

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human recombinant LXRbeta ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRalpha ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50316094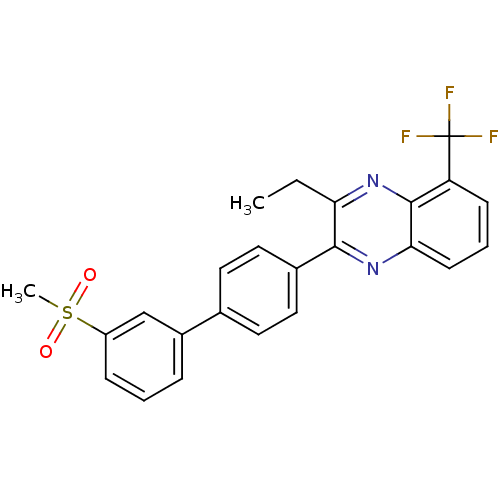 (3-Ethyl-2-[3'-(methylsulfonyl)biphenyl-4-yl]-5-(tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRbeta ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50316092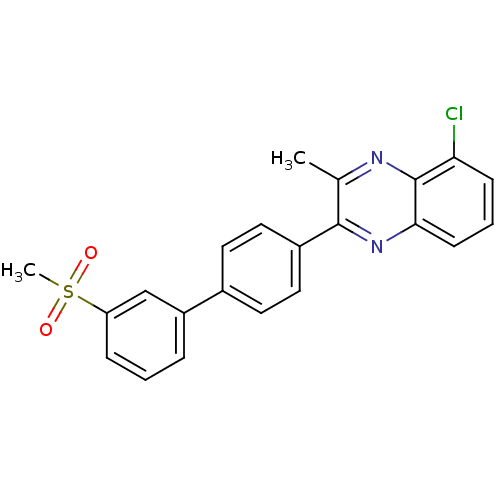 (5-Chloro-3-methyl-2-[3'-(methylsulfonyl)biphenyl-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRbeta ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50316097 (3-Methyl-2-[3'-(methylsulfonyl)biphenyl-4-yl]-5-(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRbeta ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50305494 (4-(3-(3-chloro-5-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRbeta ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50316096 (CHEMBL1090916 | N-Methyl-4'-[3-methyl-5-(trifluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRbeta ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50316102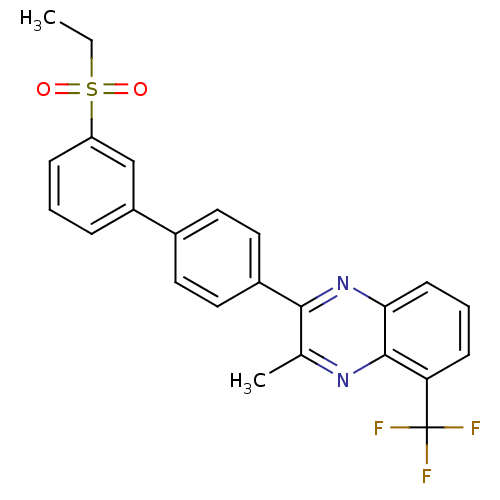 (2-[3'-(Ethylsulfonyl)biphenyl-4-yl]-3-methyl-5-(tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRbeta ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50316094 (3-Ethyl-2-[3'-(methylsulfonyl)biphenyl-4-yl]-5-(tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRalpha ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50316091 (3-Methyl-2-[3'-(methylsulfonyl)biphenyl-4-yl]quino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 299 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRbeta ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50316090 (5-Methoxy-3-methyl-2-[3'-(methylsulfonyl)biphenyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 395 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRbeta ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50316092 (5-Chloro-3-methyl-2-[3'-(methylsulfonyl)biphenyl-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 734 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRalpha ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50316095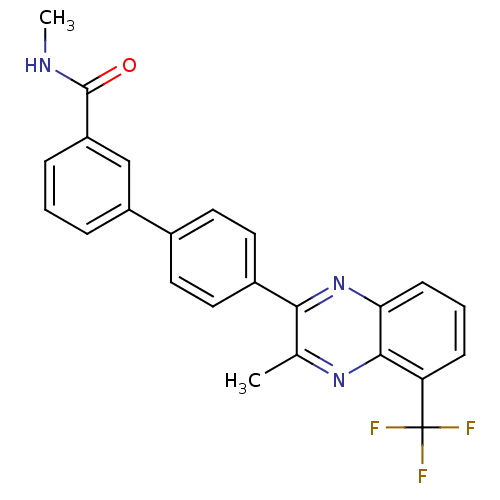 (CHEMBL1089231 | N-Methyl-4'-[3-methyl-5-(trifluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRalpha ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50316098 (3-Methyl-2-[4'-(methylsulfonyl)biphenyl-4-yl]-5-(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRbeta ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50316100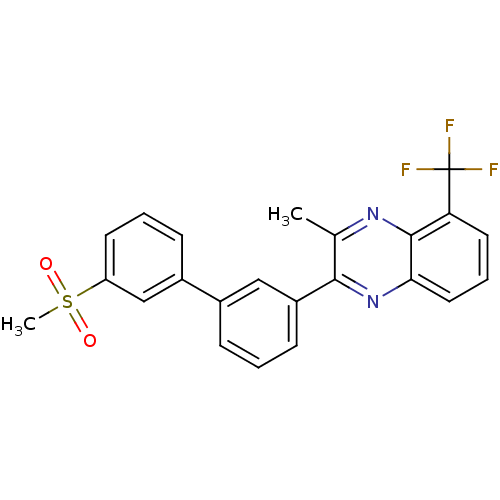 (3-Methyl-2-[3'-(methylsulfonyl)biphenyl-3-yl]-5-(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRbeta ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50316095 (CHEMBL1089231 | N-Methyl-4'-[3-methyl-5-(trifluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRbeta ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50316102 (2-[3'-(Ethylsulfonyl)biphenyl-4-yl]-3-methyl-5-(tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRalpha ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50316090 (5-Methoxy-3-methyl-2-[3'-(methylsulfonyl)biphenyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRalpha ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50316099 (3-Methyl-2-[2'-(methylsulfonyl)biphenyl-4-yl]-5-(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRalpha ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50316093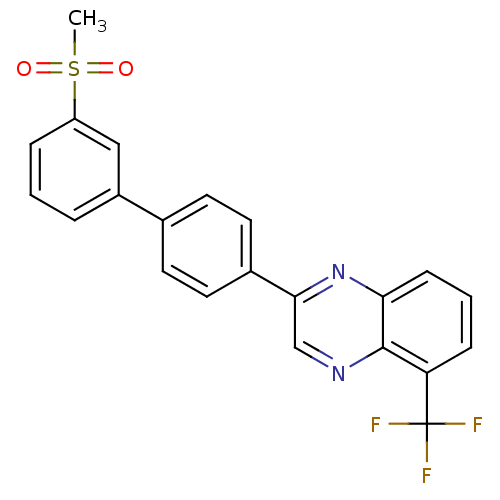 (2-[3'-(Methylsulfonyl)biphenyl-4-yl]-5-(trifluorom...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRalpha ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50316099 (3-Methyl-2-[2'-(methylsulfonyl)biphenyl-4-yl]-5-(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRbeta ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50316093 (2-[3'-(Methylsulfonyl)biphenyl-4-yl]-5-(trifluorom...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRbeta ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50316091 (3-Methyl-2-[3'-(methylsulfonyl)biphenyl-4-yl]quino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRalpha ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50316096 (CHEMBL1090916 | N-Methyl-4'-[3-methyl-5-(trifluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRalpha ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50316101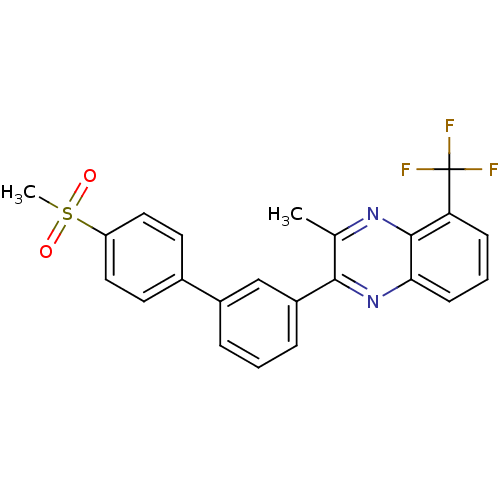 (3-Methyl-2-[4'-(methylsulfonyl)biphenyl-3-yl]-5-(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRalpha ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50316097 (3-Methyl-2-[3'-(methylsulfonyl)biphenyl-4-yl]-5-(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRalpha ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50316101 (3-Methyl-2-[4'-(methylsulfonyl)biphenyl-3-yl]-5-(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRbeta ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50316100 (3-Methyl-2-[3'-(methylsulfonyl)biphenyl-3-yl]-5-(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRalpha ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50316098 (3-Methyl-2-[4'-(methylsulfonyl)biphenyl-4-yl]-5-(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRalpha ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305494 (4-(3-(3-chloro-5-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human LXRalpha ligand binding domain | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant LXRbeta ligand binding domain in human HuH7 cells cotransfected with fused Gal4-DBD by transactivation assay | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50316097 (3-Methyl-2-[3'-(methylsulfonyl)biphenyl-4-yl]-5-(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human LXRalpha in HEK293 cells assessed as Gal4 transactivation | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50305494 (4-(3-(3-chloro-5-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human LXRalpha in HEK293 cells assessed as Gal4 transactivation | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50316097 (3-Methyl-2-[3'-(methylsulfonyl)biphenyl-4-yl]-5-(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant