 Found 52 hits of Enzyme Inhibition Constant Data
Found 52 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50323359
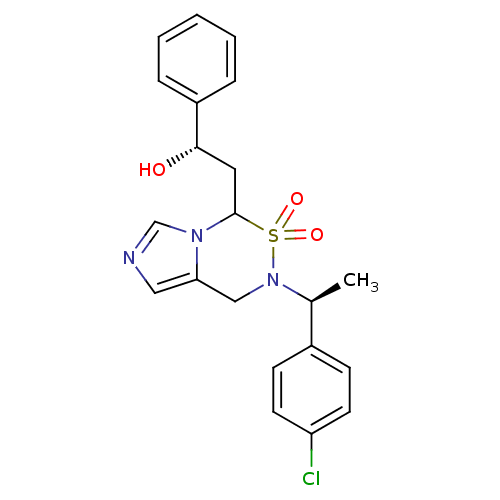
((S)-2-{6-[(S)-1-(4-Chloro-phenyl)-ethyl]-5,5-dioxo...)Show SMILES C[C@H](N1Cc2cncn2C(C[C@H](O)c2ccccc2)S1(=O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H22ClN3O3S/c1-15(16-7-9-18(22)10-8-16)25-13-19-12-23-14-24(19)21(29(25,27)28)11-20(26)17-5-3-2-4-6-17/h2-10,12,14-15,20-21,26H,11,13H2,1H3/t15-,20-,21?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50323356
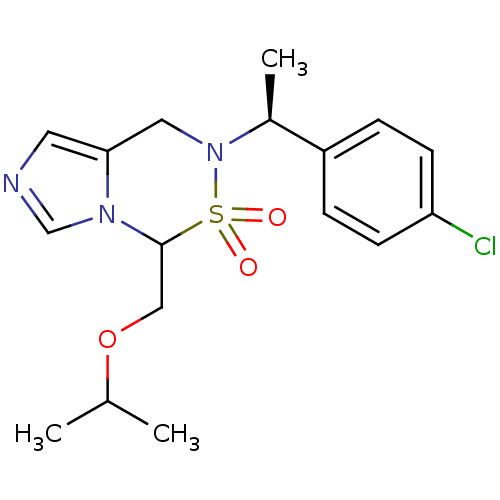
(6-[(S)-1-(4-Chloro-phenyl)-ethyl]-4-isopropoxymeth...)Show SMILES CC(C)OCC1n2cncc2CN([C@@H](C)c2ccc(Cl)cc2)S1(=O)=O |r| Show InChI InChI=1S/C17H22ClN3O3S/c1-12(2)24-10-17-20-11-19-8-16(20)9-21(25(17,22)23)13(3)14-4-6-15(18)7-5-14/h4-8,11-13,17H,9-10H2,1-3H3/t13-,17?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50323355
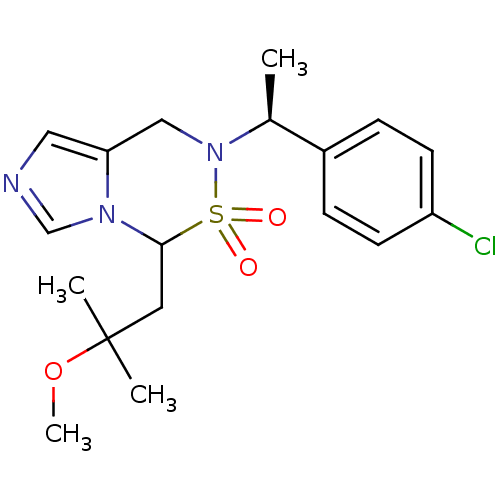
(6-[(S)-1-(4-Chloro-phenyl)-ethyl]-4-(2-methoxy-2-m...)Show SMILES COC(C)(C)CC1n2cncc2CN([C@@H](C)c2ccc(Cl)cc2)S1(=O)=O |r| Show InChI InChI=1S/C18H24ClN3O3S/c1-13(14-5-7-15(19)8-6-14)22-11-16-10-20-12-21(16)17(26(22,23)24)9-18(2,3)25-4/h5-8,10,12-13,17H,9,11H2,1-4H3/t13-,17?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50323357

