

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50317751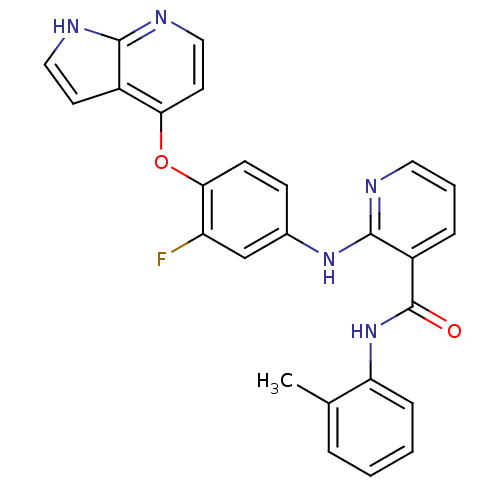 (2-(4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)-3-fluoroph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged human recombinant c-met N terminal domain expressed in Hi5 insect cells after 60 mins by liquid scintillation counting | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50317750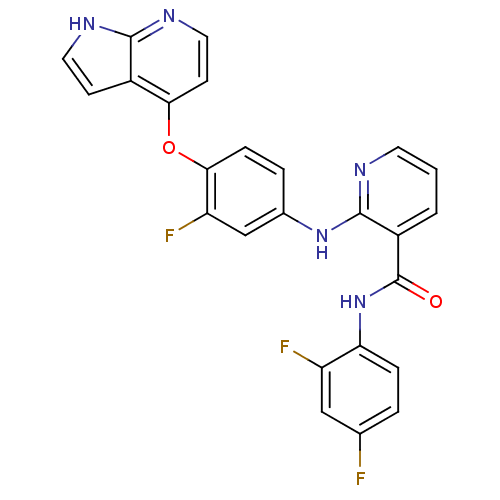 (2-(4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)-3-fluoroph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged human recombinant c-met N terminal domain expressed in Hi5 insect cells after 60 mins by liquid scintillation counting | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50317749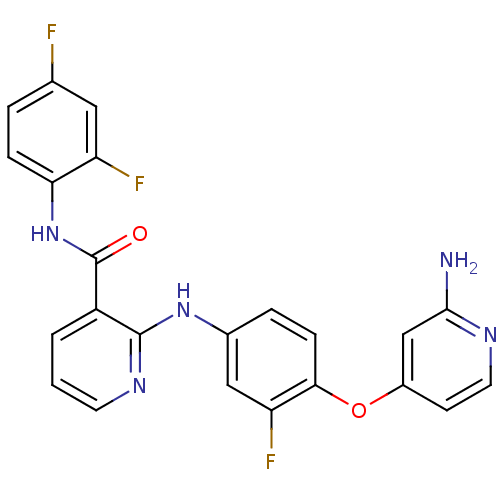 (2-(4-(2-aminopyridin-4-yloxy)-3-fluorophenylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged human recombinant c-met N terminal domain expressed in Hi5 insect cells after 60 mins by liquid scintillation counting | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50317765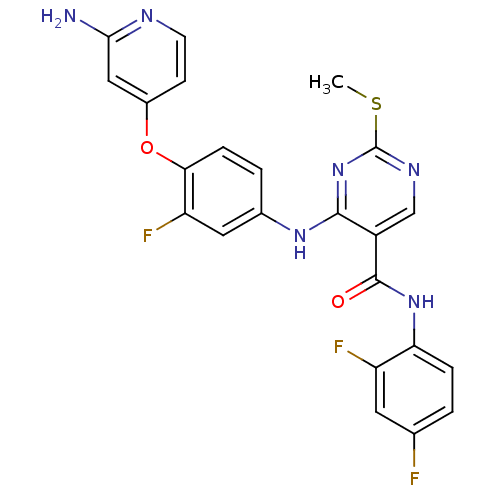 (4-(4-(2-aminopyridin-4-yloxy)-3-fluorophenylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged human recombinant c-met N terminal domain expressed in Hi5 insect cells after 60 mins by liquid scintillation counting | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50317759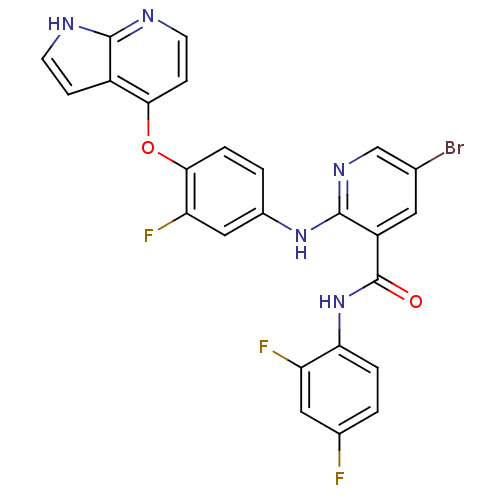 (2-(4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)-3-fluoroph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged human recombinant c-met N terminal domain expressed in Hi5 insect cells after 60 mins by liquid scintillation counting | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50317756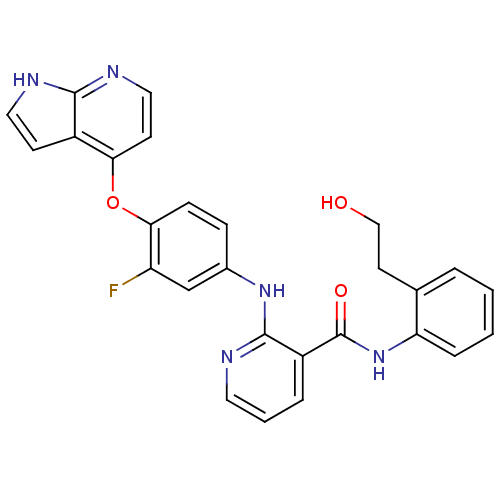 (2-(4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)-3-fluoroph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged human recombinant c-met N terminal domain expressed in Hi5 insect cells after 60 mins by liquid scintillation counting | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50317748 (2-(4-(2-aminopyridin-4-yloxy)-3-fluorophenylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged human recombinant c-met N terminal domain expressed in Hi5 insect cells after 60 mins by liquid scintillation counting | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50317758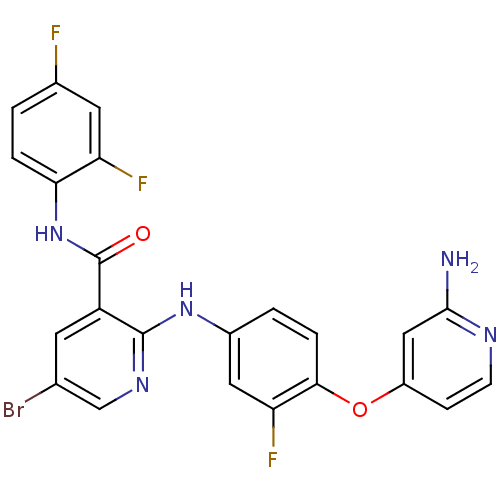 (2-(4-(2-aminopyridin-4-yloxy)-3-fluorophenylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged human recombinant c-met N terminal domain expressed in Hi5 insect cells after 60 