LXRalpha ligand binding domain in human HuH7 cells cotransfected with fused Gal4-DBD by transactivation assay | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50316094 (3-Ethyl-2-[3'-(methylsulfonyl)biphenyl-4-yl]-5-(tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.59E+3 | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant LXRalpha ligand binding domain in human HuH7 cells cotransfected with fused Gal4-DBD by transactivation assay | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50305494 (4-(3-(3-chloro-5-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant LXRalpha ligand binding domain in human HuH7 cells cotransfected with fused Gal4-DBD by transactivation assay | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human LXRbeta in HEK293 cells assessed as Gal4 transactivation | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50316097 (3-Methyl-2-[3'-(methylsulfonyl)biphenyl-4-yl]-5-(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant LXRbeta ligand binding domain in human HuH7 cells cotransfected with fused Gal4-DBD by transactivation assay | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50316092 (5-Chloro-3-methyl-2-[3'-(methylsulfonyl)biphenyl-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 860 | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant LXRbeta ligand binding domain in human HuH7 cells cotransfected with fused Gal4-DBD by transactivation assay | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50305494 (4-(3-(3-chloro-5-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 460 | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human LXRbeta in HEK293 cells assessed as Gal4 transactivation | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50316094 (3-Ethyl-2-[3'-(methylsulfonyl)biphenyl-4-yl]-5-(tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant LXRbeta ligand binding domain in human HuH7 cells cotransfected with fused Gal4-DBD by transactivation assay | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 53 | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human LXRalpha in HEK293 cells assessed as Gal4 transactivation | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50305494 (4-(3-(3-chloro-5-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant LXRbeta ligand binding domain in human HuH7 cells cotransfected with fused Gal4-DBD by transactivation assay | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50316097 (3-Methyl-2-[3'-(methylsulfonyl)biphenyl-4-yl]-5-(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 580 | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human LXRbeta in HEK293 cells assessed as Gal4 transactivation | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant LXRalpha ligand binding domain in human HuH7 cells cotransfected with fused Gal4-DBD by transactivation assay | J Med Chem 53: 3296-304 (2010) Article DOI: 10.1021/jm100034x BindingDB Entry DOI: 10.7270/Q29W0FN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||