 Found 29 hits of Enzyme Inhibition Constant Data
Found 29 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 after 5 mins |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 5 mins |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50110164
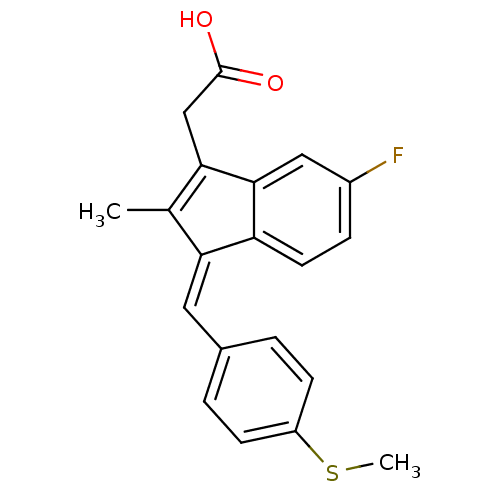
((Z)-2-(3-(4-(methylthio)benzylidene)-6-fluoro-2-me...)Show SMILES CSc1ccc(\C=C2\C(C)=C(CC(O)=O)c3cc(F)ccc23)cc1 |t:9| Show InChI InChI=1S/C20H17FO2S/c1-12-17(9-13-3-6-15(24-2)7-4-13)16-8-5-14(21)10-19(16)18(12)11-20(22)23/h3-10H,11H2,1-2H3,(H,22,23)/b17-9- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 after 5 mins |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50097346
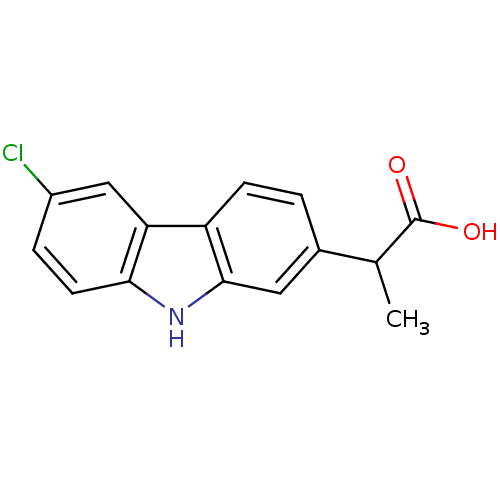
((+/-)-2-(3-chloro-9H-carbazol-7-yl)propanoic acid ...)Show InChI InChI=1S/C15H12ClNO2/c1-8(15(18)19)9-2-4-11-12-7-10(16)3-5-13(12)17-14(11)6-9/h2-8,17H,1H3,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 5 mins |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50110164

((Z)-2-(3-(4-(methylthio)benzylidene)-6-fluoro-2-me...)Show SMILES CSc1ccc(\C=C2\C(C)=C(CC(O)=O)c3cc(F)ccc23)cc1 |t:9| Show InChI InChI=1S/C20H17FO2S/c1-12-17(9-13-3-6-15(24-2)7-4-13)16-8-5-14(21)10-19(16)18(12)11-20(22)23/h3-10H,11H2,1-2H3,(H,22,23)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 5 mins |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50322460
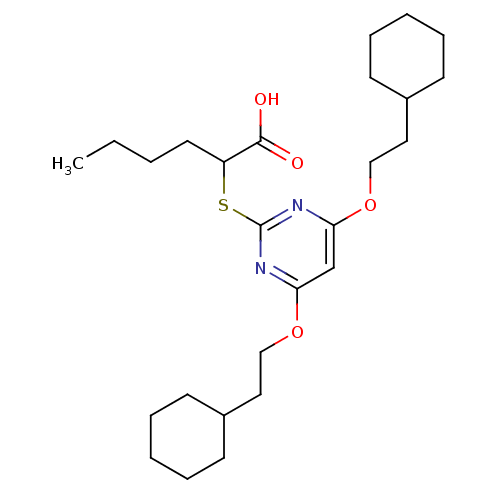
(2-(4,6-Bis(2-cyclohexylethoxy)pyrimidin-2-ylthio)h...)Show SMILES CCCCC(Sc1nc(OCCC2CCCCC2)cc(OCCC2CCCCC2)n1)C(O)=O Show InChI InChI=1S/C26H42N2O4S/c1-2-3-14-22(25(29)30)33-26-27-23(31-17-15-20-10-6-4-7-11-20)19-24(28-26)32-18-16-21-12-8-5-9-13-21/h19-22H,2-18H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 5 mins |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50009859
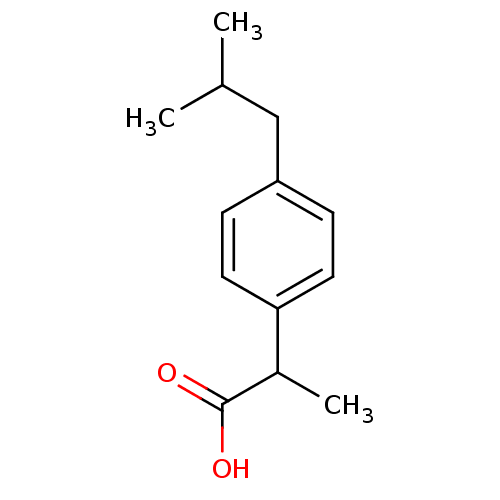
((+-)-2-(p-isobutylphenyl)propionic acid | (+-)-alp...)Show InChI InChI=1S/C13H18O2/c1-9(2)8-11-4-6-12(7-5-11)10(3)13(14)15/h4-7,9-10H,8H2,1-3H3,(H,14,15) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 after 5 mins |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50097346

