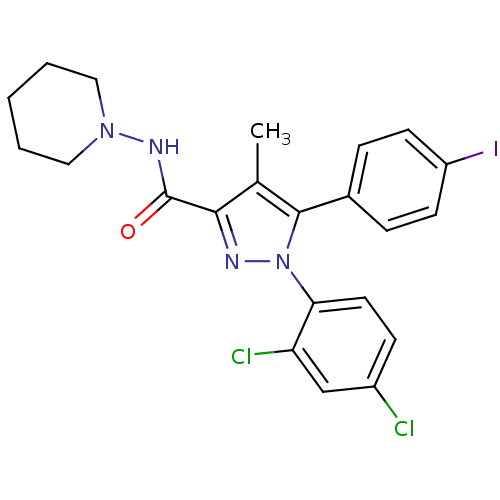
(1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N...)Show SMILES Cc1c(nn(c1-c1ccc(I)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl2IN4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-7-16(23)13-18(19)24)21(14)15-5-8-17(25)9-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to cannabinoid CB1 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50318879
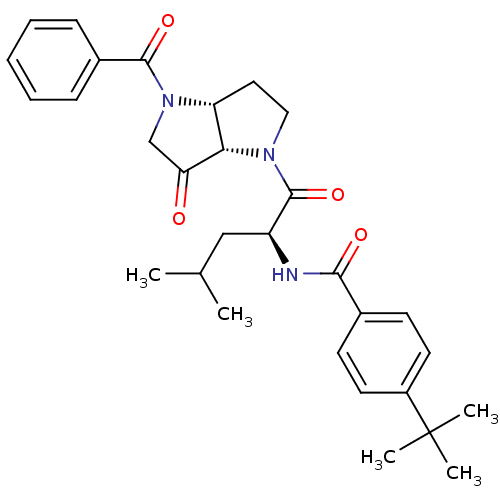
(CHEMBL607169 | N-((S)-1-((3aR,6aS)-4-benzoyl-6-oxo...)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)C(C)(C)C)C(=O)N1CC[C@@H]2[C@H]1C(=O)CN2C(=O)c1ccccc1 Show InChI InChI=1S/C30H37N3O4/c1-19(2)17-23(31-27(35)20-11-13-22(14-12-20)30(3,4)5)29(37)32-16-15-24-26(32)25(34)18-33(24)28(36)21-9-7-6-8-10-21/h6-14,19,23-24,26H,15-18H2,1-5H3,(H,31,35)/t23-,24+,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K at enzyme-substrate complex state |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50265621
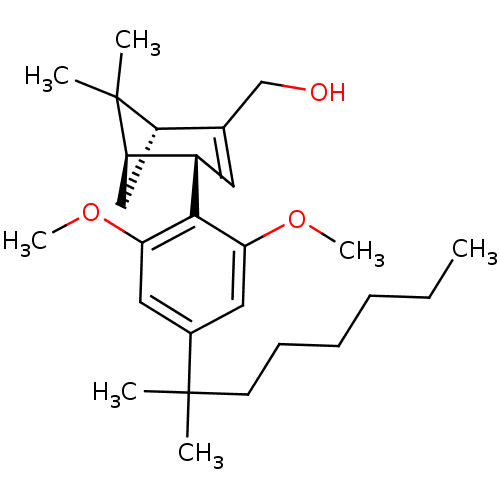
(((1R,4S,5R)-4-(2,6-dimethoxy-4-(2-methyloctan-2-yl...)Show SMILES CCCCCCC(C)(C)c1cc(OC)c([C@H]2C=C(CO)[C@@H]3C[C@H]2C3(C)C)c(OC)c1 |r,t:16| Show InChI InChI=1S/C27H42O3/c1-8-9-10-11-12-26(2,3)19-14-23(29-6)25(24(15-19)30-7)20-13-18(17-28)21-16-22(20)27(21,4)5/h13-15,20-22,28H,8-12,16-17H2,1-7H3/t20-,21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to cannabinoid CB2 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50160434
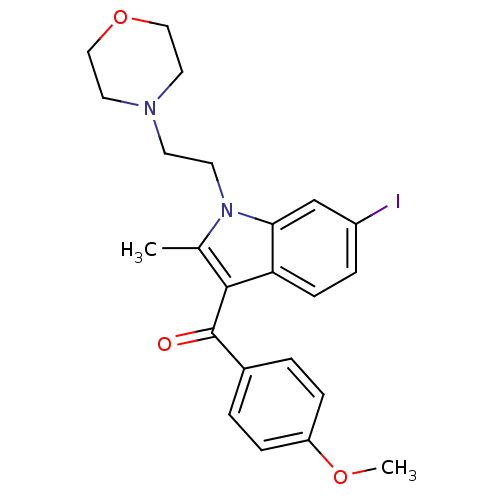
((6-iodo-2-methyl-1-(2-morpholinoethyl)-1H-indol-3-...)Show SMILES COc1ccc(cc1)C(=O)c1c(C)n(CCN2CCOCC2)c2cc(I)ccc12 Show InChI InChI=1S/C23H25IN2O3/c1-16-22(23(27)17-3-6-19(28-2)7-4-17)20-8-5-18(24)15-21(20)26(16)10-9-25-11-13-29-14-12-25/h3-8,15H,9-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to cannabinoid CB2 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50318871

(4-(2-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-3-hydroxy-4-...)Show SMILES COCc1cccc(C[C@H](O)\C=C\[C@H]2[C@H](O)CC(=O)[C@@H]2CCSCCCC(=O)OC)c1 |r| Show InChI InChI=1S/C24H34O6S/c1-29-16-18-6-3-5-17(13-18)14-19(25)8-9-20-21(23(27)15-22(20)26)10-12-31-11-4-7-24(28)30-2/h3,5-6,8-9,13,19-22,25-26H,4,7,10-12,14-16H2,1-2H3/b9-8+/t19-,20-,21-,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to mouse EP3 receptor by competitive binding assay |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Mus musculus (Mouse)) | BDBM50101858

(4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...)Show SMILES COCc1cccc(C[C@H](O)\C=C\[C@H]2[C@H](O)CC(=O)[C@@H]2CCSCCCC(O)=O)c1 Show InChI InChI=1S/C23H32O6S/c1-29-15-17-5-2-4-16(12-17)13-18(24)7-8-19-20(22(26)14-21(19)25)9-11-30-10-3-6-23(27)28/h2,4-5,7-8,12,18-21,24-25H,3,6,9-11,13-15H2,1H3,(H,27,28)/b8-7+/t18-,19-,20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to mouse EP2 receptor by competitive binding assay |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50180036

