 Found 139 hits of Enzyme Inhibition Constant Data
Found 139 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320214
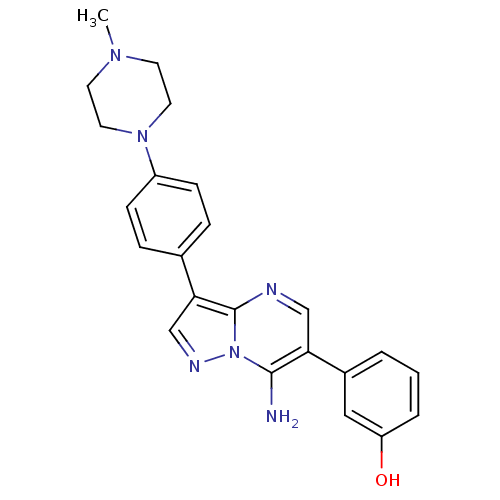
(3-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)pyr...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1cccc(O)c1 Show InChI InChI=1S/C23H24N6O/c1-27-9-11-28(12-10-27)18-7-5-16(6-8-18)21-15-26-29-22(24)20(14-25-23(21)29)17-3-2-4-19(30)13-17/h2-8,13-15,30H,9-12,24H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320225
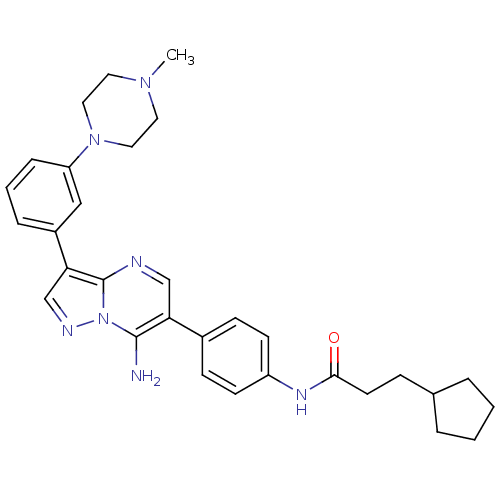
(CHEMBL1085316 | N-(4-(7-amino-3-(3-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)CCC2CCCC2)cc1 Show InChI InChI=1S/C31H37N7O/c1-36-15-17-37(18-16-36)26-8-4-7-24(19-26)28-21-34-38-30(32)27(20-33-31(28)38)23-10-12-25(13-11-23)35-29(39)14-9-22-5-2-3-6-22/h4,7-8,10-13,19-22H,2-3,5-6,9,14-18,32H2,1H3,(H,35,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320205
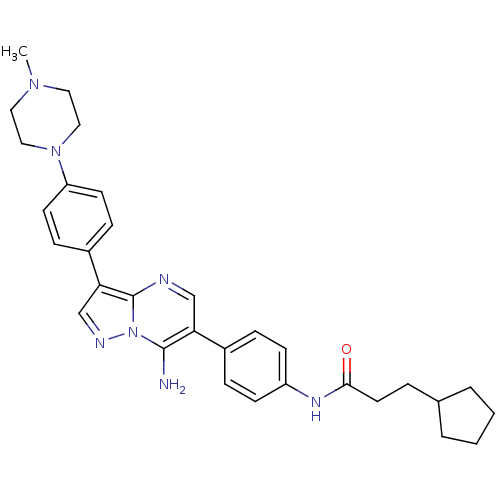
(CHEMBL1083001 | N-(4-(7-amino-3-(4-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)CCC2CCCC2)cc1 Show InChI InChI=1S/C31H37N7O/c1-36-16-18-37(19-17-36)26-13-9-24(10-14-26)28-21-34-38-30(32)27(20-33-31(28)38)23-7-11-25(12-8-23)35-29(39)15-6-22-4-2-3-5-22/h7-14,20-22H,2-6,15-19,32H2,1H3,(H,35,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320209

(1-(4-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)NCC2CCCCC2)cc1 Show InChI InChI=1S/C31H38N8O/c1-37-15-17-38(18-16-37)26-13-9-24(10-14-26)28-21-35-39-29(32)27(20-33-30(28)39)23-7-11-25(12-8-23)36-31(40)34-19-22-5-3-2-4-6-22/h7-14,20-22H,2-6,15-19,32H2,1H3,(H2,34,36,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320224
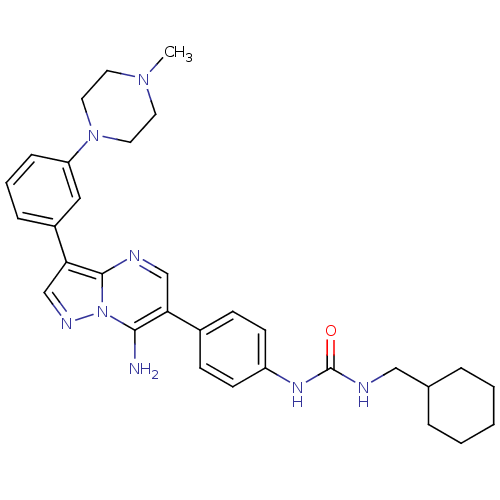
(1-(4-(7-amino-3-(3-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)NCC2CCCCC2)cc1 Show InChI InChI=1S/C31H38N8O/c1-37-14-16-38(17-15-37)26-9-5-8-24(18-26)28-21-35-39-29(32)27(20-33-30(28)39)23-10-12-25(13-11-23)36-31(40)34-19-22-6-3-2-4-7-22/h5,8-13,18,20-22H,2-4,6-7,14-17,19,32H2,1H3,(H2,34,36,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320216
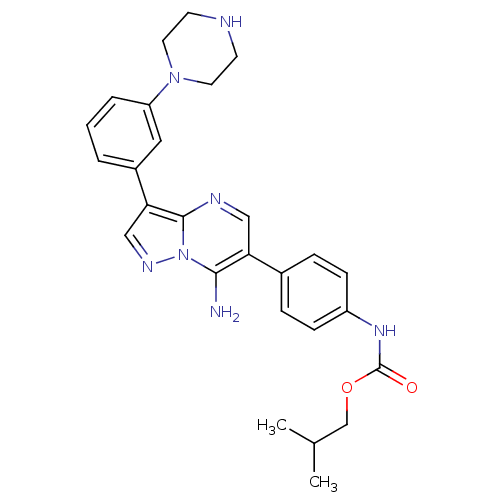
(CHEMBL1083644 | isobutyl4-(7-amino-3-(3-(piperazin...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCNCC1 Show InChI InChI=1S/C27H31N7O2/c1-18(2)17-36-27(35)32-21-8-6-19(7-9-21)23-15-30-26-24(16-31-34(26)25(23)28)20-4-3-5-22(14-20)33-12-10-29-11-13-33/h3-9,14-16,18,29H,10-13,17,28H2,1-2H3,(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320218
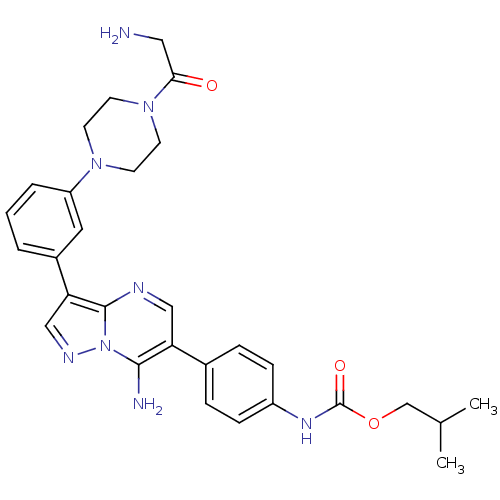
(CHEMBL1082404 | isobutyl4-(7-amino-3-(3-(4-(2-amin...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(CC1)C(=O)CN Show InChI InChI=1S/C29H34N8O3/c1-19(2)18-40-29(39)34-22-8-6-20(7-9-22)24-16-32-28-25(17-33-37(28)27(24)31)21-4-3-5-23(14-21)35-10-12-36(13-11-35)26(38)15-30/h3-9,14,16-17,19H,10-13,15,18,30-31H2,1-2H3,(H,34,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320214