((+/-)-2-(3-chloro-9H-carbazol-7-yl)propanoic acid ...)Show InChI InChI=1S/C15H12ClNO2/c1-8(15(18)19)9-2-4-11-12-7-10(16)3-5-13(12)17-14(11)6-9/h2-8,17H,1H3,(H,18,19) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 after 5 mins |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50322460

(2-(4,6-Bis(2-cyclohexylethoxy)pyrimidin-2-ylthio)h...)Show SMILES CCCCC(Sc1nc(OCCC2CCCCC2)cc(OCCC2CCCCC2)n1)C(O)=O Show InChI InChI=1S/C26H42N2O4S/c1-2-3-14-22(25(29)30)33-26-27-23(31-17-15-20-10-6-4-7-11-20)19-24(28-26)32-18-16-21-12-8-5-9-13-21/h19-22H,2-18H2,1H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 after 5 mins |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50322461
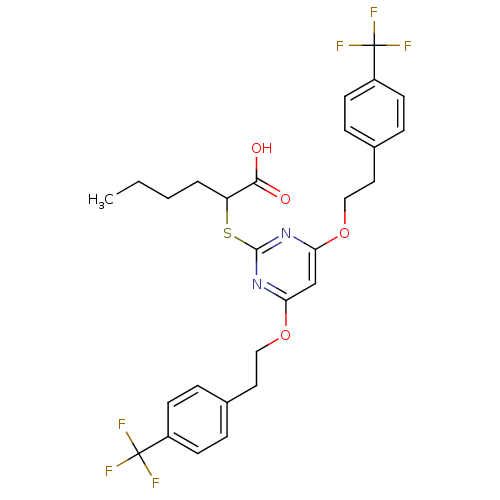
(2-(4,6-Bis(4-(trifluoromethyl)phenethoxy)pyrimidin...)Show SMILES CCCCC(Sc1nc(OCCc2ccc(cc2)C(F)(F)F)cc(OCCc2ccc(cc2)C(F)(F)F)n1)C(O)=O Show InChI InChI=1S/C28H28F6N2O4S/c1-2-3-4-22(25(37)38)41-26-35-23(39-15-13-18-5-9-20(10-6-18)27(29,30)31)17-24(36-26)40-16-14-19-7-11-21(12-8-19)28(32,33)34/h5-12,17,22H,2-4,13-16H2,1H3,(H,37,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 after 5 mins |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50273683
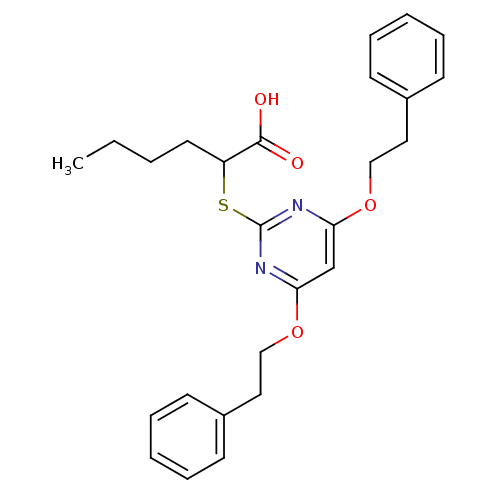
(2-(4,6-Diphenethoxypyrimidin-2-ylthio)hexanoic aci...)Show SMILES CCCCC(Sc1nc(OCCc2ccccc2)cc(OCCc2ccccc2)n1)C(O)=O Show InChI InChI=1S/C26H30N2O4S/c1-2-3-14-22(25(29)30)33-26-27-23(31-17-15-20-10-6-4-7-11-20)19-24(28-26)32-18-16-21-12-8-5-9-13-21/h4-13,19,22H,2-3,14-18H2,1H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 after 5 mins |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273683