((6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetra...)Show SMILES CCCC(C)(C)c1ccc2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |r,c:13| Show InChI InChI=1S/C22H32O/c1-7-12-21(3,4)16-9-10-17-18-13-15(2)8-11-19(18)22(5,6)23-20(17)14-16/h8-10,14,18-19H,7,11-13H2,1-6H3/t18-,19+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 677 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to cannabinoid CB1 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21279

(1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N...)Show SMILES Cc1c(nn(c1-c1ccc(I)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl2IN4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-7-16(23)13-18(19)24)21(14)15-5-8-17(25)9-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to cannabinoid CB2 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50318484
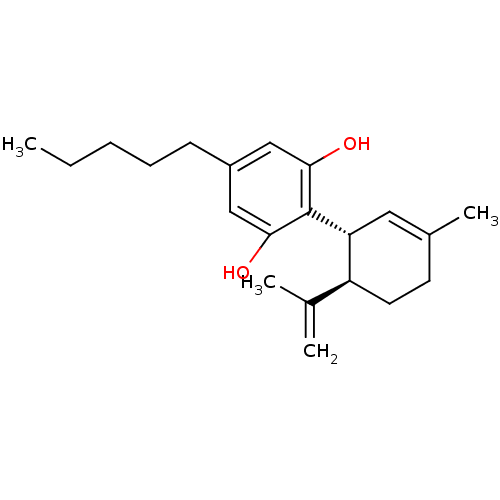
(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB1 receptor expressed in CHO cells |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160434

((6-iodo-2-methyl-1-(2-morpholinoethyl)-1H-indol-3-...)Show SMILES COc1ccc(cc1)C(=O)c1c(C)n(CCN2CCOCC2)c2cc(I)ccc12 Show InChI InChI=1S/C23H25IN2O3/c1-16-22(23(27)17-3-6-19(28-2)7-4-17)20-8-5-18(24)15-21(20)26(16)10-9-25-11-13-29-14-12-25/h3-8,15H,9-14H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to cannabinoid CB1 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB2 receptor expressed in CHO cells |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50191290

(3-((5-(2-chloro-4,5-dimethoxybenzylidene)-3-methyl...)Show SMILES COc1cc(Cl)c(\C=C2/S\C(=N\c3cccc(c3)C(O)=O)N(C)C2=O)cc1OC Show InChI InChI=1S/C20H17ClN2O5S/c1-23-18(24)17(9-12-8-15(27-2)16(28-3)10-14(12)21)29-20(23)22-13-6-4-5-11(7-13)19(25)26/h4-10H,1-3H3,(H,25,26)/b17-9-,22-20+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]echistatin from integrin alphaVbeta3 in human NCI-H1975 cells after 3 hrs by gamma counting |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50134778

((S)-3-(6-Methoxy-pyridin-3-yl)-3-{2-oxo-3-[3-(5,6,...)Show SMILES COc1ccc(cn1)[C@H](CC(O)=O)N1CCN(CCCc2ccc3CCCNc3n2)C1=O Show InChI InChI=1S/C23H29N5O4/c1-32-20-9-7-17(15-25-20)19(14-21(29)30)28-13-12-27(23(28)31)11-3-5-18-8-6-16-4-2-10-24-22(16)26-18/h6-9,15,19H,2-5,10-14H2,1H3,(H,24,26)(H,29,30)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of integrin alphaVbeta3 |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50318878
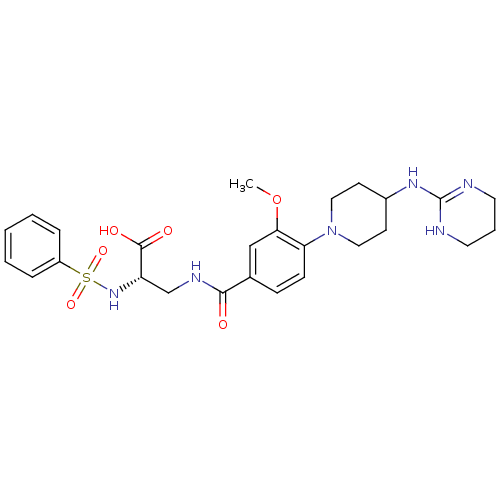
((S)-3-(3-methoxy-4-(4-(1,4,5,6-tetrahydropyrimidin...)Show SMILES COc1cc(ccc1N1CCC(CC1)NC1=NCCCN1)C(=O)NC[C@H](NS(=O)(=O)c1ccccc1)C(O)=O |r,t:17| Show InChI InChI=1S/C26H34N6O6S/c1-38-23-16-18(8-9-22(23)32-14-10-19(11-15-32)30-26-27-12-5-13-28-26)24(33)29-17-21(25(34)35)31-39(36,37)20-6-3-2-4-7-20/h2-4,6-9,16,19,21,31H,5,10-15,17H2,1H3,(H,29,33)(H,34,35)(H2,27,28,30)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of integrin alphaVbeta3 |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50255753
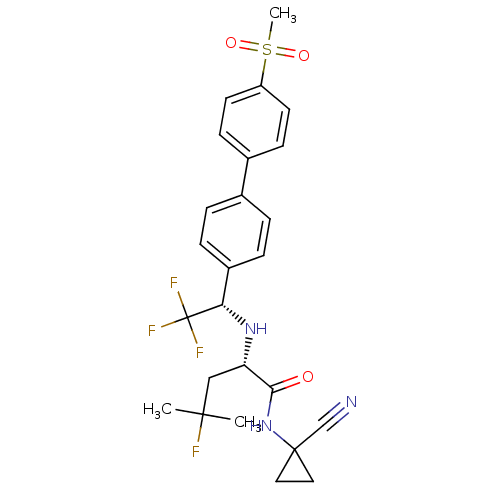
(CHEMBL481611 | MK-0822 | Odanacatib)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H27F4N3O3S/c1-23(2,26)14-20(22(33)32-24(15-30)12-13-24)31-21(25(27,28)29)18-6-4-16(5-7-18)17-8-10-19(11-9-17)36(3,34)35/h4-11,20-21,31H,12-14H2,1-3H3,(H,32,33)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50162757

