

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM233143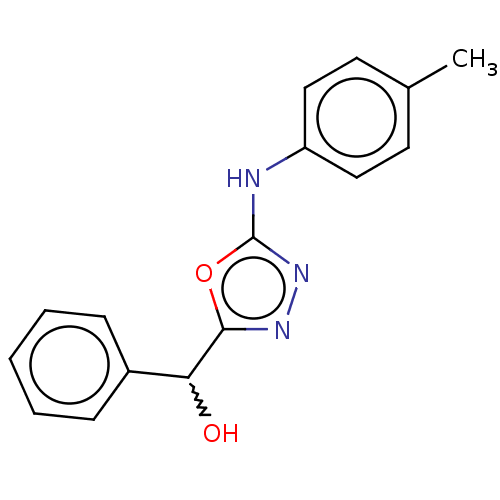 (5-(Hydroxybenzyl)-2-(4-methylphenyl)amino-1,3,4-ox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-i-Azam University | Assay Description The synthesized compounds (4a-e, 5a-e) were tested for their in vitro urease inhibition activities at 0.2 mM concentration against jack bean urease. ... | J Enzyme Inhib Med Chem 25: 572-6 (2010) Article DOI: 10.3109/14756360903389864 BindingDB Entry DOI: 10.7270/Q25H7F5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM233147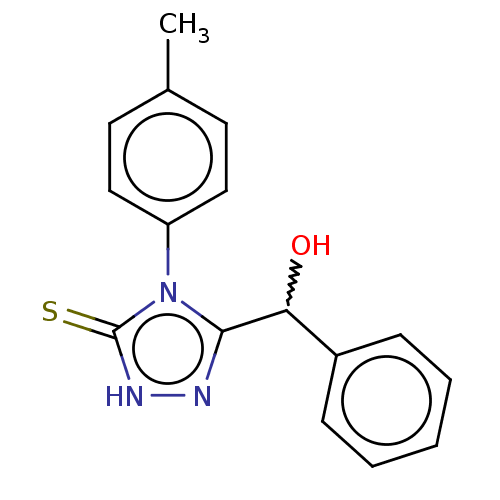 (5-(Hydroxybenzyl)-4-(4-methylphenyl)-2H-1,2,4-tria...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-i-Azam University | Assay Description The synthesized compounds (4a-e, 5a-e) were tested for their in vitro urease inhibition activities at 0.2 mM concentration against jack bean urease. ... | J Enzyme Inhib Med Chem 25: 572-6 (2010) Article DOI: 10.3109/14756360903389864 BindingDB Entry DOI: 10.7270/Q25H7F5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM233146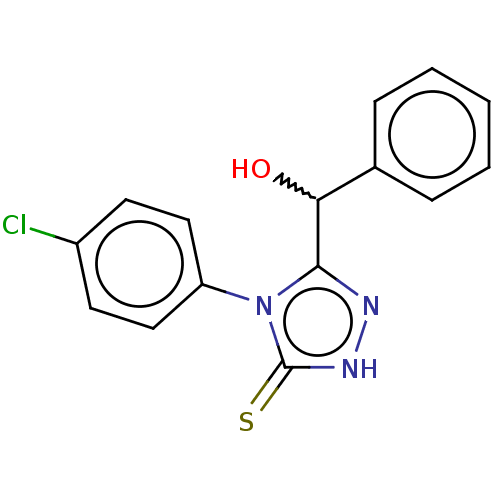 (5-(Hydroxybenzyl)-4-(4-chlorophenyl)-2H-1,2,4-tria...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-i-Azam University | Assay Description The synthesized compounds (4a-e, 5a-e) were tested for their in vitro urease inhibition activities at 0.2 mM concentration against jack bean urease. ... | J Enzyme Inhib Med Chem 25: 572-6 (2010) Article DOI: 10.3109/14756360903389864 BindingDB Entry DOI: 10.7270/Q25H7F5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50229993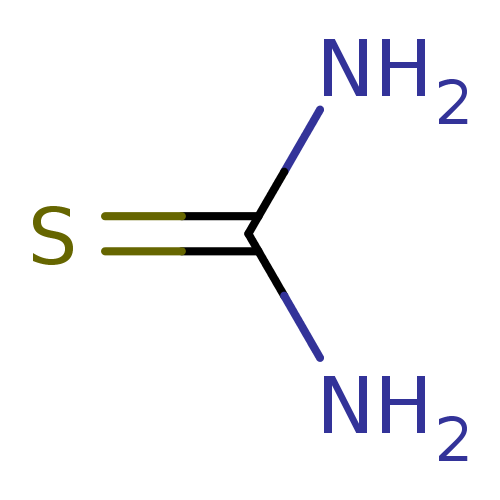 (2-thiourea | CHEMBL260876 | Thiocarbamid | Thiohar...