(2-(4,6-Diphenethoxypyrimidin-2-ylthio)hexanoic aci...)Show SMILES CCCCC(Sc1nc(OCCc2ccccc2)cc(OCCc2ccccc2)n1)C(O)=O Show InChI InChI=1S/C26H30N2O4S/c1-2-3-14-22(25(29)30)33-26-27-23(31-17-15-20-10-6-4-7-11-20)19-24(28-26)32-18-16-21-12-8-5-9-13-21/h4-13,19,22H,2-3,14-18H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 5 mins |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50322461

(2-(4,6-Bis(4-(trifluoromethyl)phenethoxy)pyrimidin...)Show SMILES CCCCC(Sc1nc(OCCc2ccc(cc2)C(F)(F)F)cc(OCCc2ccc(cc2)C(F)(F)F)n1)C(O)=O Show InChI InChI=1S/C28H28F6N2O4S/c1-2-3-4-22(25(37)38)41-26-35-23(39-15-13-18-5-9-20(10-6-18)27(29,30)31)17-24(36-26)40-16-14-19-7-11-21(12-8-19)28(32,33)34/h5-12,17,22H,2-4,13-16H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 5 mins |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50009859

((+-)-2-(p-isobutylphenyl)propionic acid | (+-)-alp...)Show InChI InChI=1S/C13H18O2/c1-9(2)8-11-4-6-12(7-5-11)10(3)13(14)15/h4-7,9-10H,8H2,1-3H3,(H,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 5 mins |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50322462
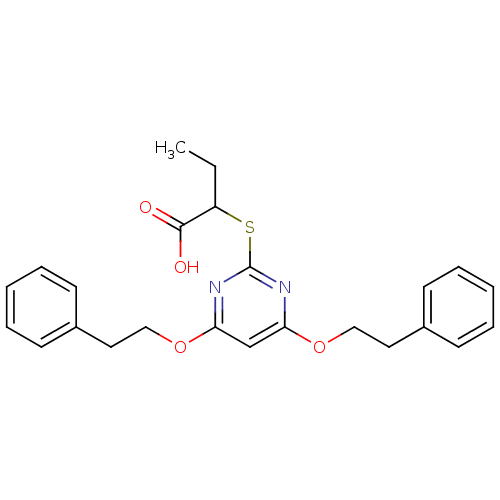
(2-(4,6-Diphenethoxypyrimidin-2-ylthio)butanoic aci...)Show SMILES CCC(Sc1nc(OCCc2ccccc2)cc(OCCc2ccccc2)n1)C(O)=O Show InChI InChI=1S/C24H26N2O4S/c1-2-20(23(27)28)31-24-25-21(29-15-13-18-9-5-3-6-10-18)17-22(26-24)30-16-14-19-11-7-4-8-12-19/h3-12,17,20H,2,13-16H2,1H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma-LBD expressed in COS7 cells cotransfected with Gal4 by luciferase based transactivation assay |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50322463

((S)-2-(4,6-diphenethoxypyrimidin-2-ylthio)hexanoic...)Show SMILES CCCC[C@H](Sc1nc(OCCc2ccccc2)cc(OCCc2ccccc2)n1)C(O)=O |r| Show InChI InChI=1S/C26H30N2O4S/c1-2-3-14-22(25(29)30)33-26-27-23(31-17-15-20-10-6-4-7-11-20)19-24(28-26)32-18-16-21-12-8-5-9-13-21/h4-13,19,22H,2-3,14-18H2,1H3,(H,29,30)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma-LBD expressed in COS7 cells cotransfected with Gal4 by luciferase based transactivation assay |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50273683