(3-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)pyr...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1cccc(O)c1 Show InChI InChI=1S/C23H24N6O/c1-27-9-11-28(12-10-27)18-7-5-16(6-8-18)21-15-26-29-22(24)20(14-25-23(21)29)17-3-2-4-19(30)13-17/h2-8,13-15,30H,9-12,24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320204

(3-(4-(7-amino-3-(3-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN(C1CCCCC1)C(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C31H38N8O/c1-36-15-17-38(18-16-36)26-10-6-7-23(19-26)28-21-34-39-29(32)27(20-33-30(28)39)22-11-13-24(14-12-22)35-31(40)37(2)25-8-4-3-5-9-25/h6-7,10-14,19-21,25H,3-5,8-9,15-18,32H2,1-2H3,(H,35,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320214

(3-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)pyr...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1cccc(O)c1 Show InChI InChI=1S/C23H24N6O/c1-27-9-11-28(12-10-27)18-7-5-16(6-8-18)21-15-26-29-22(24)20(14-25-23(21)29)17-3-2-4-19(30)13-17/h2-8,13-15,30H,9-12,24H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320223
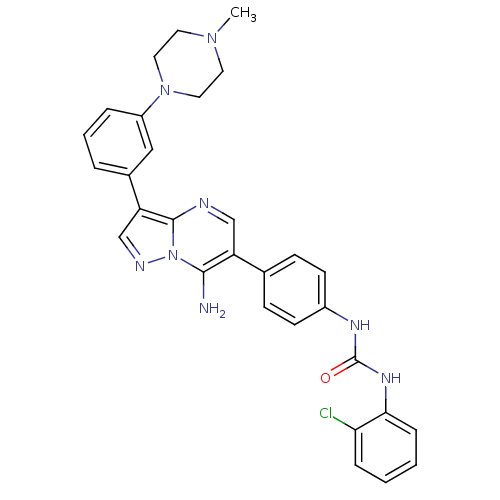
(1-(4-(7-amino-3-(3-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)Nc2ccccc2Cl)cc1 Show InChI InChI=1S/C30H29ClN8O/c1-37-13-15-38(16-14-37)23-6-4-5-21(17-23)25-19-34-39-28(32)24(18-33-29(25)39)20-9-11-22(12-10-20)35-30(40)36-27-8-3-2-7-26(27)31/h2-12,17-19H,13-16,32H2,1H3,(H2,35,36,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320210
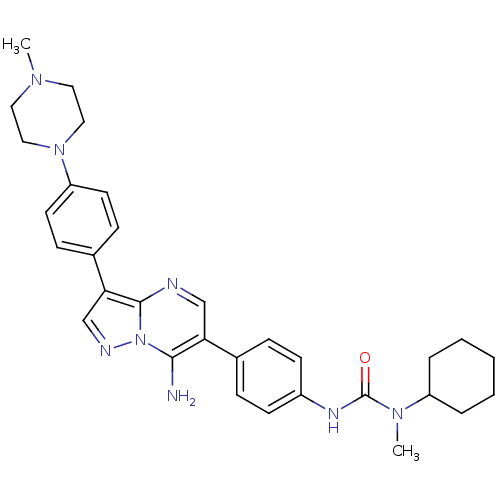
(3-(4-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN(C1CCCCC1)C(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C31H38N8O/c1-36-16-18-38(19-17-36)26-14-10-23(11-15-26)28-21-34-39-29(32)27(20-33-30(28)39)22-8-12-24(13-9-22)35-31(40)37(2)25-6-4-3-5-7-25/h8-15,20-21,25H,3-7,16-19,32H2,1-2H3,(H,35,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320209

(1-(4-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)NCC2CCCCC2)cc1 Show InChI InChI=1S/C31H38N8O/c1-37-15-17-38(18-16-37)26-13-9-24(10-14-26)28-21-35-39-29(32)27(20-33-30(28)39)23-7-11-25(12-8-23)36-31(40)34-19-22-5-3-2-4-6-22/h7-14,20-22H,2-6,15-19,32H2,1H3,(H2,34,36,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320205

(CHEMBL1083001 | N-(4-(7-amino-3-(4-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)CCC2CCCC2)cc1 Show InChI InChI=1S/C31H37N7O/c1-36-16-18-37(19-17-36)26-13-9-24(10-14-26)28-21-34-38-30(32)27(20-33-31(28)38)23-7-11-25(12-8-23)35-29(39)15-6-22-4-2-3-5-22/h7-14,20-22H,2-6,15-19,32H2,1H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320207
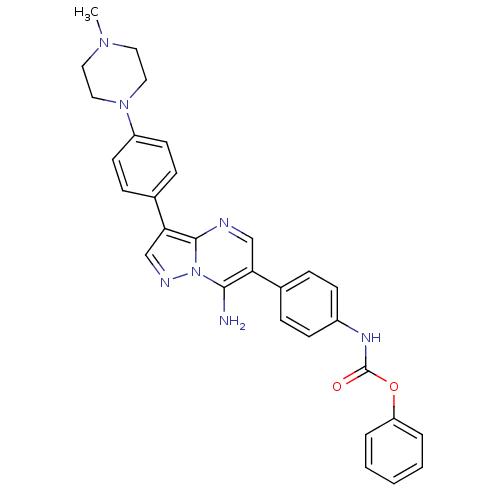
(CHEMBL1085512 | phenyl4-(7-amino-3-(4-(4-methylpip...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)Oc2ccccc2)cc1 Show InChI InChI=1S/C30H29N7O2/c1-35-15-17-36(18-16-35)24-13-9-22(10-14-24)27-20-33-37-28(31)26(19-32-29(27)37)21-7-11-23(12-8-21)34-30(38)39-25-5-3-2-4-6-25/h2-14,19-20H,15-18,31H2,1H3,(H,34,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320222
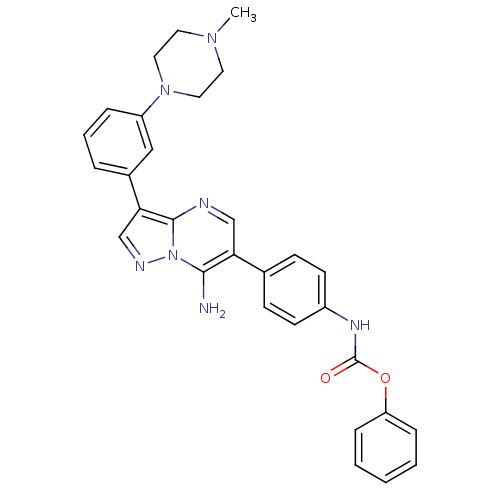
(CHEMBL1085564 | phenyl4-(7-amino-3-(3-(4-methylpip...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)Oc2ccccc2)cc1 Show InChI InChI=1S/C30H29N7O2/c1-35-14-16-36(17-15-35)24-7-5-6-22(18-24)27-20-33-37-28(31)26(19-32-29(27)37)21-10-12-23(13-11-21)34-30(38)39-25-8-3-2-4-9-25/h2-13,18-20H,14-17,31H2,1H3,(H,34,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320224

(1-(4-(7-amino-3-(3-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)NCC2CCCCC2)cc1 Show InChI InChI=1S/C31H38N8O/c1-37-14-16-38(17-15-37)26-9-5-8-24(18-26)28-21-35-39-29(32)27(20-33-30(28)39)23-10-12-25(13-11-23)36-31(40)34-19-22-6-3-2-4-7-22/h5,8-13,18,20-22H,2-4,6-7,14-17,19,32H2,1H3,(H2,34,36,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320209

(1-(4-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)NCC2CCCCC2)cc1 Show InChI InChI=1S/C31H38N8O/c1-37-15-17-38(18-16-37)26-13-9-24(10-14-26)28-21-35-39-29(32)27(20-33-30(28)39)23-7-11-25(12-8-23)36-31(40)34-19-22-5-3-2-4-6-22/h7-14,20-22H,2-6,15-19,32H2,1H3,(H2,34,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320226