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-i-Azam University | Assay Description The synthesized compounds (4a-e, 5a-e) were tested for their in vitro urease inhibition activities at 0.2 mM concentration against jack bean urease. ... | J Enzyme Inhib Med Chem 25: 572-6 (2010) Article DOI: 10.3109/14756360903389864 BindingDB Entry DOI: 10.7270/Q25H7F5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM233145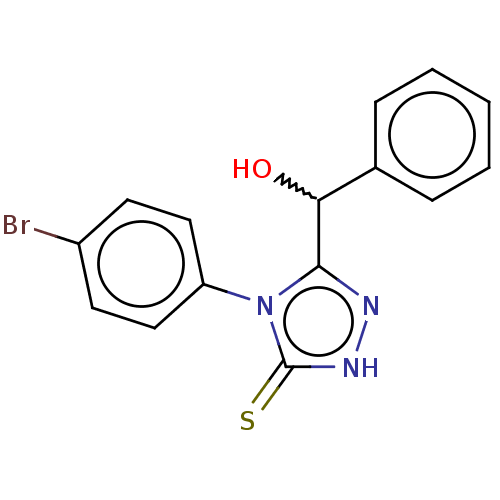 (5-(Hydroxybenzyl)-4-(4-bromophenyl)-2H-1,2,4-triaz...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-i-Azam University | Assay Description The synthesized compounds (4a-e, 5a-e) were tested for their in vitro urease inhibition activities at 0.2 mM concentration against jack bean urease. ... | J Enzyme Inhib Med Chem 25: 572-6 (2010) Article DOI: 10.3109/14756360903389864 BindingDB Entry DOI: 10.7270/Q25H7F5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM233144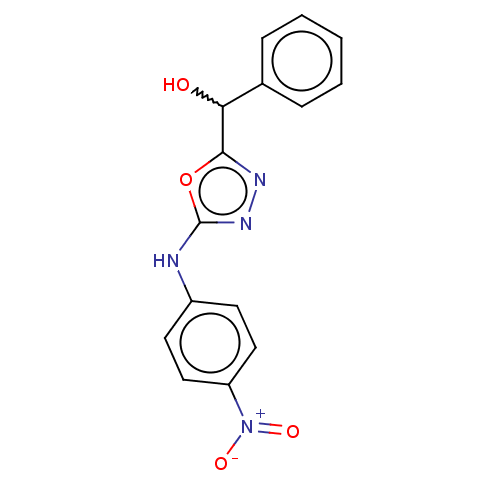 (5-(Hydroxybenzyl)-2-(4-nitrophenyl)amino-1,3,4-oxa...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-i-Azam University | Assay Description The synthesized compounds (4a-e, 5a-e) were tested for their in vitro urease inhibition activities at 0.2 mM concentration against jack bean urease. ... | J Enzyme Inhib Med Chem 25: 572-6 (2010) Article DOI: 10.3109/14756360903389864 BindingDB Entry DOI: 10.7270/Q25H7F5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM233148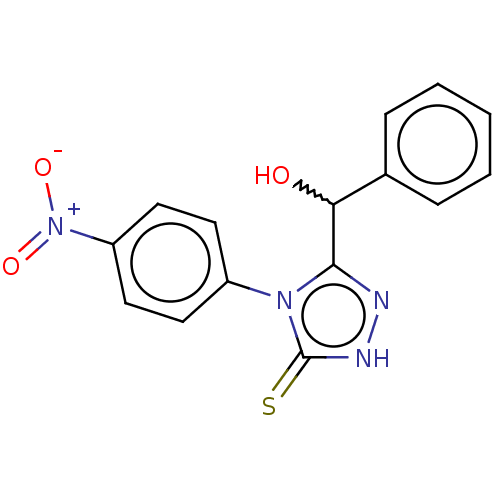 (5-(Hydroxybenzyl)-4-(4-nitrophenyl)-2H-1,2,4-triaz...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.32E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-i-Azam University | Assay Description The synthesized compounds (4a-e, 5a-e) were tested for their in vitro urease inhibition activities at 0.2 mM concentration against jack bean urease. ... | J Enzyme Inhib Med Chem 25: 572-6 (2010) Article DOI: 10.3109/14756360903389864 BindingDB Entry DOI: 10.7270/Q25H7F5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||