(2-(4,6-Diphenethoxypyrimidin-2-ylthio)hexanoic aci...)Show SMILES CCCCC(Sc1nc(OCCc2ccccc2)cc(OCCc2ccccc2)n1)C(O)=O Show InChI InChI=1S/C26H30N2O4S/c1-2-3-14-22(25(29)30)33-26-27-23(31-17-15-20-10-6-4-7-11-20)19-24(28-26)32-18-16-21-12-8-5-9-13-21/h4-13,19,22H,2-3,14-18H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma-LBD expressed in COS7 cells cotransfected with Gal4 by luciferase based transactivation assay |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50322467
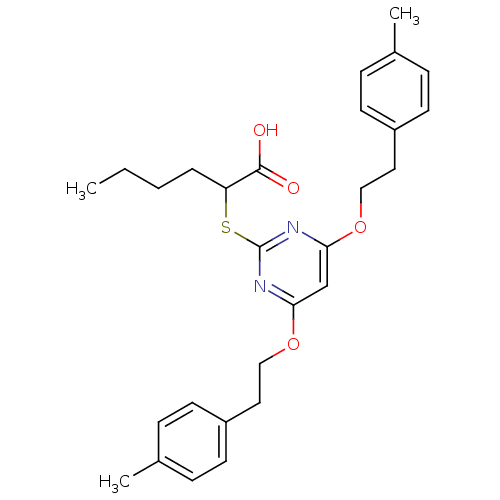
(2-(4,6-Bis(4-methylphenethoxy)pyrimidin-2-ylthio)-...)Show SMILES CCCCC(Sc1nc(OCCc2ccc(C)cc2)cc(OCCc2ccc(C)cc2)n1)C(O)=O Show InChI InChI=1S/C28H34N2O4S/c1-4-5-6-24(27(31)32)35-28-29-25(33-17-15-22-11-7-20(2)8-12-22)19-26(30-28)34-18-16-23-13-9-21(3)10-14-23/h7-14,19,24H,4-6,15-18H2,1-3H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma-LBD expressed in COS7 cells cotransfected with Gal4 by luciferase based transactivation assay |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50322468
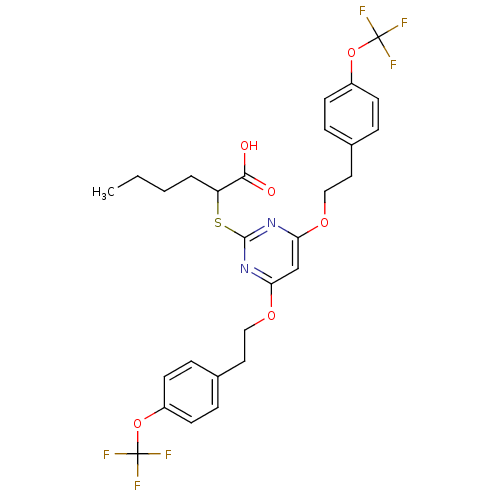
(2-(4,6-Bis(4-(trifluoromethoxy)phenethoxy)pyrimidi...)Show SMILES CCCCC(Sc1nc(OCCc2ccc(OC(F)(F)F)cc2)cc(OCCc2ccc(OC(F)(F)F)cc2)n1)C(O)=O Show InChI InChI=1S/C28H28F6N2O6S/c1-2-3-4-22(25(37)38)43-26-35-23(39-15-13-18-5-9-20(10-6-18)41-27(29,30)31)17-24(36-26)40-16-14-19-7-11-21(12-8-19)42-28(32,33)34/h5-12,17,22H,2-4,13-16H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma-LBD expressed in COS7 cells cotransfected with Gal4 by luciferase based transactivation assay |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50322469

(2-(4,6-Bis(4-cyanophenethoxy)pyrimidin-2-ylthio)he...)Show SMILES CCCCC(Sc1nc(OCCc2ccc(cc2)C#N)cc(OCCc2ccc(cc2)C#N)n1)C(O)=O Show InChI InChI=1S/C28H28N4O4S/c1-2-3-4-24(27(33)34)37-28-31-25(35-15-13-20-5-9-22(18-29)10-6-20)17-26(32-28)36-16-14-21-7-11-23(19-30)12-8-21/h5-12,17,24H,2-4,13-16H2,1H3,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma-LBD expressed in COS7 cells cotransfected with Gal4 by luciferase based transactivation assay |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50322466

(2-(4,6-Bis(4-phenylbutoxy)pyrimidin-2-ylthio)hexan...)Show SMILES CCCCC(Sc1nc(OCCCCc2ccccc2)cc(OCCCCc2ccccc2)n1)C(O)=O Show InChI InChI=1S/C30H38N2O4S/c1-2-3-20-26(29(33)34)37-30-31-27(35-21-12-10-18-24-14-6-4-7-15-24)23-28(32-30)36-22-13-11-19-25-16-8-5-9-17-25/h4-9,14-17,23,26H,2-3,10-13,18-22H2,1H3,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma-LBD expressed in COS7 cells cotransfected with Gal4 by luciferase based transactivation assay |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50322460