(6-[(S)-1-(4-Chloro-phenyl)-ethyl]-4-cyclopropylmet...)Show SMILES C[C@H](N1Cc2cncn2C(CC2CC2)S1(=O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C17H20ClN3O2S/c1-12(14-4-6-15(18)7-5-14)21-10-16-9-19-11-20(16)17(24(21,22)23)8-13-2-3-13/h4-7,9,11-13,17H,2-3,8,10H2,1H3/t12-,17?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50047262
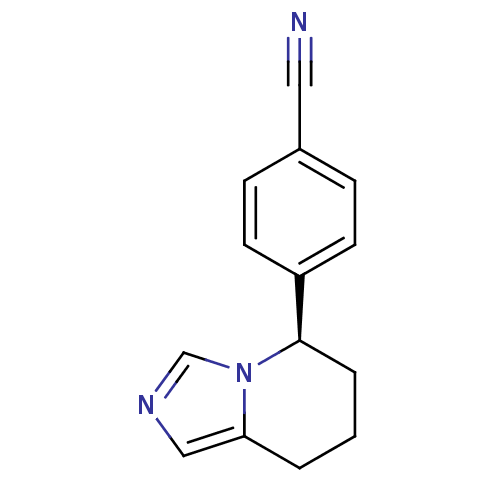
((R)-4-(5,6,7,8-tetrahydroimidazo[1,5-a]pyridin-5-y...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50323360
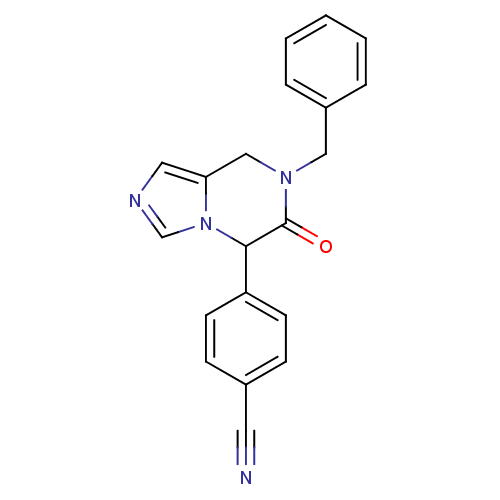
(4-(7-benzyl-6-oxo-5,6,7,8-tetrahydroimidazo[1,5-a]...)Show InChI InChI=1S/C20H16N4O/c21-10-15-6-8-17(9-7-15)19-20(25)23(12-16-4-2-1-3-5-16)13-18-11-22-14-24(18)19/h1-9,11,14,19H,12-13H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B1 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50323358

((R)-2-{6-[(S)-1-(4-Chloro-phenyl)-ethyl]-5,5-dioxo...)Show SMILES C[C@H](N1Cc2cncn2C(C[C@@H](O)c2ccccc2)S1(=O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H22ClN3O3S/c1-15(16-7-9-18(22)10-8-16)25-13-19-12-23-14-24(19)21(29(25,27)28)11-20(26)17-5-3-2-4-6-17/h2-10,12,14-15,20-21,26H,11,13H2,1H3/t15-,20+,21?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50323342

(6-(4-Fluoro-benzyl)-4-isobutyl-6,7-dihydro-5-thia-...)Show InChI InChI=1S/C16H20FN3O2S/c1-12(2)7-16-20-11-18-8-15(20)10-19(23(16,21)22)9-13-3-5-14(17)6-4-13/h3-6,8,11-12,16H,7,9-10H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50323343

(6-(4-Chloro-benzyl)-4-isobutyl-6,7-dihydro-5-thia-...)Show InChI InChI=1S/C16H20ClN3O2S/c1-12(2)7-16-20-11-18-8-15(20)10-19(23(16,21)22)9-13-3-5-14(17)6-4-13/h3-6,8,11-12,16H,7,9-10H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50323354
(6-[(S)-1-(4-Chloro-phenyl)-ethyl]-4-(3-methyl-oxet...)Show SMILES C[C@H](N1Cc2cncn2C(CC2(C)COC2)S1(=O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C18H22ClN3O3S/c1-13(14-3-5-15(19)6-4-14)22-9-16-8-20-12-21(16)17(26(22,23)24)7-18(2)10-25-11-18/h3-6,8,12-13,17H,7,9-11H2,1-2H3/t13-,17?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50323351