((3S,7S)-7-Hydroxy-3-(6-methoxy-pyridin-3-yl)-9-(5,...)Show SMILES COc1ccc(cn1)[C@@H](CCC[C@H](O)CCc1ccc2CCCNc2n1)CC(O)=O Show InChI InChI=1S/C23H31N3O4/c1-30-21-12-8-18(15-25-21)17(14-22(28)29)4-2-6-20(27)11-10-19-9-7-16-5-3-13-24-23(16)26-19/h7-9,12,15,17,20,27H,2-6,10-11,13-14H2,1H3,(H,24,26)(H,28,29)/t17-,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of integrin alphaVbeta3 |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50177628
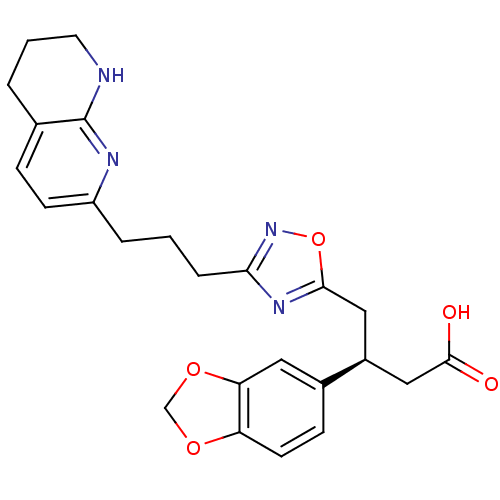
((S)-3-(benzo[d][1,3]dioxol-5-yl)-4-(3-(3-(5,6,7,8-...)Show SMILES OC(=O)C[C@H](Cc1nc(CCCc2ccc3CCCNc3n2)no1)c1ccc2OCOc2c1 Show InChI InChI=1S/C24H26N4O5/c29-23(30)13-17(16-7-9-19-20(11-16)32-14-31-19)12-22-27-21(28-33-22)5-1-4-18-8-6-15-3-2-10-25-24(15)26-18/h6-9,11,17H,1-5,10,12-14H2,(H,25,26)(H,29,30)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of integrin alphaVbeta3 |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50201116
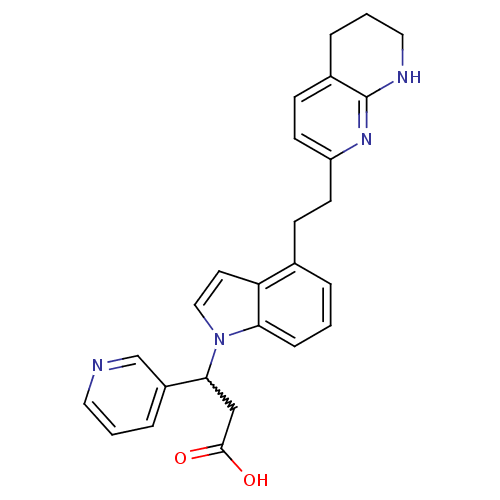
(3-(3-pyridyl)-3-[4-[2-(5,6,7,8-tetrahydro[1,8]naph...)Show SMILES OC(=O)CC(c1cccnc1)n1ccc2c(CCc3ccc4CCCNc4n3)cccc12 |w:4.3| Show InChI InChI=1S/C26H26N4O2/c31-25(32)16-24(20-6-2-13-27-17-20)30-15-12-22-18(4-1-7-23(22)30)8-10-21-11-9-19-5-3-14-28-26(19)29-21/h1-2,4,6-7,9,11-13,15,17,24H,3,5,8,10,14,16H2,(H,28,29)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of integrin alphaVbeta3 |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50235980