(2-(4,6-Bis(2-cyclohexylethoxy)pyrimidin-2-ylthio)h...)Show SMILES CCCCC(Sc1nc(OCCC2CCCCC2)cc(OCCC2CCCCC2)n1)C(O)=O Show InChI InChI=1S/C26H42N2O4S/c1-2-3-14-22(25(29)30)33-26-27-23(31-17-15-20-10-6-4-7-11-20)19-24(28-26)32-18-16-21-12-8-5-9-13-21/h19-22H,2-18H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma-LBD expressed in COS7 cells cotransfected with Gal4 by luciferase based transactivation assay |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50322464
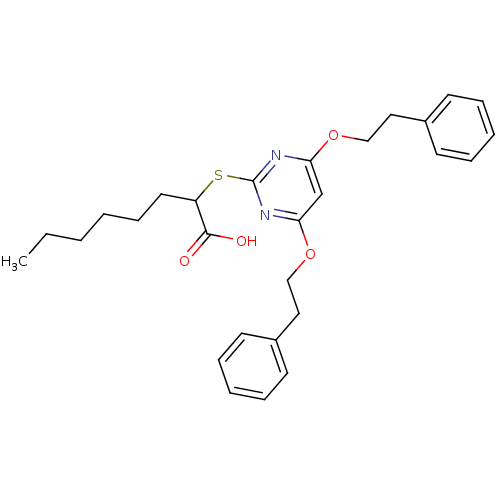
(2-(4,6-diphenethoxypyrimidin-2-ylthio)octanoic aci...)Show SMILES CCCCCCC(Sc1nc(OCCc2ccccc2)cc(OCCc2ccccc2)n1)C(O)=O Show InChI InChI=1S/C28H34N2O4S/c1-2-3-4-11-16-24(27(31)32)35-28-29-25(33-19-17-22-12-7-5-8-13-22)21-26(30-28)34-20-18-23-14-9-6-10-15-23/h5-10,12-15,21,24H,2-4,11,16-20H2,1H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma-LBD expressed in COS7 cells cotransfected with Gal4 by luciferase based transactivation assay |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50322472
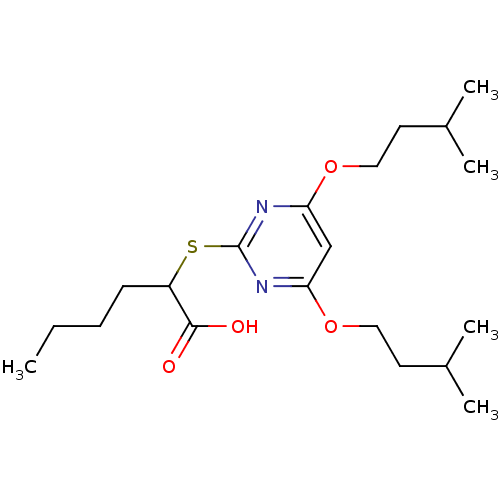
(2-(4,6-Bis(isopentyloxy)pyrimidin-2-ylthio)hexanoi...)Show InChI InChI=1S/C20H34N2O4S/c1-6-7-8-16(19(23)24)27-20-21-17(25-11-9-14(2)3)13-18(22-20)26-12-10-15(4)5/h13-16H,6-12H2,1-5H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma-LBD expressed in COS7 cells cotransfected with Gal4 by luciferase based transactivation assay |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50322471

(2-(4,6-Bis(2-cyclopropylethoxy)pyrimidin-2-ylthio)...)Show InChI InChI=1S/C20H30N2O4S/c1-2-3-4-16(19(23)24)27-20-21-17(25-11-9-14-5-6-14)13-18(22-20)26-12-10-15-7-8-15/h13-16H,2-12H2,1H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma-LBD expressed in COS7 cells cotransfected with Gal4 by luciferase based transactivation assay |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50322461