(CHEMBL1085240 | N-(4-(7-amino-3-(3-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)c2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C31H28F3N7O/c1-39-13-15-40(16-14-39)25-4-2-3-22(17-25)27-19-37-41-28(35)26(18-36-29(27)41)20-7-11-24(12-8-20)38-30(42)21-5-9-23(10-6-21)31(32,33)34/h2-12,17-19H,13-16,35H2,1H3,(H,38,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320212
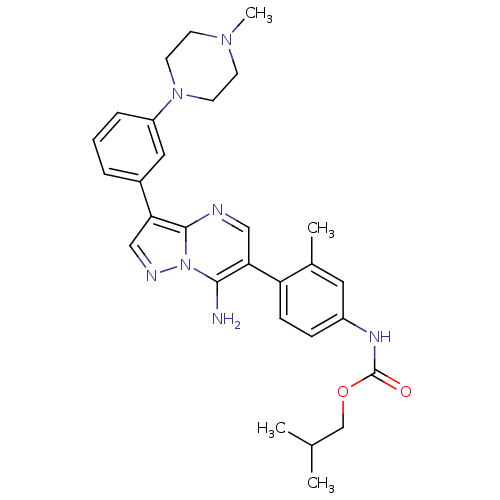
(CHEMBL1085996 | isobutyl4-(7-amino-3-(3-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(c(C)c1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C29H35N7O2/c1-19(2)18-38-29(37)33-22-8-9-24(20(3)14-22)26-16-31-28-25(17-32-36(28)27(26)30)21-6-5-7-23(15-21)35-12-10-34(4)11-13-35/h5-9,14-17,19H,10-13,18,30H2,1-4H3,(H,33,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320221
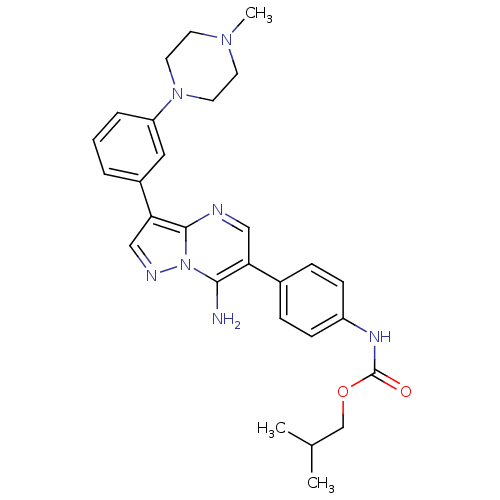
(CHEMBL1085317 | isobutyl4-(7-amino-3-(3-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-9-7-20(8-10-22)24-16-30-27-25(17-31-35(27)26(24)29)21-5-4-6-23(15-21)34-13-11-33(3)12-14-34/h4-10,15-17,19H,11-14,18,29H2,1-3H3,(H,32,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320208

(1-(4-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)Nc2ccccc2Cl)cc1 Show InChI InChI=1S/C30H29ClN8O/c1-37-14-16-38(17-15-37)23-12-8-21(9-13-23)25-19-34-39-28(32)24(18-33-29(25)39)20-6-10-22(11-7-20)35-30(40)36-27-5-3-2-4-26(27)31/h2-13,18-19H,14-17,32H2,1H3,(H2,35,36,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320229

(CHEMBL1084974 | N-(4-(7-amino-3-(3-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NS(=O)(=O)c2cccc(Cl)c2Cl)cc1 Show InChI InChI=1S/C29H27Cl2N7O2S/c1-36-12-14-37(15-13-36)22-5-2-4-20(16-22)24-18-34-38-28(32)23(17-33-29(24)38)19-8-10-21(11-9-19)35-41(39,40)26-7-3-6-25(30)27(26)31/h2-11,16-18,35H,12-15,32H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320227
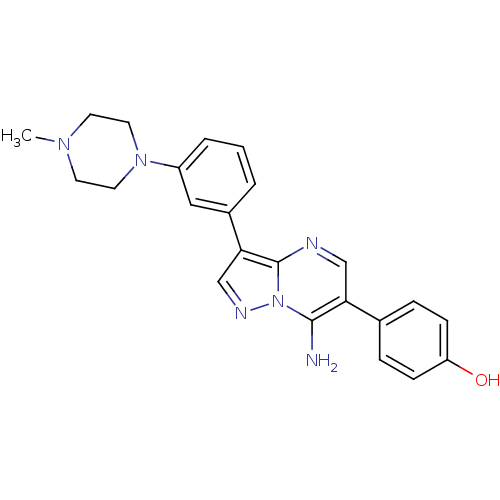
(4-(7-amino-3-(3-(4-methylpiperazin-1-yl)phenyl)pyr...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(O)cc1 Show InChI InChI=1S/C23H24N6O/c1-27-9-11-28(12-10-27)18-4-2-3-17(13-18)21-15-26-29-22(24)20(14-25-23(21)29)16-5-7-19(30)8-6-16/h2-8,13-15,30H,9-12,24H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320223

(1-(4-(7-amino-3-(3-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)Nc2ccccc2Cl)cc1 Show InChI InChI=1S/C30H29ClN8O/c1-37-13-15-38(16-14-37)23-6-4-5-21(17-23)25-19-34-39-28(32)24(18-33-29(25)39)20-9-11-22(12-10-20)35-30(40)36-27-8-3-2-7-26(27)31/h2-12,17-19H,13-16,32H2,1H3,(H2,35,36,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320225

(CHEMBL1085316 | N-(4-(7-amino-3-(3-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)CCC2CCCC2)cc1 Show InChI InChI=1S/C31H37N7O/c1-36-15-17-37(18-16-36)26-8-4-7-24(19-26)28-21-34-38-30(32)27(20-33-31(28)38)23-10-12-25(13-11-23)35-29(39)14-9-22-5-2-3-6-22/h4,7-8,10-13,19-22H,2-3,5-6,9,14-18,32H2,1H3,(H,35,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320228

(4-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)pyr...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(O)cc1 Show InChI InChI=1S/C23H24N6O/c1-27-10-12-28(13-11-27)18-6-2-17(3-7-18)21-15-26-29-22(24)20(14-25-23(21)29)16-4-8-19(30)9-5-16/h2-9,14-15,30H,10-13,24H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320219
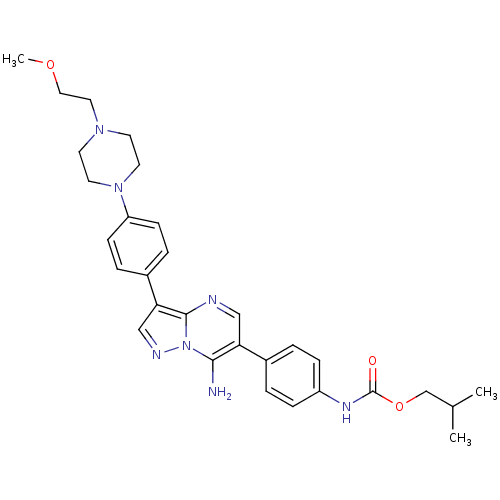
(CHEMBL1082958 | isobutyl4-(7-amino-3-(4-(4-(2-meth...)Show SMILES COCCN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)OCC(C)C)cc1 Show InChI InChI=1S/C30H37N7O3/c1-21(2)20-40-30(38)34-24-8-4-22(5-9-24)26-18-32-29-27(19-33-37(29)28(26)31)23-6-10-25(11-7-23)36-14-12-35(13-15-36)16-17-39-3/h4-11,18-19,21H,12-17,20,31H2,1-3H3,(H,34,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320225

(CHEMBL1085316 | N-(4-(7-amino-3-(3-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)CCC2CCCC2)cc1 Show InChI InChI=1S/C31H37N7O/c1-36-15-17-37(18-16-36)26-8-4-7-24(19-26)28-21-34-38-30(32)27(20-33-31(28)38)23-10-12-25(13-11-23)35-29(39)14-9-22-5-2-3-6-22/h4,7-8,10-13,19-22H,2-3,5-6,9,14-18,32H2,1H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320207