(2-((2S,5R,8S,11S)-5-benzyl-11-(3-guanidinopropyl)-...)Show SMILES [#6]-[#6](-[#6])-[#6@@H]-1-[#7](-[#6])-[#6](=O)-[#6@@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6]-1=O |r| Show InChI InChI=1S/C27H40N8O7/c1-15(2)22-25(41)33-17(10-7-11-30-27(28)29)23(39)31-14-20(36)32-18(13-21(37)38)24(40)34-19(26(42)35(22)3)12-16-8-5-4-6-9-16/h4-6,8-9,15,17-19,22H,7,10-14H2,1-3H3,(H,31,39)(H,32,36)(H,33,41)(H,34,40)(H,37,38)(H4,28,29,30)/t17-,18-,19+,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of integrin alphaVbeta3 |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50253098
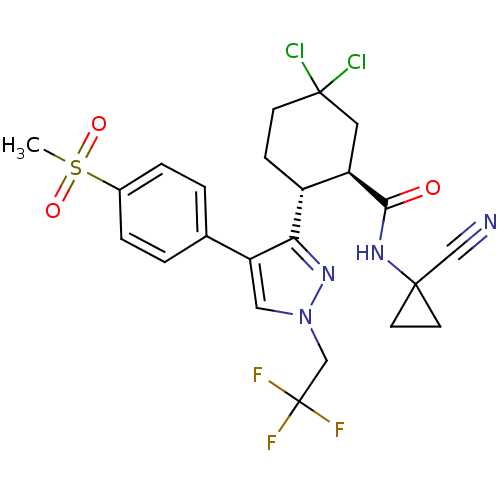
((1R,2R)-5,5-Dichloro-N-(1-cyanocyclopropyl)-2-[4-[...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cn(CC(F)(F)F)nc1[C@@H]1CCC(Cl)(Cl)C[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C23H23Cl2F3N4O3S/c1-36(34,35)15-4-2-14(3-5-15)18-11-32(13-23(26,27)28)31-19(18)16-6-7-22(24,25)10-17(16)20(33)30-21(12-29)8-9-21/h2-5,11,16-17H,6-10,13H2,1H3,(H,30,33)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150698
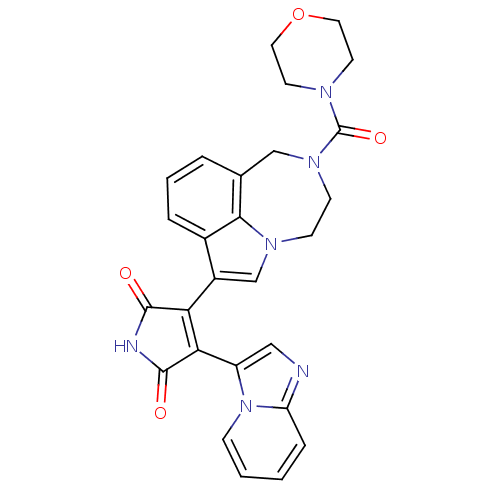
(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19855
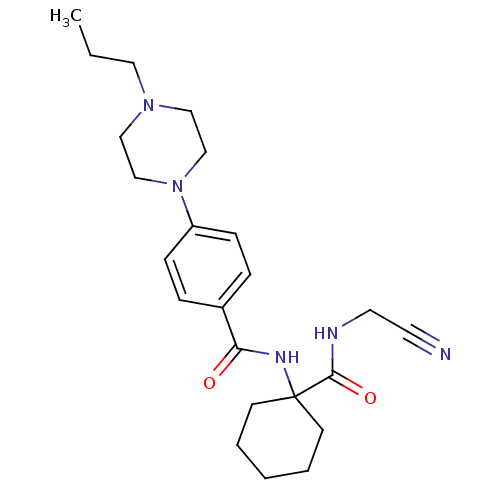
(Balicatib | CHEMBL371064 | N-[1-(cyanomethylcarbam...)Show SMILES CCCN1CCN(CC1)c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C23H33N5O2/c1-2-14-27-15-17-28(18-16-27)20-8-6-19(7-9-20)21(29)26-23(10-4-3-5-11-23)22(30)25-13-12-24/h6-9H,2-5,10-11,13-18H2,1H3,(H,25,30)(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50246060
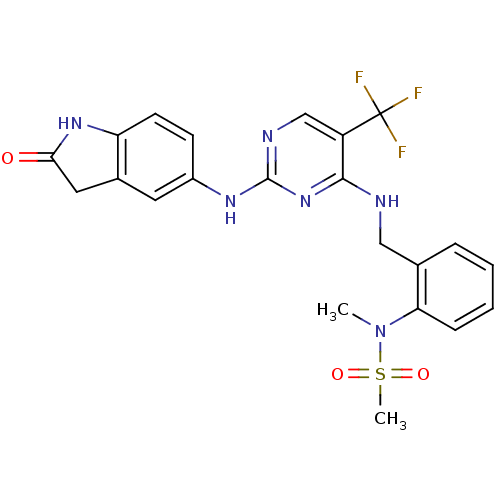
(CHEMBL472212 | CHEMBL541649 | D3RKN_6 | N-methyl-N...)Show SMILES CN(c1ccccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C22H21F3N6O3S/c1-31(35(2,33)34)18-6-4-3-5-13(18)11-26-20-16(22(23,24)25)12-27-21(30-20)28-15-7-8-17-14(9-15)10-19(32)29-17/h3-9,12H,10-11H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FAK |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50318884
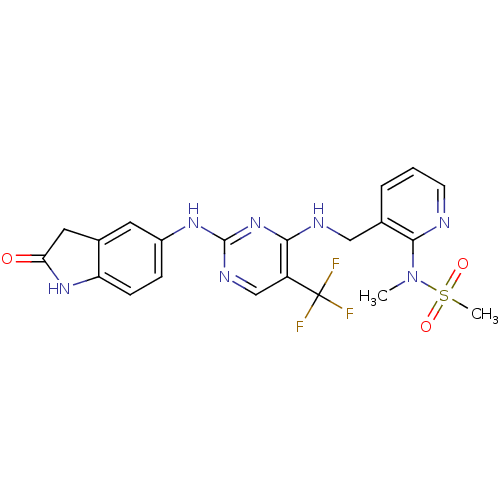
(CHEMBL1084546 | CHEMBL2430359 | N-methyl-N-(3-((2-...)Show SMILES CN(c1ncccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C21H20F3N7O3S/c1-31(35(2,33)34)19-12(4-3-7-25-19)10-26-18-15(21(22,23)24)11-27-20(30-18)28-14-5-6-16-13(8-14)9-17(32)29-16/h3-8,11H,9-10H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FAK |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM12255
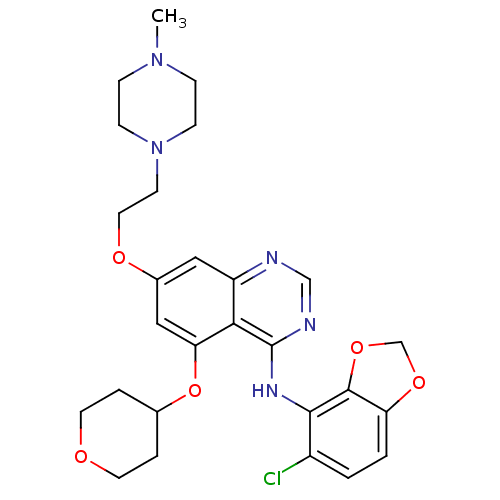
(AZD0530 | CHEMBL217092 | Compound 33 | N-(5-chloro...)Show SMILES CN1CCN(CCOc2cc(OC3CCOCC3)c3c(Nc4c5OCOc5ccc4Cl)ncnc3c2)CC1 Show InChI InChI=1S/C27H32ClN5O5/c1-32-6-8-33(9-7-32)10-13-35-19-14-21-24(23(15-19)38-18-4-11-34-12-5-18)27(30-16-29-21)31-25-20(28)2-3-22-26(25)37-17-36-22/h2-3,14-16,18H,4-13,17H2,1H3,(H,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM12255

(AZD0530 | CHEMBL217092 | Compound 33 | N-(5-chloro...)Show SMILES CN1CCN(CCOc2cc(OC3CCOCC3)c3c(Nc4c5OCOc5ccc4Cl)ncnc3c2)CC1 Show InChI InChI=1S/C27H32ClN5O5/c1-32-6-8-33(9-7-32)10-13-35-19-14-21-24(23(15-19)38-18-4-11-34-12-5-18)27(30-16-29-21)31-25-20(28)2-3-22-26(25)37-17-36-22/h2-3,14-16,18H,4-13,17H2,1H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of YES |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50318880
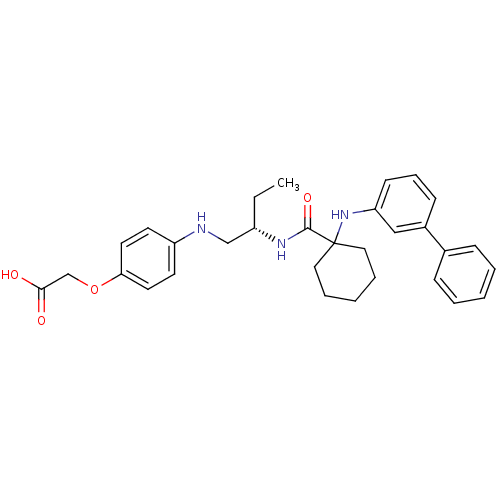
((S)-2-(4-(2-(1-(biphenyl-3-ylamino)cyclohexanecarb...)Show SMILES CC[C@@H](CNc1ccc(OCC(O)=O)cc1)NC(=O)C1(CCCCC1)Nc1cccc(c1)-c1ccccc1 |r| Show InChI InChI=1S/C31H37N3O4/c1-2-25(21-32-26-14-16-28(17-15-26)38-22-29(35)36)33-30(37)31(18-7-4-8-19-31)34-27-13-9-12-24(20-27)23-10-5-3-6-11-23/h3,5-6,9-17,20,25,32,34H,2,4,7-8,18-19,21-22H2,1H3,(H,33,37)(H,35,36)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50318880