(6-[(S)-1-(4-Chloro-phenyl)-ethyl]-4-isobutyl-6,7-d...)Show SMILES CC(C)CC1n2cncc2CN([C@@H](C)c2ccc(Cl)cc2)S1(=O)=O |r| Show InChI InChI=1S/C17H22ClN3O2S/c1-12(2)8-17-20-11-19-9-16(20)10-21(24(17,22)23)13(3)14-4-6-15(18)7-5-14/h4-7,9,11-13,17H,8,10H2,1-3H3/t13-,17?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50047262

((R)-4-(5,6,7,8-tetrahydroimidazo[1,5-a]pyridin-5-y...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B1 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50323353
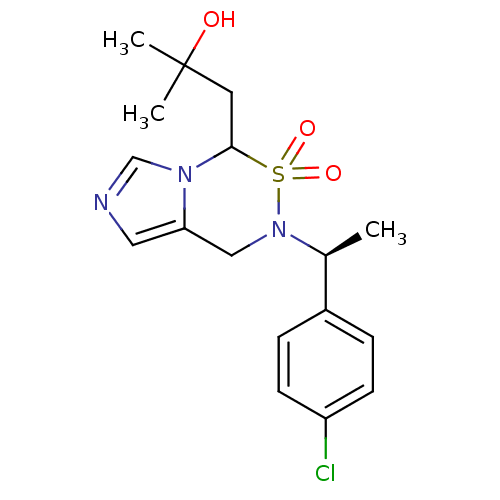
(1-{6-[(S)-1-(4-Chloro-phenyl)-ethyl]-5,5-dioxo-4,5...)Show SMILES C[C@H](N1Cc2cncn2C(CC(C)(C)O)S1(=O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C17H22ClN3O3S/c1-12(13-4-6-14(18)7-5-13)21-10-15-9-19-11-20(15)16(25(21,23)24)8-17(2,3)22/h4-7,9,11-12,16,22H,8,10H2,1-3H3/t12-,16?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50323344
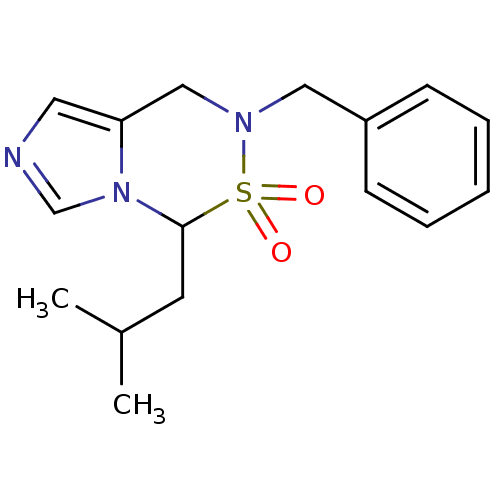
(6-Benzyl-4-isobutyl-6,7-dihydro-5-thia-2,3a,6-tria...)Show InChI InChI=1S/C16H21N3O2S/c1-13(2)8-16-19-12-17-9-15(19)11-18(22(16,20)21)10-14-6-4-3-5-7-14/h3-7,9,12-13,16H,8,10-11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50323355

(6-[(S)-1-(4-Chloro-phenyl)-ethyl]-4-(2-methoxy-2-m...)Show SMILES COC(C)(C)CC1n2cncc2CN([C@@H](C)c2ccc(Cl)cc2)S1(=O)=O |r| Show InChI InChI=1S/C18H24ClN3O3S/c1-13(14-5-7-15(19)8-6-14)22-11-16-10-20-12-21(16)17(26(22,23)24)9-18(2,3)25-4/h5-8,10,12-13,17H,9,11H2,1-4H3/t13-,17?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B1 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50323342

(6-(4-Fluoro-benzyl)-4-isobutyl-6,7-dihydro-5-thia-...)Show InChI InChI=1S/C16H20FN3O2S/c1-12(2)7-16-20-11-18-8-15(20)10-19(23(16,21)22)9-13-3-5-14(17)6-4-13/h3-6,8,11-12,16H,7,9-10H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B1 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50323360

(4-(7-benzyl-6-oxo-5,6,7,8-tetrahydroimidazo[1,5-a]...)Show InChI InChI=1S/C20H16N4O/c21-10-15-6-8-17(9-7-15)19-20(25)23(12-16-4-2-1-3-5-16)13-18-11-22-14-24(18)19/h1-9,11,14,19H,12-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50323343