(CHEMBL1085512 | phenyl4-(7-amino-3-(4-(4-methylpip...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)Oc2ccccc2)cc1 Show InChI InChI=1S/C30H29N7O2/c1-35-15-17-36(18-16-35)24-13-9-22(10-14-24)27-20-33-37-28(31)26(19-32-29(27)37)21-7-11-23(12-8-21)34-30(38)39-25-5-3-2-4-6-25/h2-14,19-20H,15-18,31H2,1H3,(H,34,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320221

(CHEMBL1085317 | isobutyl4-(7-amino-3-(3-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-9-7-20(8-10-22)24-16-30-27-25(17-31-35(27)26(24)29)21-5-4-6-23(15-21)34-13-11-33(3)12-14-34/h4-10,15-17,19H,11-14,18,29H2,1-3H3,(H,32,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320224

(1-(4-(7-amino-3-(3-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)NCC2CCCCC2)cc1 Show InChI InChI=1S/C31H38N8O/c1-37-14-16-38(17-15-37)26-9-5-8-24(18-26)28-21-35-39-29(32)27(20-33-30(28)39)23-10-12-25(13-11-23)36-31(40)34-19-22-6-3-2-4-7-22/h5,8-13,18,20-22H,2-4,6-7,14-17,19,32H2,1H3,(H2,34,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320206
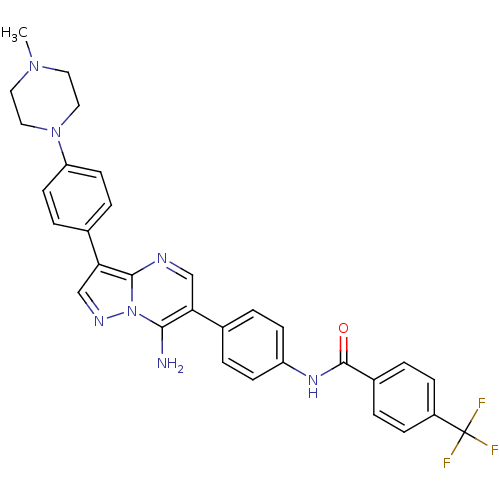
(CHEMBL1085733 | N-(4-(7-amino-3-(4-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)c2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C31H28F3N7O/c1-39-14-16-40(17-15-39)25-12-6-21(7-13-25)27-19-37-41-28(35)26(18-36-29(27)41)20-4-10-24(11-5-20)38-30(42)22-2-8-23(9-3-22)31(32,33)34/h2-13,18-19H,14-17,35H2,1H3,(H,38,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320205

(CHEMBL1083001 | N-(4-(7-amino-3-(4-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)CCC2CCCC2)cc1 Show InChI InChI=1S/C31H37N7O/c1-36-16-18-37(19-17-36)26-13-9-24(10-14-26)28-21-34-38-30(32)27(20-33-31(28)38)23-7-11-25(12-8-23)35-29(39)15-6-22-4-2-3-5-22/h7-14,20-22H,2-6,15-19,32H2,1H3,(H,35,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320228

(4-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)pyr...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(O)cc1 Show InChI InChI=1S/C23H24N6O/c1-27-10-12-28(13-11-27)18-6-2-17(3-7-18)21-15-26-29-22(24)20(14-25-23(21)29)16-4-8-19(30)9-5-16/h2-9,14-15,30H,10-13,24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320207

(CHEMBL1085512 | phenyl4-(7-amino-3-(4-(4-methylpip...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)Oc2ccccc2)cc1 Show InChI InChI=1S/C30H29N7O2/c1-35-15-17-36(18-16-35)24-13-9-22(10-14-24)27-20-33-37-28(31)26(19-32-29(27)37)21-7-11-23(12-8-21)34-30(38)39-25-5-3-2-4-6-25/h2-14,19-20H,15-18,31H2,1H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cKIt |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320208

(1-(4-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)Nc2ccccc2Cl)cc1 Show InChI InChI=1S/C30H29ClN8O/c1-37-14-16-38(17-15-37)23-12-8-21(9-13-23)25-19-34-39-28(32)24(18-33-29(25)39)20-6-10-22(11-7-20)35-30(40)36-27-5-3-2-4-26(27)31/h2-13,18-19H,14-17,32H2,1H3,(H2,35,36,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320214

(3-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)pyr...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1cccc(O)c1 Show InChI InChI=1S/C23H24N6O/c1-27-9-11-28(12-10-27)18-7-5-16(6-8-18)21-15-26-29-22(24)20(14-25-23(21)29)17-3-2-4-19(30)13-17/h2-8,13-15,30H,9-12,24H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320206

(CHEMBL1085733 | N-(4-(7-amino-3-(4-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)c2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C31H28F3N7O/c1-39-14-16-40(17-15-39)25-12-6-21(7-13-25)27-19-37-41-28(35)26(18-36-29(27)41)20-4-10-24(11-5-20)38-30(42)22-2-8-23(9-3-22)31(32,33)34/h2-13,18-19H,14-17,35H2,1H3,(H,38,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320204

(3-(4-(7-amino-3-(3-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN(C1CCCCC1)C(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C31H38N8O/c1-36-15-17-38(18-16-36)26-10-6-7-23(19-26)28-21-34-39-29(32)27(20-33-30(28)39)22-11-13-24(14-12-22)35-31(40)37(2)25-8-4-3-5-9-25/h6-7,10-14,19-21,25H,3-5,8-9,15-18,32H2,1-2H3,(H,35,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320211

(CHEMBL1085995 | isobutyl4-(7-amino-5-methyl-3-(3-(...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1c(C)nc2c(cnn2c1N)-c1cccc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C29H35N7O2/c1-19(2)18-38-29(37)33-23-10-8-21(9-11-23)26-20(3)32-28-25(17-31-36(28)27(26)30)22-6-5-7-24(16-22)35-14-12-34(4)13-15-35/h5-11,16-17,19H,12-15,18,30H2,1-4H3,(H,33,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320210

(3-(4-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN(C1CCCCC1)C(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C31H38N8O/c1-36-16-18-38(19-17-36)26-14-10-23(11-15-26)28-21-34-39-29(32)27(20-33-30(28)39)22-8-12-24(13-9-22)35-31(40)37(2)25-6-4-3-5-7-25/h8-15,20-21,25H,3-7,16-19,32H2,1-2H3,(H,35,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320224

(1-(4-(7-amino-3-(3-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)NCC2CCCCC2)cc1 Show InChI InChI=1S/C31H38N8O/c1-37-14-16-38(17-15-37)26-9-5-8-24(18-26)28-21-35-39-29(32)27(20-33-30(28)39)23-10-12-25(13-11-23)36-31(40)34-19-22-6-3-2-4-7-22/h5,8-13,18,20-22H,2-4,6-7,14-17,19,32H2,1H3,(H2,34,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320225

(CHEMBL1085316 | N-(4-(7-amino-3-(3-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)CCC2CCCC2)cc1 Show InChI InChI=1S/C31H37N7O/c1-36-15-17-37(18-16-36)26-8-4-7-24(19-26)28-21-34-38-30(32)27(20-33-31(28)38)23-10-12-25(13-11-23)35-29(39)14-9-22-5-2-3-6-22/h4,7-8,10-13,19-22H,2-3,5-6,9,14-18,32H2,1H3,(H,35,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320222

(CHEMBL1085564 | phenyl4-(7-amino-3-(3-(4-methylpip...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)Oc2ccccc2)cc1 Show InChI InChI=1S/C30H29N7O2/c1-35-14-16-36(17-15-35)24-7-5-6-22(18-24)27-20-33-37-28(31)26(19-32-29(27)37)21-10-12-23(13-11-21)34-30(38)39-25-8-3-2-4-9-25/h2-13,18-20H,14-17,31H2,1H3,(H,34,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320205