mins by liquid scintillation counting | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50317757 (2-(4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)-3-fluoroph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged human recombinant c-met N terminal domain expressed in Hi5 insect cells after 60 mins by liquid scintillation counting | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50317755 (2-(4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)-3-fluoroph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged human recombinant c-met N terminal domain expressed in Hi5 insect cells after 60 mins by liquid scintillation counting | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50317764 (4-(4-(2-aminopyridin-4-yloxy)-3-fluorophenylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged human recombinant c-met N terminal domain expressed in Hi5 insect cells after 60 mins by liquid scintillation counting | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50317752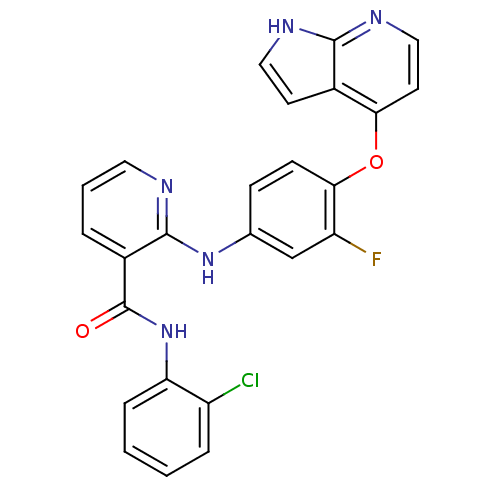 (2-(4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)-3-fluoroph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged human recombinant c-met N terminal domain expressed in Hi5 insect cells after 60 mins by liquid scintillation counting | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50317754 (2-(4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)-3-fluoroph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged human recombinant c-met N terminal domain expressed in Hi5 insect cells after 60 mins by liquid scintillation counting | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50317763 (3-(4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)-3-fluoroph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged human recombinant c-met N terminal domain expressed in Hi5 insect cells after 60 mins by liquid scintillation counting | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50317762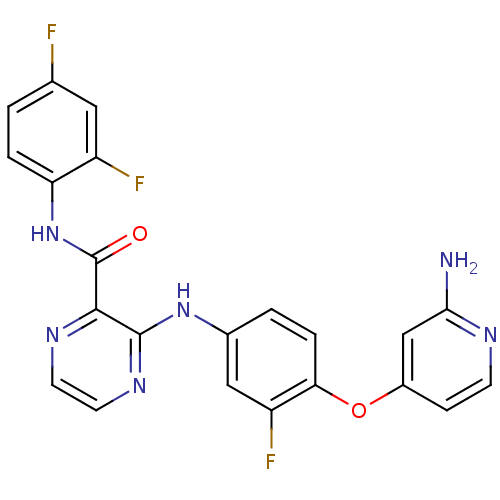 (3-(4-(2-aminopyridin-4-yloxy)-3-fluorophenylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged human recombinant c-met N terminal domain expressed in Hi5 insect cells after 60 mins by liquid scintillation counting | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50317750 (2-(4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)-3-fluoroph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of FLT3 | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50317766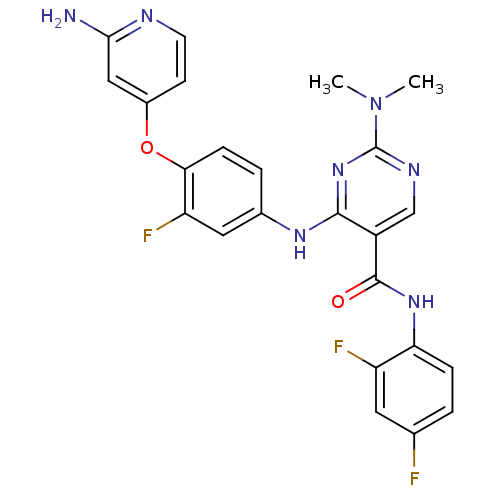 (4-(4-(2-aminopyridin-4-yloxy)-3-fluorophenylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged human recombinant c-met N terminal domain expressed in Hi5 insect cells after 60 mins by liquid scintillation counting | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50317753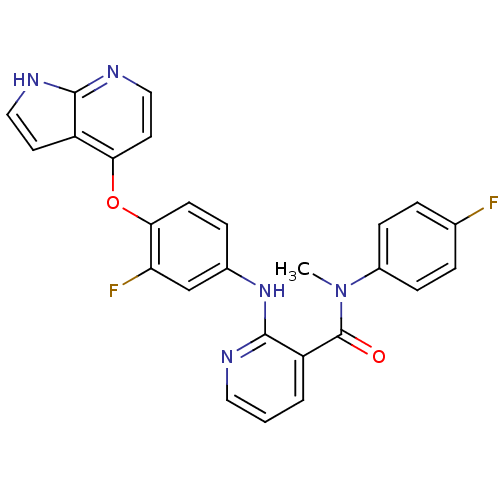 (2-(4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)-3-fluoroph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged human recombinant c-met N terminal domain expressed in Hi5 insect cells after 60 mins by liquid scintillation counting | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50317761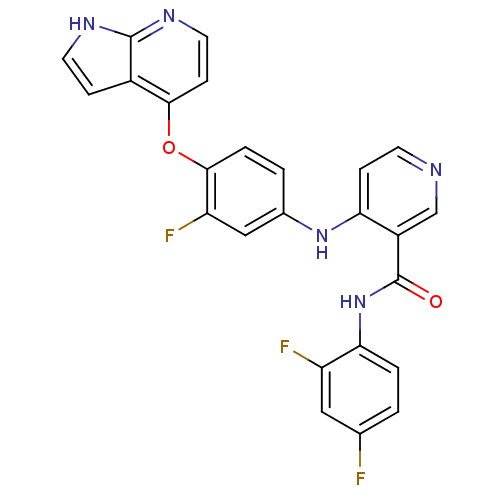 (4-(4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)-3-fluoroph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged human recombinant c-met N terminal domain expressed in Hi5 insect cells after 60 mins by liquid scintillation counting | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50317750 (2-(4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)-3-fluoroph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of TrkA | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50317760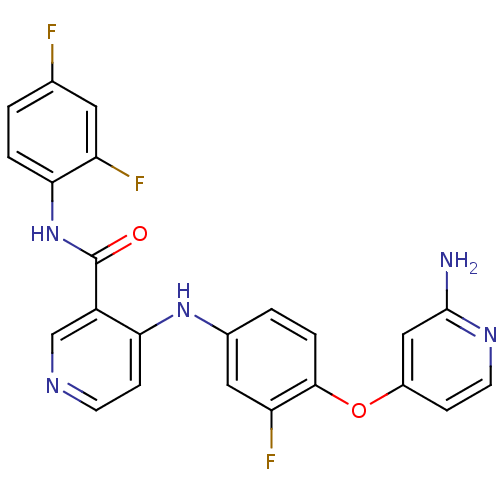 (4-(4-(2-aminopyridin-4-yloxy)-3-fluorophenylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged human recombinant c-met N terminal domain expressed in Hi5 insect cells after 60 mins by liquid scintillation counting | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50317750 (2-(4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)-3-fluoroph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of LcK | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity immunoglobulin gamma Fc receptor I (Homo sapiens (Human)) | BDBM50317750 (2-(4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)-3-fluoroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of IGFR1 | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50317750 (2-(4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)-3-fluoroph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of InsR | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50317750 (2-(4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)-3-fluoroph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of PKCalpha | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-activated protein kinase 2 (Homo sapiens (Human)) | BDBM50317750 (2-(4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)-3-fluoroph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of MK2 | Bioorg Med Chem Lett 20: 2998-3002 (2010) Article DOI: 10.1016/j.bmcl.2010.01.042 BindingDB Entry DOI: 10.7270/Q2PN95TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||