(6-(4-Chloro-benzyl)-4-isobutyl-6,7-dihydro-5-thia-...)Show InChI InChI=1S/C16H20ClN3O2S/c1-12(2)7-16-20-11-18-8-15(20)10-19(23(16,21)22)9-13-3-5-14(17)6-4-13/h3-6,8,11-12,16H,7,9-10H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B1 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50323349

(4-Isobutyl-6-thiophen-2-ylmethyl-6,7-dihydro-5-thi...)Show InChI InChI=1S/C14H19N3O2S2/c1-11(2)6-14-17-10-15-7-12(17)8-16(21(14,18)19)9-13-4-3-5-20-13/h3-5,7,10-11,14H,6,8-9H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B1 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50323352
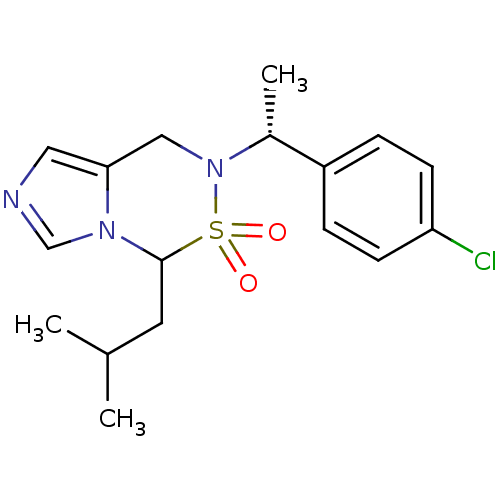
(6-[(R)-1-(4-Chloro-phenyl)-ethyl]-4-isobutyl-6,7-d...)Show SMILES CC(C)CC1n2cncc2CN([C@H](C)c2ccc(Cl)cc2)S1(=O)=O |r| Show InChI InChI=1S/C17H22ClN3O2S/c1-12(2)8-17-20-11-19-9-16(20)10-21(24(17,22)23)13(3)14-4-6-15(18)7-5-14/h4-7,9,11-13,17H,8,10H2,1-3H3/t13-,17?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50323351

(6-[(S)-1-(4-Chloro-phenyl)-ethyl]-4-isobutyl-6,7-d...)Show SMILES CC(C)CC1n2cncc2CN([C@@H](C)c2ccc(Cl)cc2)S1(=O)=O |r| Show InChI InChI=1S/C17H22ClN3O2S/c1-12(2)8-17-20-11-19-9-16(20)10-21(24(17,22)23)13(3)14-4-6-15(18)7-5-14/h4-7,9,11-13,17H,8,10H2,1-3H3/t13-,17?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B1 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50323343

(6-(4-Chloro-benzyl)-4-isobutyl-6,7-dihydro-5-thia-...)Show InChI InChI=1S/C16H20ClN3O2S/c1-12(2)7-16-20-11-18-8-15(20)10-19(23(16,21)22)9-13-3-5-14(17)6-4-13/h3-6,8,11-12,16H,7,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4-mediated testosterone oxidation |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50323344

(6-Benzyl-4-isobutyl-6,7-dihydro-5-thia-2,3a,6-tria...)Show InChI InChI=1S/C16H21N3O2S/c1-13(2)8-16-19-12-17-9-15(19)11-18(22(16,20)21)10-14-6-4-3-5-7-14/h3-7,9,12-13,16H,8,10-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4-mediated midazolam oxidation |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50323358

((R)-2-{6-[(S)-1-(4-Chloro-phenyl)-ethyl]-5,5-dioxo...)Show SMILES C[C@H](N1Cc2cncn2C(C[C@@H](O)c2ccccc2)S1(=O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H22ClN3O3S/c1-15(16-7-9-18(22)10-8-16)25-13-19-12-23-14-24(19)21(29(25,27)28)11-20(26)17-5-3-2-4-6-17/h2-10,12,14-15,20-21,26H,11,13H2,1H3/t15-,20+,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4-mediated midazolam oxidation |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50323344