(CHEMBL1083001 | N-(4-(7-amino-3-(4-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)CCC2CCCC2)cc1 Show InChI InChI=1S/C31H37N7O/c1-36-16-18-37(19-17-36)26-13-9-24(10-14-26)28-21-34-38-30(32)27(20-33-31(28)38)23-7-11-25(12-8-23)35-29(39)15-6-22-4-2-3-5-22/h7-14,20-22H,2-6,15-19,32H2,1H3,(H,35,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320209

(1-(4-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)NCC2CCCCC2)cc1 Show InChI InChI=1S/C31H38N8O/c1-37-15-17-38(18-16-37)26-13-9-24(10-14-26)28-21-35-39-29(32)27(20-33-30(28)39)23-7-11-25(12-8-23)36-31(40)34-19-22-5-3-2-4-6-22/h7-14,20-22H,2-6,15-19,32H2,1H3,(H2,34,36,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320223

(1-(4-(7-amino-3-(3-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)Nc2ccccc2Cl)cc1 Show InChI InChI=1S/C30H29ClN8O/c1-37-13-15-38(16-14-37)23-6-4-5-21(17-23)25-19-34-39-28(32)24(18-33-29(25)39)20-9-11-22(12-10-20)35-30(40)36-27-8-3-2-7-26(27)31/h2-12,17-19H,13-16,32H2,1H3,(H2,35,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320210

(3-(4-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN(C1CCCCC1)C(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C31H38N8O/c1-36-16-18-38(19-17-36)26-14-10-23(11-15-26)28-21-34-39-29(32)27(20-33-30(28)39)22-8-12-24(13-9-22)35-31(40)37(2)25-6-4-3-5-7-25/h8-15,20-21,25H,3-7,16-19,32H2,1-2H3,(H,35,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 181 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320222

(CHEMBL1085564 | phenyl4-(7-amino-3-(3-(4-methylpip...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)Oc2ccccc2)cc1 Show InChI InChI=1S/C30H29N7O2/c1-35-14-16-36(17-15-35)24-7-5-6-22(18-24)27-20-33-37-28(31)26(19-32-29(27)37)21-10-12-23(13-11-21)34-30(38)39-25-8-3-2-4-9-25/h2-13,18-20H,14-17,31H2,1H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 193 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320217

(CHEMBL1082403 | isobutyl4-(7-amino-3-(3-(4-(methyl...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C28H33N7O4S/c1-19(2)18-39-28(36)32-22-9-7-20(8-10-22)24-16-30-27-25(17-31-35(27)26(24)29)21-5-4-6-23(15-21)33-11-13-34(14-12-33)40(3,37)38/h4-10,15-17,19H,11-14,18,29H2,1-3H3,(H,32,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 209 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320208

(1-(4-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)Nc2ccccc2Cl)cc1 Show InChI InChI=1S/C30H29ClN8O/c1-37-14-16-38(17-15-37)23-12-8-21(9-13-23)25-19-34-39-28(32)24(18-33-29(25)39)20-6-10-22(11-7-20)35-30(40)36-27-5-3-2-4-26(27)31/h2-13,18-19H,14-17,32H2,1H3,(H2,35,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320224

(1-(4-(7-amino-3-(3-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)NCC2CCCCC2)cc1 Show InChI InChI=1S/C31H38N8O/c1-37-14-16-38(17-15-37)26-9-5-8-24(18-26)28-21-35-39-29(32)27(20-33-30(28)39)23-10-12-25(13-11-23)36-31(40)34-19-22-6-3-2-4-7-22/h5,8-13,18,20-22H,2-4,6-7,14-17,19,32H2,1H3,(H2,34,36,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 227 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320206

(CHEMBL1085733 | N-(4-(7-amino-3-(4-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)c2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C31H28F3N7O/c1-39-14-16-40(17-15-39)25-12-6-21(7-13-25)27-19-37-41-28(35)26(18-36-29(27)41)20-4-10-24(11-5-20)38-30(42)22-2-8-23(9-3-22)31(32,33)34/h2-13,18-19H,14-17,35H2,1H3,(H,38,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 256 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320218

(CHEMBL1082404 | isobutyl4-(7-amino-3-(3-(4-(2-amin...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(CC1)C(=O)CN Show InChI InChI=1S/C29H34N8O3/c1-19(2)18-40-29(39)34-22-8-6-20(7-9-22)24-16-32-28-25(17-33-37(28)27(24)31)21-4-3-5-23(14-21)35-10-12-36(13-11-35)26(38)15-30/h3-9,14,16-17,19H,10-13,15,18,30-31H2,1-2H3,(H,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320226

(CHEMBL1085240 | N-(4-(7-amino-3-(3-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)c2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C31H28F3N7O/c1-39-13-15-40(16-14-39)25-4-2-3-22(17-25)27-19-37-41-28(35)26(18-36-29(27)41)20-7-11-24(12-8-20)38-30(42)21-5-9-23(10-6-21)31(32,33)34/h2-12,17-19H,13-16,35H2,1H3,(H,38,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 295 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320216

(CHEMBL1083644 | isobutyl4-(7-amino-3-(3-(piperazin...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCNCC1 Show InChI InChI=1S/C27H31N7O2/c1-18(2)17-36-27(35)32-21-8-6-19(7-9-21)23-15-30-26-24(16-31-34(26)25(23)28)20-4-3-5-22(14-20)33-12-10-29-11-13-33/h3-9,14-16,18,29H,10-13,17,28H2,1-2H3,(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320226

(CHEMBL1085240 | N-(4-(7-amino-3-(3-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)c2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C31H28F3N7O/c1-39-13-15-40(16-14-39)25-4-2-3-22(17-25)27-19-37-41-28(35)26(18-36-29(27)41)20-7-11-24(12-8-20)38-30(42)21-5-9-23(10-6-21)31(32,33)34/h2-12,17-19H,13-16,35H2,1H3,(H,38,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 323 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320204

(3-(4-(7-amino-3-(3-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN(C1CCCCC1)C(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C31H38N8O/c1-36-15-17-38(18-16-36)26-10-6-7-23(19-26)28-21-34-39-29(32)27(20-33-30(28)39)22-11-13-24(14-12-22)35-31(40)37(2)25-8-4-3-5-9-25/h6-7,10-14,19-21,25H,3-5,8-9,15-18,32H2,1-2H3,(H,35,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 333 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320227

(4-(7-amino-3-(3-(4-methylpiperazin-1-yl)phenyl)pyr...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(O)cc1 Show InChI InChI=1S/C23H24N6O/c1-27-9-11-28(12-10-27)18-4-2-3-17(13-18)21-15-26-29-22(24)20(14-25-23(21)29)16-5-7-19(30)8-6-16/h2-8,13-15,30H,9-12,24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 342 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320219

(CHEMBL1082958 | isobutyl4-(7-amino-3-(4-(4-(2-meth...)Show SMILES COCCN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)OCC(C)C)cc1 Show InChI InChI=1S/C30H37N7O3/c1-21(2)20-40-30(38)34-24-8-4-22(5-9-24)26-18-32-29-27(19-33-37(29)28(26)31)23-6-10-25(11-7-23)36-14-12-35(13-15-36)16-17-39-3/h4-11,18-19,21H,12-17,20,31H2,1-3H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 349 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320207

(CHEMBL1085512 | phenyl4-(7-amino-3-(4-(4-methylpip...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)Oc2ccccc2)cc1 Show InChI InChI=1S/C30H29N7O2/c1-35-15-17-36(18-16-35)24-13-9-22(10-14-24)27-20-33-37-28(31)26(19-32-29(27)37)21-7-11-23(12-8-21)34-30(38)39-25-5-3-2-4-6-25/h2-14,19-20H,15-18,31H2,1H3,(H,34,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 394 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320228

(4-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)pyr...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(O)cc1 Show InChI InChI=1S/C23H24N6O/c1-27-10-12-28(13-11-27)18-6-2-17(3-7-18)21-15-26-29-22(24)20(14-25-23(21)29)16-4-8-19(30)9-5-16/h2-9,14-15,30H,10-13,24H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 396 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320210