((S)-2-(4-(2-(1-(biphenyl-3-ylamino)cyclohexanecarb...)Show SMILES CC[C@@H](CNc1ccc(OCC(O)=O)cc1)NC(=O)C1(CCCCC1)Nc1cccc(c1)-c1ccccc1 |r| Show InChI InChI=1S/C31H37N3O4/c1-2-25(21-32-26-14-16-28(17-15-26)38-22-29(35)36)33-30(37)31(18-7-4-8-19-31)34-27-13-9-12-24(20-27)23-10-5-3-6-11-23/h3,5-6,9-17,20,25,32,34H,2,4,7-8,18-19,21-22H2,1H3,(H,33,37)(H,35,36)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Rattus norvegicus) | BDBM50181298
(5-(3-((S)-2-((R)-3-hydroxy-4-(3-(trifluoromethyl)p...)Show SMILES O[C@H](CC[C@H]1CCC(=O)N1CCCc1ccc(s1)C(O)=O)Cc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H26F3NO4S/c24-23(25,26)16-4-1-3-15(13-16)14-18(28)8-6-17-7-11-21(29)27(17)12-2-5-19-9-10-20(32-19)22(30)31/h1,3-4,9-10,13,17-18,28H,2,5-8,11-12,14H2,(H,30,31)/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to rat EP4 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to EP4 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50246060

(CHEMBL472212 | CHEMBL541649 | D3RKN_6 | N-methyl-N...)Show SMILES CN(c1ccccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C22H21F3N6O3S/c1-31(35(2,33)34)18-6-4-3-5-13(18)11-26-20-16(22(23,24)25)12-27-21(30-20)28-15-7-8-17-14(9-15)10-19(32)29-17/h3-9,12H,10-11H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PYK2 |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50318884

(CHEMBL1084546 | CHEMBL2430359 | N-methyl-N-(3-((2-...)Show SMILES CN(c1ncccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C21H20F3N7O3S/c1-31(35(2,33)34)19-12(4-3-7-25-19)10-26-18-15(21(22,23)24)11-27-20(30-18)28-14-5-6-16-13(8-14)9-17(32)29-16/h3-8,11H,9-10H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PYK2 |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50249410
(5-amino-4-(isobutylamino)-6-morpholinopyrimidine-2...)Show InChI InChI=1S/C13H20N6O/c1-9(2)8-16-12-11(15)13(18-10(7-14)17-12)19-3-5-20-6-4-19/h9H,3-6,8,15H2,1-2H3,(H,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Homo sapiens (Human)) | BDBM50318873

((1S,2S,3R,4R,5S)-6-{2-[(1R)-1-[(2R)-3-{[1-(3-fluor...)Show SMILES C[C@@H](OC[C@H](O)CNC(C)(C)Cc1ccc(C)c(F)c1)c1ccccc1C1C[C@@H]2C[C@H]1[C@@H]1[C@H]2[C@H]1C(O)=O |r| Show InChI InChI=1S/C31H40FNO4/c1-17-9-10-19(11-26(17)32)14-31(3,4)33-15-21(34)16-37-18(2)22-7-5-6-8-23(22)24-12-20-13-25(24)28-27(20)29(28)30(35)36/h5-11,18,20-21,24-25,27-29,33-34H,12-16H2,1-4H3,(H,35,36)/t18-,20-,21-,24?,25-,27?,28-,29-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CaSR |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM12255

(AZD0530 | CHEMBL217092 | Compound 33 | N-(5-chloro...)Show SMILES CN1CCN(CCOc2cc(OC3CCOCC3)c3c(Nc4c5OCOc5ccc4Cl)ncnc3c2)CC1 Show InChI InChI=1S/C27H32ClN5O5/c1-32-6-8-33(9-7-32)10-13-35-19-14-21-24(23(15-19)38-18-4-11-34-12-5-18)27(30-16-29-21)31-25-20(28)2-3-22-26(25)37-17-36-22/h2-3,14-16,18H,4-13,17H2,1H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50191290

(3-((5-(2-chloro-4,5-dimethoxybenzylidene)-3-methyl...)Show SMILES COc1cc(Cl)c(\C=C2/S\C(=N\c3cccc(c3)C(O)=O)N(C)C2=O)cc1OC Show InChI InChI=1S/C20H17ClN2O5S/c1-23-18(24)17(9-12-8-15(27-2)16(28-3)10-14(12)21)29-20(23)22-13-6-4-5-11(7-13)19(25)26/h4-10H,1-3H3,(H,25,26)/b17-9-,22-20+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of integrin alphaVbeta3 |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50100971
(CHEMBL40929 | N-(3,5-dichlorophenyl)imidodicarboni...)Show InChI InChI=1S/C8H9Cl2N5/c9-4-1-5(10)3-6(2-4)14-8(13)15-7(11)12/h1-3H,(H6,11,12,13,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of integrin alphaVbeta3-mediated adhesion of human cells M21 cells |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50318883
(CHEMBL1085282 | VEL-0230 | sodium(2S,3S)-3-((S)-1-...)Show SMILES CC(C)COC[C@H](CC(C)C)NC(=O)[C@H]1O[C@@H]1C([O-])=O |r| Show InChI InChI=1S/C14H25NO5/c1-8(2)5-10(7-19-6-9(3)4)15-13(16)11-12(20-11)14(17)18/h8-12H,5-7H2,1-4H3,(H,15,16)(H,17,18)/p-1/t10-,11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 34.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 37.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to EP2 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Extracellular calcium-sensing receptor
(Homo sapiens (Human)) | BDBM50161093
((R)-2-chloro-6-(2-hydroxy-3-(2-methyl-1-(naphthale...)Show SMILES CC(C)(Cc1ccc2ccccc2c1)NC[C@@H](O)COc1cccc(Cl)c1C#N |r| Show InChI InChI=1S/C24H25ClN2O2/c1-24(2,13-17-10-11-18-6-3-4-7-19(18)12-17)27-15-20(28)16-29-23-9-5-8-22(25)21(23)14-26/h3-12,20,27-28H,13,15-16H2,1-2H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human CaSR expressed in HEK293 cells |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Rattus norvegicus) | BDBM50318880