(2-(4,6-Bis(4-(trifluoromethyl)phenethoxy)pyrimidin...)Show SMILES CCCCC(Sc1nc(OCCc2ccc(cc2)C(F)(F)F)cc(OCCc2ccc(cc2)C(F)(F)F)n1)C(O)=O Show InChI InChI=1S/C28H28F6N2O4S/c1-2-3-4-22(25(37)38)41-26-35-23(39-15-13-18-5-9-20(10-6-18)27(29,30)31)17-24(36-26)40-16-14-19-7-11-21(12-8-19)28(32,33)34/h5-12,17,22H,2-4,13-16H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma-LBD expressed in COS7 cells cotransfected with Gal4 by luciferase based transactivation assay |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50322465
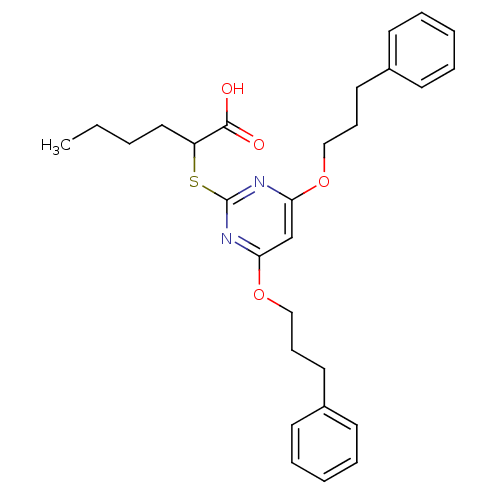
(2-(4,6-Bis(3-phenylpropoxy)pyrimidin-2-ylthio)hexa...)Show SMILES CCCCC(Sc1nc(OCCCc2ccccc2)cc(OCCCc2ccccc2)n1)C(O)=O Show InChI InChI=1S/C28H34N2O4S/c1-2-3-18-24(27(31)32)35-28-29-25(33-19-10-16-22-12-6-4-7-13-22)21-26(30-28)34-20-11-17-23-14-8-5-9-15-23/h4-9,12-15,21,24H,2-3,10-11,16-20H2,1H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma-LBD expressed in COS7 cells cotransfected with Gal4 by luciferase based transactivation assay |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50322470
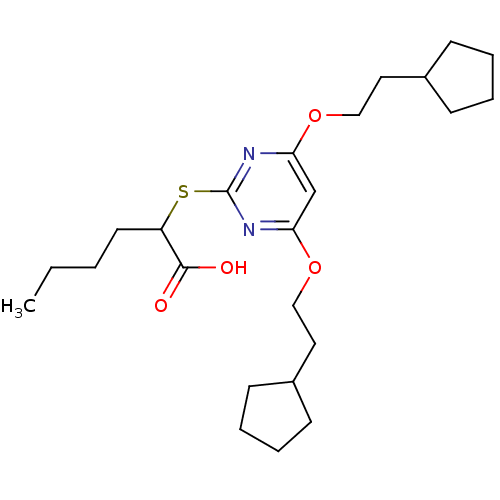
(2-(4,6-Bis(2-cyclopentylethoxy)pyrimidin-2-ylthio)...)Show SMILES CCCCC(Sc1nc(OCCC2CCCC2)cc(OCCC2CCCC2)n1)C(O)=O Show InChI InChI=1S/C24H38N2O4S/c1-2-3-12-20(23(27)28)31-24-25-21(29-15-13-18-8-4-5-9-18)17-22(26-24)30-16-14-19-10-6-7-11-19/h17-20H,2-16H2,1H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma-LBD expressed in COS7 cells cotransfected with Gal4 by luciferase based transactivation assay |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50273682
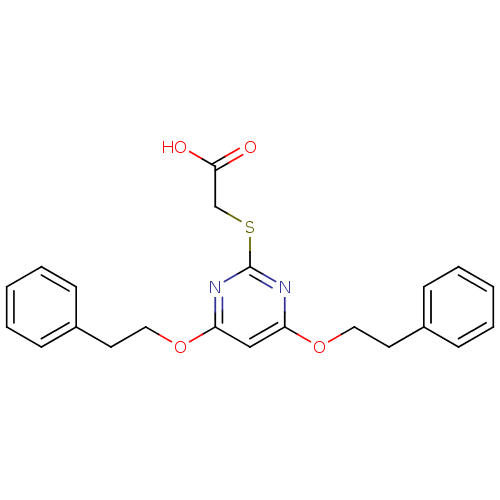
(2-(4,6-Diphenethoxypyrimidin-2-ylthio)acetic acid ...)Show InChI InChI=1S/C22H22N2O4S/c25-21(26)16-29-22-23-19(27-13-11-17-7-3-1-4-8-17)15-20(24-22)28-14-12-18-9-5-2-6-10-18/h1-10,15H,11-14,16H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma-LBD expressed in COS7 cells cotransfected with Gal4 by luciferase based transactivation assay |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data