(3-(4-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN(C1CCCCC1)C(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C31H38N8O/c1-36-16-18-38(19-17-36)26-14-10-23(11-15-26)28-21-34-39-29(32)27(20-33-30(28)39)22-8-12-24(13-9-22)35-31(40)37(2)25-6-4-3-5-7-25/h8-15,20-21,25H,3-7,16-19,32H2,1-2H3,(H,35,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 435 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320227

(4-(7-amino-3-(3-(4-methylpiperazin-1-yl)phenyl)pyr...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(O)cc1 Show InChI InChI=1S/C23H24N6O/c1-27-9-11-28(12-10-27)18-4-2-3-17(13-18)21-15-26-29-22(24)20(14-25-23(21)29)16-5-7-19(30)8-6-16/h2-8,13-15,30H,9-12,24H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 442 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320212

(CHEMBL1085996 | isobutyl4-(7-amino-3-(3-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(c(C)c1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C29H35N7O2/c1-19(2)18-38-29(37)33-22-8-9-24(20(3)14-22)26-16-31-28-25(17-32-36(28)27(26)30)21-6-5-7-23(15-21)35-12-10-34(4)11-13-35/h5-9,14-17,19H,10-13,18,30H2,1-4H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 456 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320212

(CHEMBL1085996 | isobutyl4-(7-amino-3-(3-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(c(C)c1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C29H35N7O2/c1-19(2)18-38-29(37)33-22-8-9-24(20(3)14-22)26-16-31-28-25(17-32-36(28)27(26)30)21-6-5-7-23(15-21)35-12-10-34(4)11-13-35/h5-9,14-17,19H,10-13,18,30H2,1-4H3,(H,33,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 462 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320227

(4-(7-amino-3-(3-(4-methylpiperazin-1-yl)phenyl)pyr...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(O)cc1 Show InChI InChI=1S/C23H24N6O/c1-27-9-11-28(12-10-27)18-4-2-3-17(13-18)21-15-26-29-22(24)20(14-25-23(21)29)16-5-7-19(30)8-6-16/h2-8,13-15,30H,9-12,24H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320210

(3-(4-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN(C1CCCCC1)C(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C31H38N8O/c1-36-16-18-38(19-17-36)26-14-10-23(11-15-26)28-21-34-39-29(32)27(20-33-30(28)39)22-8-12-24(13-9-22)35-31(40)37(2)25-6-4-3-5-7-25/h8-15,20-21,25H,3-7,16-19,32H2,1-2H3,(H,35,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 497 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320215

(3-(7-amino-2-methyl-3-(4-(4-methylpiperazin-1-yl)p...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1c(C)nn2c(N)c(cnc12)-c1cccc(O)c1 Show InChI InChI=1S/C24H26N6O/c1-16-22(17-6-8-19(9-7-17)29-12-10-28(2)11-13-29)24-26-15-21(23(25)30(24)27-16)18-4-3-5-20(31)14-18/h3-9,14-15,31H,10-13,25H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320213

(CHEMBL1083022 | isobutyl4-(7-amino-3-(3-(4-methylp...)Show SMILES COc1cc(ccc1NC(=O)OCC(C)C)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C29H35N7O3/c1-19(2)18-39-29(37)33-25-9-8-21(15-26(25)38-4)23-16-31-28-24(17-32-36(28)27(23)30)20-6-5-7-22(14-20)35-12-10-34(3)11-13-35/h5-9,14-17,19H,10-13,18,30H2,1-4H3,(H,33,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 546 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320204

(3-(4-(7-amino-3-(3-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN(C1CCCCC1)C(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C31H38N8O/c1-36-15-17-38(18-16-36)26-10-6-7-23(19-26)28-21-34-39-29(32)27(20-33-30(28)39)22-11-13-24(14-12-22)35-31(40)37(2)25-8-4-3-5-9-25/h6-7,10-14,19-21,25H,3-5,8-9,15-18,32H2,1-2H3,(H,35,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 554 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320221

(CHEMBL1085317 | isobutyl4-(7-amino-3-(3-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-9-7-20(8-10-22)24-16-30-27-25(17-31-35(27)26(24)29)21-5-4-6-23(15-21)34-13-11-33(3)12-14-34/h4-10,15-17,19H,11-14,18,29H2,1-3H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 594 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320216

(CHEMBL1083644 | isobutyl4-(7-amino-3-(3-(piperazin...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCNCC1 Show InChI InChI=1S/C27H31N7O2/c1-18(2)17-36-27(35)32-21-8-6-19(7-9-21)23-15-30-26-24(16-31-34(26)25(23)28)20-4-3-5-22(14-20)33-12-10-29-11-13-33/h3-9,14-16,18,29H,10-13,17,28H2,1-2H3,(H,32,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 642 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 662 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320217

(CHEMBL1082403 | isobutyl4-(7-amino-3-(3-(4-(methyl...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C28H33N7O4S/c1-19(2)18-39-28(36)32-22-9-7-20(8-10-22)24-16-30-27-25(17-31-35(27)26(24)29)21-5-4-6-23(15-21)33-11-13-34(14-12-33)40(3,37)38/h4-10,15-17,19H,11-14,18,29H2,1-3H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 676 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320222

(CHEMBL1085564 | phenyl4-(7-amino-3-(3-(4-methylpip...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)Oc2ccccc2)cc1 Show InChI InChI=1S/C30H29N7O2/c1-35-14-16-36(17-15-35)24-7-5-6-22(18-24)27-20-33-37-28(31)26(19-32-29(27)37)21-10-12-23(13-11-21)34-30(38)39-25-8-3-2-4-9-25/h2-13,18-20H,14-17,31H2,1H3,(H,34,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 688 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320225

(CHEMBL1085316 | N-(4-(7-amino-3-(3-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)CCC2CCCC2)cc1 Show InChI InChI=1S/C31H37N7O/c1-36-15-17-37(18-16-36)26-8-4-7-24(19-26)28-21-34-38-30(32)27(20-33-31(28)38)23-10-12-25(13-11-23)35-29(39)14-9-22-5-2-3-6-22/h4,7-8,10-13,19-22H,2-3,5-6,9,14-18,32H2,1H3,(H,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320220

(CHEMBL1082959 | isobutyl4-(7-amino-3-(3-morpholino...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCOCC1 Show InChI InChI=1S/C27H30N6O3/c1-18(2)17-36-27(34)31-21-8-6-19(7-9-21)23-15-29-26-24(16-30-33(26)25(23)28)20-4-3-5-22(14-20)32-10-12-35-13-11-32/h3-9,14-16,18H,10-13,17,28H2,1-2H3,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 699 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320218

(CHEMBL1082404 | isobutyl4-(7-amino-3-(3-(4-(2-amin...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(CC1)C(=O)CN Show InChI InChI=1S/C29H34N8O3/c1-19(2)18-40-29(39)34-22-8-6-20(7-9-22)24-16-32-28-25(17-33-37(28)27(24)31)21-4-3-5-23(14-21)35-10-12-36(13-11-35)26(38)15-30/h3-9,14,16-17,19H,10-13,15,18,30-31H2,1-2H3,(H,34,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 762 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of tie2 |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320220

(CHEMBL1082959 | isobutyl4-(7-amino-3-(3-morpholino...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCOCC1 Show InChI InChI=1S/C27H30N6O3/c1-18(2)17-36-27(34)31-21-8-6-19(7-9-21)23-15-29-26-24(16-30-33(26)25(23)28)20-4-3-5-22(14-20)32-10-12-35-13-11-32/h3-9,14-16,18H,10-13,17,28H2,1-2H3,(H,31,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320211

(CHEMBL1085995 | isobutyl4-(7-amino-5-methyl-3-(3-(...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1c(C)nc2c(cnn2c1N)-c1cccc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C29H35N7O2/c1-19(2)18-38-29(37)33-23-10-8-21(9-11-23)26-20(3)32-28-25(17-31-36(28)27(26)30)22-6-5-7-24(16-22)35-14-12-34(4)13-15-35/h5-11,16-17,19H,12-15,18,30H2,1-4H3,(H,33,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320229