((S)-2-(4-(2-(1-(biphenyl-3-ylamino)cyclohexanecarb...)Show SMILES CC[C@@H](CNc1ccc(OCC(O)=O)cc1)NC(=O)C1(CCCCC1)Nc1cccc(c1)-c1ccccc1 |r| Show InChI InChI=1S/C31H37N3O4/c1-2-25(21-32-26-14-16-28(17-15-26)38-22-29(35)36)33-30(37)31(18-7-4-8-19-31)34-27-13-9-12-24(20-27)23-10-5-3-6-11-23/h3,5-6,9-17,20,25,32,34H,2,4,7-8,18-19,21-22H2,1H3,(H,33,37)(H,35,36)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat cathepsin K |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50318870
(((4-(2-(cis-4-aminocyclohexyl)-9-ethyl-9H-purin-6-...)Show SMILES CCn1cnc2c(Nc3ccc(cc3)P(O)(=O)CP(O)(O)=O)nc(nc12)[C@H]1CC[C@H](N)CC1 |r,wU:26.28,wD:29.32,(30.94,-9.82,;29.4,-9.81,;28.64,-8.49,;29.54,-7.24,;28.63,-6,;27.17,-6.48,;25.83,-5.71,;25.82,-4.17,;27.15,-3.4,;28.49,-4.17,;29.82,-3.4,;29.82,-1.86,;28.47,-1.09,;27.14,-1.87,;31.15,-1.08,;31.03,-2.56,;31.14,.46,;32.48,-1.85,;33.81,-1.07,;35.15,-1.84,;33.8,-2.61,;33.81,.47,;24.5,-6.48,;24.5,-8.02,;25.83,-8.79,;27.17,-8.02,;23.17,-8.79,;23.17,-10.33,;21.82,-11.1,;20.49,-10.33,;19.16,-11.1,;20.49,-8.79,;21.82,-8.01,)| Show InChI InChI=1S/C20H28N6O5P2/c1-2-26-11-22-17-19(24-18(25-20(17)26)13-3-5-14(21)6-4-13)23-15-7-9-16(10-8-15)32(27,28)12-33(29,30)31/h7-11,13-14H,2-6,12,21H2,1H3,(H,27,28)(H,23,24,25)(H2,29,30,31)/t13-,14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50318870

(((4-(2-(cis-4-aminocyclohexyl)-9-ethyl-9H-purin-6-...)Show SMILES CCn1cnc2c(Nc3ccc(cc3)P(O)(=O)CP(O)(O)=O)nc(nc12)[C@H]1CC[C@H](N)CC1 |r,wU:26.28,wD:29.32,(30.94,-9.82,;29.4,-9.81,;28.64,-8.49,;29.54,-7.24,;28.63,-6,;27.17,-6.48,;25.83,-5.71,;25.82,-4.17,;27.15,-3.4,;28.49,-4.17,;29.82,-3.4,;29.82,-1.86,;28.47,-1.09,;27.14,-1.87,;31.15,-1.08,;31.03,-2.56,;31.14,.46,;32.48,-1.85,;33.81,-1.07,;35.15,-1.84,;33.8,-2.61,;33.81,.47,;24.5,-6.48,;24.5,-8.02,;25.83,-8.79,;27.17,-8.02,;23.17,-8.79,;23.17,-10.33,;21.82,-11.1,;20.49,-10.33,;19.16,-11.1,;20.49,-8.79,;21.82,-8.01,)| Show InChI InChI=1S/C20H28N6O5P2/c1-2-26-11-22-17-19(24-18(25-20(17)26)13-3-5-14(21)6-4-13)23-15-7-9-16(10-8-15)32(27,28)12-33(29,30)31/h7-11,13-14H,2-6,12,21H2,1H3,(H,27,28)(H,23,24,25)(H2,29,30,31)/t13-,14- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Homo sapiens (Human)) | BDBM50318876
((R)-3-(4-cyano-3-(3-(1-(2,3-dihydro-1H-inden-2-yl)...)Show SMILES CC(C)(CC1Cc2ccccc2C1)NC[C@@H](O)COc1cc(CCC(O)=O)ccc1C#N |r| Show InChI InChI=1S/C26H32N2O4/c1-26(2,14-19-11-20-5-3-4-6-21(20)12-19)28-16-23(29)17-32-24-13-18(8-10-25(30)31)7-9-22(24)15-27/h3-7,9,13,19,23,28-29H,8,10-12,14,16-17H2,1-2H3,(H,30,31)/t23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CaSR |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Homo sapiens (Human)) | BDBM50318874
(6-ethyl-3-(2-fluorophenethyl)-2-(2-hydroxyphenyl)-...)Show SMILES CCc1cc2c(cc1C)nc(-c1ccccc1O)n(CCc1ccccc1F)c2=O Show InChI InChI=1S/C25H23FN2O2/c1-3-17-15-20-22(14-16(17)2)27-24(19-9-5-7-11-23(19)29)28(25(20)30)13-12-18-8-4-6-10-21(18)26/h4-11,14-15,29H,3,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CaSR |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM12255

(AZD0530 | CHEMBL217092 | Compound 33 | N-(5-chloro...)Show SMILES CN1CCN(CCOc2cc(OC3CCOCC3)c3c(Nc4c5OCOc5ccc4Cl)ncnc3c2)CC1 Show InChI InChI=1S/C27H32ClN5O5/c1-32-6-8-33(9-7-32)10-13-35-19-14-21-24(23(15-19)38-18-4-11-34-12-5-18)27(30-16-29-21)31-25-20(28)2-3-22-26(25)37-17-36-22/h2-3,14-16,18H,4-13,17H2,1H3,(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of KIT |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Homo sapiens (Human)) | BDBM50162554

(2-(2-Hydroxy-phenyl)-3-phenethyl-3H-quinazolin-4-o...)Show InChI InChI=1S/C22H18N2O2/c25-20-13-7-5-11-18(20)21-23-19-12-6-4-10-17(19)22(26)24(21)15-14-16-8-2-1-3-9-16/h1-13,25H,14-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CaSR |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50318877
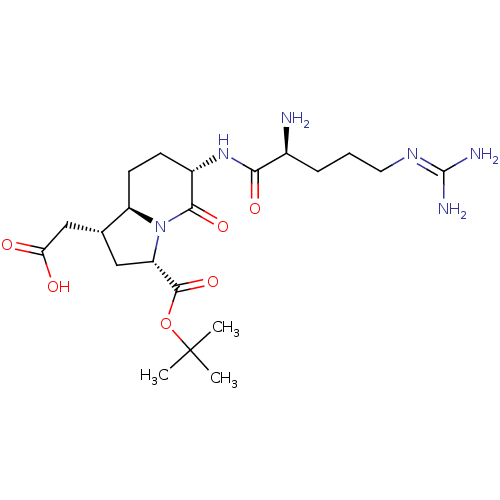
(2-((1R,3S,6S,8aR)-6-((S)-2-amino-5-guanidinopentan...)Show SMILES [#6]C([#6])([#6])[#8]-[#6](=O)-[#6@@H]-1-[#6]-[#6@H](-[#6]-[#6](-[#8])=O)-[#6@H]-2-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-2 |r| Show InChI InChI=1S/C21H36N6O6/c1-21(2,3)33-19(32)15-9-11(10-16(28)29)14-7-6-13(18(31)27(14)15)26-17(30)12(22)5-4-8-25-20(23)24/h11-15H,4-10,22H2,1-3H3,(H,26,30)(H,28,29)(H4,23,24,25)/t11-,12+,13+,14-,15+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 304 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of integrin alphaVbeta3 |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Rattus norvegicus) | BDBM50181298