(CHEMBL1084974 | N-(4-(7-amino-3-(3-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NS(=O)(=O)c2cccc(Cl)c2Cl)cc1 Show InChI InChI=1S/C29H27Cl2N7O2S/c1-36-12-14-37(15-13-36)22-5-2-4-20(16-22)24-18-34-38-28(32)23(17-33-29(24)38)19-8-10-21(11-9-19)35-41(39,40)26-7-3-6-25(30)27(26)31/h2-11,16-18,35H,12-15,32H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320207

(CHEMBL1085512 | phenyl4-(7-amino-3-(4-(4-methylpip...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)Oc2ccccc2)cc1 Show InChI InChI=1S/C30H29N7O2/c1-35-15-17-36(18-16-35)24-13-9-22(10-14-24)27-20-33-37-28(31)26(19-32-29(27)37)21-7-11-23(12-8-21)34-30(38)39-25-5-3-2-4-6-25/h2-14,19-20H,15-18,31H2,1H3,(H,34,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320215

(3-(7-amino-2-methyl-3-(4-(4-methylpiperazin-1-yl)p...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1c(C)nn2c(N)c(cnc12)-c1cccc(O)c1 Show InChI InChI=1S/C24H26N6O/c1-16-22(17-6-8-19(9-7-17)29-12-10-28(2)11-13-29)24-26-15-21(23(25)30(24)27-16)18-4-3-5-20(31)14-18/h3-9,14-15,31H,10-13,25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320215

(3-(7-amino-2-methyl-3-(4-(4-methylpiperazin-1-yl)p...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1c(C)nn2c(N)c(cnc12)-c1cccc(O)c1 Show InChI InChI=1S/C24H26N6O/c1-16-22(17-6-8-19(9-7-17)29-12-10-28(2)11-13-29)24-26-15-21(23(25)30(24)27-16)18-4-3-5-20(31)14-18/h3-9,14-15,31H,10-13,25H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK in human Jurkat cells assessed as ZAP70 phosphorylation by FACS assay |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320213

(CHEMBL1083022 | isobutyl4-(7-amino-3-(3-(4-methylp...)Show SMILES COc1cc(ccc1NC(=O)OCC(C)C)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C29H35N7O3/c1-19(2)18-39-29(37)33-25-9-8-21(15-26(25)38-4)23-16-31-28-24(17-32-36(28)27(23)30)20-6-5-7-22(14-20)35-12-10-34(3)11-13-35/h5-9,14-17,19H,10-13,18,30H2,1-4H3,(H,33,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320229

(CHEMBL1084974 | N-(4-(7-amino-3-(3-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NS(=O)(=O)c2cccc(Cl)c2Cl)cc1 Show InChI InChI=1S/C29H27Cl2N7O2S/c1-36-12-14-37(15-13-36)22-5-2-4-20(16-22)24-18-34-38-28(32)23(17-33-29(24)38)19-8-10-21(11-9-19)35-41(39,40)26-7-3-6-25(30)27(26)31/h2-11,16-18,35H,12-15,32H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320217

(CHEMBL1082403 | isobutyl4-(7-amino-3-(3-(4-(methyl...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C28H33N7O4S/c1-19(2)18-39-28(36)32-22-9-7-20(8-10-22)24-16-30-27-25(17-31-35(27)26(24)29)21-5-4-6-23(15-21)33-11-13-34(14-12-33)40(3,37)38/h4-10,15-17,19H,11-14,18,29H2,1-3H3,(H,32,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320223

(1-(4-(7-amino-3-(3-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)Nc2ccccc2Cl)cc1 Show InChI InChI=1S/C30H29ClN8O/c1-37-13-15-38(16-14-37)23-6-4-5-21(17-23)25-19-34-39-28(32)24(18-33-29(25)39)20-9-11-22(12-10-20)35-30(40)36-27-8-3-2-7-26(27)31/h2-12,17-19H,13-16,32H2,1H3,(H2,35,36,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320208

(1-(4-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)Nc2ccccc2Cl)cc1 Show InChI InChI=1S/C30H29ClN8O/c1-37-14-16-38(17-15-37)23-12-8-21(9-13-23)25-19-34-39-28(32)24(18-33-29(25)39)20-6-10-22(11-7-20)35-30(40)36-27-5-3-2-4-26(27)31/h2-13,18-19H,14-17,32H2,1H3,(H2,35,36,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320226

(CHEMBL1085240 | N-(4-(7-amino-3-(3-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)c2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C31H28F3N7O/c1-39-13-15-40(16-14-39)25-4-2-3-22(17-25)27-19-37-41-28(35)26(18-36-29(27)41)20-7-11-24(12-8-20)38-30(42)21-5-9-23(10-6-21)31(32,33)34/h2-12,17-19H,13-16,35H2,1H3,(H,38,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320206

(CHEMBL1085733 | N-(4-(7-amino-3-(4-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)c2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C31H28F3N7O/c1-39-14-16-40(17-15-39)25-12-6-21(7-13-25)27-19-37-41-28(35)26(18-36-29(27)41)20-4-10-24(11-5-20)38-30(42)22-2-8-23(9-3-22)31(32,33)34/h2-13,18-19H,14-17,35H2,1H3,(H,38,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320219

(CHEMBL1082958 | isobutyl4-(7-amino-3-(4-(4-(2-meth...)Show SMILES COCCN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)OCC(C)C)cc1 Show InChI InChI=1S/C30H37N7O3/c1-21(2)20-40-30(38)34-24-8-4-22(5-9-24)26-18-32-29-27(19-33-37(29)28(26)31)23-6-10-25(11-7-23)36-14-12-35(13-15-36)16-17-39-3/h4-11,18-19,21H,12-17,20,31H2,1-3H3,(H,34,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320213

(CHEMBL1083022 | isobutyl4-(7-amino-3-(3-(4-methylp...)Show SMILES COc1cc(ccc1NC(=O)OCC(C)C)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C29H35N7O3/c1-19(2)18-39-29(37)33-25-9-8-21(15-26(25)38-4)23-16-31-28-24(17-32-36(28)27(23)30)20-6-5-7-22(14-20)35-12-10-34(3)11-13-35/h5-9,14-17,19H,10-13,18,30H2,1-4H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320220

(CHEMBL1082959 | isobutyl4-(7-amino-3-(3-morpholino...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCOCC1 Show InChI InChI=1S/C27H30N6O3/c1-18(2)17-36-27(34)31-21-8-6-19(7-9-21)23-15-29-26-24(16-30-33(26)25(23)28)20-4-3-5-22(14-20)32-10-12-35-13-11-32/h3-9,14-16,18H,10-13,17,28H2,1-2H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50320211

(CHEMBL1085995 | isobutyl4-(7-amino-5-methyl-3-(3-(...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1c(C)nc2c(cnn2c1N)-c1cccc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C29H35N7O2/c1-19(2)18-38-29(37)33-23-10-8-21(9-11-23)26-20(3)32-28-25(17-31-36(28)27(26)30)22-6-5-7-24(16-22)35-14-12-34(4)13-15-35/h5-11,16-17,19H,12-15,18,30H2,1-4H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320215

(3-(7-amino-2-methyl-3-(4-(4-methylpiperazin-1-yl)p...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1c(C)nn2c(N)c(cnc12)-c1cccc(O)c1 Show InChI InChI=1S/C24H26N6O/c1-16-22(17-6-8-19(9-7-17)29-12-10-28(2)11-13-29)24-26-15-21(23(25)30(24)27-16)18-4-3-5-20(31)14-18/h3-9,14-15,31H,10-13,25H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320205

(CHEMBL1083001 | N-(4-(7-amino-3-(4-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)CCC2CCCC2)cc1 Show InChI InChI=1S/C31H37N7O/c1-36-16-18-37(19-17-36)26-13-9-24(10-14-26)28-21-34-38-30(32)27(20-33-31(28)38)23-7-11-25(12-8-23)35-29(39)15-6-22-4-2-3-5-22/h7-14,20-22H,2-6,15-19,32H2,1H3,(H,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320228