(5-(3-((S)-2-((R)-3-hydroxy-4-(3-(trifluoromethyl)p...)Show SMILES O[C@H](CC[C@H]1CCC(=O)N1CCCc1ccc(s1)C(O)=O)Cc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H26F3NO4S/c24-23(25,26)16-4-1-3-15(13-16)14-18(28)8-6-17-7-11-21(29)27(17)12-2-5-19-9-10-20(32-19)22(30)31/h1,3-4,9-10,13,17-18,28H,2,5-8,11-12,14H2,(H,30,31)/t17-,18+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 483 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to rat EP2 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Homo sapiens (Human)) | BDBM50318875

((R)-ethyl 3-(4-cyano-3-(3-(1-(2,3-dihydro-1H-inden...)Show SMILES CCOC(=O)CCc1ccc(C#N)c(OC[C@H](O)CNC(C)(C)CC2Cc3ccccc3C2)c1 |r| Show InChI InChI=1S/C28H36N2O4/c1-4-33-27(32)12-10-20-9-11-24(17-29)26(15-20)34-19-25(31)18-30-28(2,3)16-21-13-22-7-5-6-8-23(22)14-21/h5-9,11,15,21,25,30-31H,4,10,12-14,16,18-19H2,1-3H3/t25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CaSR |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50318872
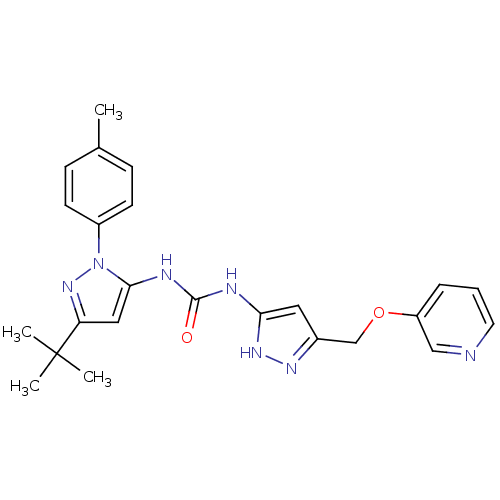
(1-(3-tert-butyl-1-p-tolyl-1H-pyrazol-5-yl)-3-(3-((...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1cc(COc2cccnc2)n[nH]1)C(C)(C)C Show InChI InChI=1S/C24H27N7O2/c1-16-7-9-18(10-8-16)31-22(13-20(30-31)24(2,3)4)27-23(32)26-21-12-17(28-29-21)15-33-19-6-5-11-25-14-19/h5-14H,15H2,1-4H3,(H3,26,27,28,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 637 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PYK2 |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM12255

(AZD0530 | CHEMBL217092 | Compound 33 | N-(5-chloro...)Show SMILES CN1CCN(CCOc2cc(OC3CCOCC3)c3c(Nc4c5OCOc5ccc4Cl)ncnc3c2)CC1 Show InChI InChI=1S/C27H32ClN5O5/c1-32-6-8-33(9-7-32)10-13-35-19-14-21-24(23(15-19)38-18-4-11-34-12-5-18)27(30-16-29-21)31-25-20(28)2-3-22-26(25)37-17-36-22/h2-3,14-16,18H,4-13,17H2,1H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CSK |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50160434

((6-iodo-2-methyl-1-(2-morpholinoethyl)-1H-indol-3-...)Show SMILES COc1ccc(cc1)C(=O)c1c(C)n(CCN2CCOCC2)c2cc(I)ccc12 Show InChI InChI=1S/C23H25IN2O3/c1-16-22(23(27)17-3-6-19(28-2)7-4-17)20-8-5-18(24)15-21(20)26(16)10-9-25-11-13-29-14-12-25/h3-8,15H,9-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at cannabinoid CB2 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Rattus norvegicus) | BDBM50181298

(5-(3-((S)-2-((R)-3-hydroxy-4-(3-(trifluoromethyl)p...)Show SMILES O[C@H](CC[C@H]1CCC(=O)N1CCCc1ccc(s1)C(O)=O)Cc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H26F3NO4S/c24-23(25,26)16-4-1-3-15(13-16)14-18(28)8-6-17-7-11-21(29)27(17)12-2-5-19-9-10-20(32-19)22(30)31/h1,3-4,9-10,13,17-18,28H,2,5-8,11-12,14H2,(H,30,31)/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at rat EP4 receptor expressed in HEK293 cells assessed as cAMP activation |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Mus musculus (Mouse)) | BDBM50318871

(4-(2-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-3-hydroxy-4-...)Show SMILES COCc1cccc(C[C@H](O)\C=C\[C@H]2[C@H](O)CC(=O)[C@@H]2CCSCCCC(=O)OC)c1 |r| Show InChI InChI=1S/C24H34O6S/c1-29-16-18-6-3-5-17(13-18)14-19(25)8-9-20-21(23(27)15-22(20)26)10-12-31-11-4-7-24(28)30-2/h3,5-6,8-9,13,19-22,25-26H,4,7,10-12,14-16H2,1-2H3/b9-8+/t19-,20-,21-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse EP4 receptor expressed in CHO cells assessed as cAMP production |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50318886

(1,3-dibutyl-5-(diaminomethylene)pyrimidine-2,4,6(1...)Show InChI InChI=1S/C13H22N4O3/c1-3-5-7-16-11(18)9(10(14)15)12(19)17(13(16)20)8-6-4-2/h9H,3-8H2,1-2H3,(H3,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at PTH1R overexpressed in HEK293 cells assessed as intracellular cAMP mobilization |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Bos taurus) | BDBM50161093