(4-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)pyr...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(O)cc1 Show InChI InChI=1S/C23H24N6O/c1-27-10-12-28(13-11-27)18-6-2-17(3-7-18)21-15-26-29-22(24)20(14-25-23(21)29)16-4-8-19(30)9-5-16/h2-9,14-15,30H,10-13,24H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320221

(CHEMBL1085317 | isobutyl4-(7-amino-3-(3-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-9-7-20(8-10-22)24-16-30-27-25(17-31-35(27)26(24)29)21-5-4-6-23(15-21)34-13-11-33(3)12-14-34/h4-10,15-17,19H,11-14,18,29H2,1-3H3,(H,32,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320218

(CHEMBL1082404 | isobutyl4-(7-amino-3-(3-(4-(2-amin...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(CC1)C(=O)CN Show InChI InChI=1S/C29H34N8O3/c1-19(2)18-40-29(39)34-22-8-6-20(7-9-22)24-16-32-28-25(17-33-37(28)27(24)31)21-4-3-5-23(14-21)35-10-12-36(13-11-35)26(38)15-30/h3-9,14,16-17,19H,10-13,15,18,30-31H2,1-2H3,(H,34,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320216

(CHEMBL1083644 | isobutyl4-(7-amino-3-(3-(piperazin...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCNCC1 Show InChI InChI=1S/C27H31N7O2/c1-18(2)17-36-27(35)32-21-8-6-19(7-9-21)23-15-30-26-24(16-31-34(26)25(23)28)20-4-3-5-22(14-20)33-12-10-29-11-13-33/h3-9,14-16,18,29H,10-13,17,28H2,1-2H3,(H,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320219

(CHEMBL1082958 | isobutyl4-(7-amino-3-(4-(4-(2-meth...)Show SMILES COCCN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)OCC(C)C)cc1 Show InChI InChI=1S/C30H37N7O3/c1-21(2)20-40-30(38)34-24-8-4-22(5-9-24)26-18-32-29-27(19-33-37(29)28(26)31)23-6-10-25(11-7-23)36-14-12-35(13-15-36)16-17-39-3/h4-11,18-19,21H,12-17,20,31H2,1-3H3,(H,34,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320226

(CHEMBL1085240 | N-(4-(7-amino-3-(3-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)c2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C31H28F3N7O/c1-39-13-15-40(16-14-39)25-4-2-3-22(17-25)27-19-37-41-28(35)26(18-36-29(27)41)20-7-11-24(12-8-20)38-30(42)21-5-9-23(10-6-21)31(32,33)34/h2-12,17-19H,13-16,35H2,1H3,(H,38,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320212

(CHEMBL1085996 | isobutyl4-(7-amino-3-(3-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(c(C)c1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C29H35N7O2/c1-19(2)18-38-29(37)33-22-8-9-24(20(3)14-22)26-16-31-28-25(17-32-36(28)27(26)30)21-6-5-7-23(15-21)35-12-10-34(4)11-13-35/h5-9,14-17,19H,10-13,18,30H2,1-4H3,(H,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320222

(CHEMBL1085564 | phenyl4-(7-amino-3-(3-(4-methylpip...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)Oc2ccccc2)cc1 Show InChI InChI=1S/C30H29N7O2/c1-35-14-16-36(17-15-35)24-7-5-6-22(18-24)27-20-33-37-28(31)26(19-32-29(27)37)21-10-12-23(13-11-21)34-30(38)39-25-8-3-2-4-9-25/h2-13,18-20H,14-17,31H2,1H3,(H,34,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Ret |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320221

(CHEMBL1085317 | isobutyl4-(7-amino-3-(3-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-9-7-20(8-10-22)24-16-30-27-25(17-31-35(27)26(24)29)21-5-4-6-23(15-21)34-13-11-33(3)12-14-34/h4-10,15-17,19H,11-14,18,29H2,1-3H3,(H,32,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320229

(CHEMBL1084974 | N-(4-(7-amino-3-(3-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NS(=O)(=O)c2cccc(Cl)c2Cl)cc1 Show InChI InChI=1S/C29H27Cl2N7O2S/c1-36-12-14-37(15-13-36)22-5-2-4-20(16-22)24-18-34-38-28(32)23(17-33-29(24)38)19-8-10-21(11-9-19)35-41(39,40)26-7-3-6-25(30)27(26)31/h2-11,16-18,35H,12-15,32H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320204

(3-(4-(7-amino-3-(3-(4-methylpiperazin-1-yl)phenyl)...)Show SMILES CN(C1CCCCC1)C(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C31H38N8O/c1-36-15-17-38(18-16-36)26-10-6-7-23(19-26)28-21-34-39-29(32)27(20-33-30(28)39)22-11-13-24(14-12-22)35-31(40)37(2)25-8-4-3-5-9-25/h6-7,10-14,19-21,25H,3-5,8-9,15-18,32H2,1-2H3,(H,35,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320220

(CHEMBL1082959 | isobutyl4-(7-amino-3-(3-morpholino...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCOCC1 Show InChI InChI=1S/C27H30N6O3/c1-18(2)17-36-27(34)31-21-8-6-19(7-9-21)23-15-29-26-24(16-30-33(26)25(23)28)20-4-3-5-22(14-20)32-10-12-35-13-11-32/h3-9,14-16,18H,10-13,17,28H2,1-2H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320206

(CHEMBL1085733 | N-(4-(7-amino-3-(4-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)c2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C31H28F3N7O/c1-39-14-16-40(17-15-39)25-12-6-21(7-13-25)27-19-37-41-28(35)26(18-36-29(27)41)20-4-10-24(11-5-20)38-30(42)22-2-8-23(9-3-22)31(32,33)34/h2-13,18-19H,14-17,35H2,1H3,(H,38,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320213

(CHEMBL1083022 | isobutyl4-(7-amino-3-(3-(4-methylp...)Show SMILES COc1cc(ccc1NC(=O)OCC(C)C)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C29H35N7O3/c1-19(2)18-39-29(37)33-25-9-8-21(15-26(25)38-4)23-16-31-28-24(17-32-36(28)27(23)30)20-6-5-7-22(14-20)35-12-10-34(3)11-13-35/h5-9,14-17,19H,10-13,18,30H2,1-4H3,(H,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Lysine--tRNA ligase
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Syk |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cMet |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Her2 |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Alk |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Her1 |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320211

(CHEMBL1085995 | isobutyl4-(7-amino-5-methyl-3-(3-(...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1c(C)nc2c(cnn2c1N)-c1cccc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C29H35N7O2/c1-19(2)18-38-29(37)33-23-10-8-21(9-11-23)26-20(3)32-28-25(17-31-36(28)27(26)30)22-6-5-7-24(16-22)35-14-12-34(4)13-15-35/h5-11,16-17,19H,12-15,18,30H2,1-4H3,(H,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50320217

(CHEMBL1082403 | isobutyl4-(7-amino-3-(3-(4-(methyl...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1cccc(c1)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C28H33N7O4S/c1-19(2)18-39-28(36)32-22-9-7-20(8-10-22)24-16-30-27-25(17-31-35(27)26(24)29)21-5-4-6-23(15-21)33-11-13-34(14-12-33)40(3,37)38/h4-10,15-17,19H,11-14,18,29H2,1-3H3,(H,32,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FGFR3K |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKBalpha |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ZAP70 |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclinA |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50320203

(CHEMBL1085511 | isobutyl4-(7-amino-3-(4-(4-methylp...)Show SMILES CC(C)COC(=O)Nc1ccc(cc1)-c1cnc2c(cnn2c1N)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C28H33N7O2/c1-19(2)18-37-28(36)32-22-8-4-20(5-9-22)24-16-30-27-25(17-31-35(27)26(24)29)21-6-10-23(11-7-21)34-14-12-33(3)13-15-34/h4-11,16-17,19H,12-15,18,29H2,1-3H3,(H,32,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of INSR |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data