((R)-2-chloro-6-(2-hydroxy-3-(2-methyl-1-(naphthale...)Show SMILES CC(C)(Cc1ccc2ccccc2c1)NC[C@@H](O)COc1cccc(Cl)c1C#N |r| Show InChI InChI=1S/C24H25ClN2O2/c1-24(2,13-17-10-11-18-6-3-4-7-19(18)12-17)27-15-20(28)16-29-23-9-5-8-22(25)21(23)14-26/h3-12,20,27-28H,13,15-16H2,1-2H3/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 41 | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CaSR in bovine parathyroid cells assessed as increase in PTH level |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50318885
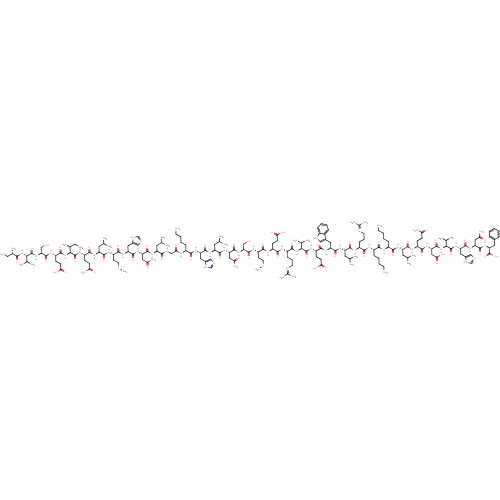
(CHEMBL525610 | teriparatide)Show SMILES [H][C@](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@@H](N)CO)C(C)C)([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C181H291N55O51S2/c1-21-96(18)146(236-160(267)114(48-53-141(250)251)212-174(281)132(84-239)232-177(284)143(93(12)13)233-147(254)103(185)82-237)178(285)216-111(45-50-134(187)241)155(262)219-119(65-90(6)7)163(270)213-116(55-62-289-20)158(265)224-124(71-100-79-196-86-203-100)167(274)226-126(73-135(188)242)169(276)217-117(63-88(2)3)148(255)201-81-138(245)205-105(39-27-30-56-182)149(256)223-123(70-99-78-195-85-202-99)166(273)221-121(67-92(10)11)164(271)225-128(75-137(190)244)171(278)231-131(83-238)173(280)214-115(54-61-288-19)157(264)210-112(46-51-139(246)247)153(260)208-109(43-34-60-199-181(193)194)159(266)234-144(94(14)15)175(282)215-113(47-52-140(248)249)156(263)222-122(69-98-77-200-104-38-26-25-37-102(98)104)165(272)220-120(66-91(8)9)161(268)209-108(42-33-59-198-180(191)192)151(258)206-106(40-28-31-57-183)150(257)207-107(41-29-32-58-184)152(259)218-118(64-89(4)5)162(269)211-110(44-49-133(186)240)154(261)228-129(76-142(252)253)172(279)235-145(95(16)17)176(283)229-125(72-101-80-197-87-204-101)168(275)227-127(74-136(189)243)170(277)230-130(179(286)287)68-97-35-23-22-24-36-97/h22-26,35-38,77-80,85-96,103,105-132,143-146,200,237-239H,21,27-34,39-76,81-84,182-185H2,1-20H3,(H2,186,240)(H2,187,241)(H2,188,242)(H2,189,243)(H2,190,244)(H,195,202)(H,196,203)(H,197,204)(H,201,255)(H,205,245)(H,206,258)(H,207,257)(H,208,260)(H,209,268)(H,210,264)(H,211,269)(H,212,281)(H,213,270)(H,214,280)(H,215,282)(H,216,285)(H,217,276)(H,218,259)(H,219,262)(H,220,272)(H,221,273)(H,222,263)(H,223,256)(H,224,265)(H,225,271)(H,226,274)(H,227,275)(H,228,261)(H,229,283)(H,230,277)(H,231,278)(H,232,284)(H,233,254)(H,234,266)(H,235,279)(H,236,267)(H,246,247)(H,248,249)(H,250,251)(H,252,253)(H,286,287)(H4,191,192,198)(H4,193,194,199)/t96-,103-,105-,106-,107-,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,143-,144-,145-,146-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at PTH1R overexpressed in HEK293 cells assessed as intracellular cAMP mobilization |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50318885

(CHEMBL525610 | teriparatide)Show SMILES [H][C@](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@@H](N)CO)C(C)C)([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C181H291N55O51S2/c1-21-96(18)146(236-160(267)114(48-53-141(250)251)212-174(281)132(84-239)232-177(284)143(93(12)13)233-147(254)103(185)82-237)178(285)216-111(45-50-134(187)241)155(262)219-119(65-90(6)7)163(270)213-116(55-62-289-20)158(265)224-124(71-100-79-196-86-203-100)167(274)226-126(73-135(188)242)169(276)217-117(63-88(2)3)148(255)201-81-138(245)205-105(39-27-30-56-182)149(256)223-123(70-99-78-195-85-202-99)166(273)221-121(67-92(10)11)164(271)225-128(75-137(190)244)171(278)231-131(83-238)173(280)214-115(54-61-288-19)157(264)210-112(46-51-139(246)247)153(260)208-109(43-34-60-199-181(193)194)159(266)234-144(94(14)15)175(282)215-113(47-52-140(248)249)156(263)222-122(69-98-77-200-104-38-26-25-37-102(98)104)165(272)220-120(66-91(8)9)161(268)209-108(42-33-59-198-180(191)192)151(258)206-106(40-28-31-57-183)150(257)207-107(41-29-32-58-184)152(259)218-118(64-89(4)5)162(269)211-110(44-49-133(186)240)154(261)228-129(76-142(252)253)172(279)235-145(95(16)17)176(283)229-125(72-101-80-197-87-204-101)168(275)227-127(74-136(189)243)170(277)230-130(179(286)287)68-97-35-23-22-24-36-97/h22-26,35-38,77-80,85-96,103,105-132,143-146,200,237-239H,21,27-34,39-76,81-84,182-185H2,1-20H3,(H2,186,240)(H2,187,241)(H2,188,242)(H2,189,243)(H2,190,244)(H,195,202)(H,196,203)(H,197,204)(H,201,255)(H,205,245)(H,206,258)(H,207,257)(H,208,260)(H,209,268)(H,210,264)(H,211,269)(H,212,281)(H,213,270)(H,214,280)(H,215,282)(H,216,285)(H,217,276)(H,218,259)(H,219,262)(H,220,272)(H,221,273)(H,222,263)(H,223,256)(H,224,265)(H,225,271)(H,226,274)(H,227,275)(H,228,261)(H,229,283)(H,230,277)(H,231,278)(H,232,284)(H,233,254)(H,234,266)(H,235,279)(H,236,267)(H,246,247)(H,248,249)(H,250,251)(H,252,253)(H,286,287)(H4,191,192,198)(H4,193,194,199)/t96-,103-,105-,106-,107-,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,143-,144-,145-,146-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at PTH1R overexpressed in HEK293 cells assessed as intracellular cAMP production |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
G-protein coupled receptor 55
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 445 | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to GPR55 |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data