(6-Benzyl-4-isobutyl-6,7-dihydro-5-thia-2,3a,6-tria...)Show InChI InChI=1S/C16H21N3O2S/c1-13(2)8-16-19-12-17-9-15(19)11-18(22(16,20)21)10-14-6-4-3-5-7-14/h3-7,9,12-13,16H,8,10-11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B1 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50323345

(4-Isobutyl-6-(4-trifluoromethoxy-benzyl)-6,7-dihyd...)Show SMILES CC(C)CC1n2cncc2CN(Cc2ccc(OC(F)(F)F)cc2)S1(=O)=O Show InChI InChI=1S/C17H20F3N3O3S/c1-12(2)7-16-23-11-21-8-14(23)10-22(27(16,24)25)9-13-3-5-15(6-4-13)26-17(18,19)20/h3-6,8,11-12,16H,7,9-10H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50323349

(4-Isobutyl-6-thiophen-2-ylmethyl-6,7-dihydro-5-thi...)Show InChI InChI=1S/C14H19N3O2S2/c1-11(2)6-14-17-10-15-7-12(17)8-16(21(14,18)19)9-13-4-3-5-20-13/h3-5,7,10-11,14H,6,8-9H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50323350

(6-[1-(4-Chloro-phenyl)-cyclopropyl]-4-isobutyl-6,7...)Show SMILES CC(C)CC1n2cncc2CN(C2(CC2)c2ccc(Cl)cc2)S1(=O)=O Show InChI InChI=1S/C18H22ClN3O2S/c1-13(2)9-17-21-12-20-10-16(21)11-22(25(17,23)24)18(7-8-18)14-3-5-15(19)6-4-14/h3-6,10,12-13,17H,7-9,11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B1 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50323356

(6-[(S)-1-(4-Chloro-phenyl)-ethyl]-4-isopropoxymeth...)Show SMILES CC(C)OCC1n2cncc2CN([C@@H](C)c2ccc(Cl)cc2)S1(=O)=O |r| Show InChI InChI=1S/C17H22ClN3O3S/c1-12(2)24-10-17-20-11-19-8-16(20)9-21(25(17,22)23)13(3)14-4-6-15(18)7-5-14/h4-8,11-13,17H,9-10H2,1-3H3/t13-,17?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4-mediated testosterone oxidation |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50323346

(CHEMBL1209556 | rac-4-Isobutyl-6-(4-trifluoromethy...)Show SMILES CC(C)CC1n2cncc2CN(Cc2ccc(cc2)C(F)(F)F)S1(=O)=O Show InChI InChI=1S/C17H20F3N3O2S/c1-12(2)7-16-23-11-21-8-15(23)10-22(26(16,24)25)9-13-3-5-14(6-4-13)17(18,19)20/h3-6,8,11-12,16H,7,9-10H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50323348
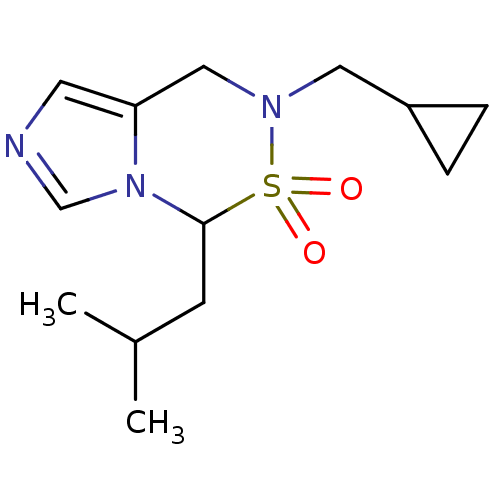
(6-Cyclopropylmethyl-4-isobutyl-6,7-dihydro-5-thia-...)Show InChI InChI=1S/C13H21N3O2S/c1-10(2)5-13-16-9-14-6-12(16)8-15(19(13,17)18)7-11-3-4-11/h6,9-11,13H,3-5,7-8H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B1 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50323357

(6-[(S)-1-(4-Chloro-phenyl)-ethyl]-4-cyclopropylmet...)Show SMILES C[C@H](N1Cc2cncn2C(CC2CC2)S1(=O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C17H20ClN3O2S/c1-12(14-4-6-15(18)7-5-14)21-10-16-9-19-11-20(16)17(24(21,22)23)8-13-2-3-13/h4-7,9,11-13,17H,2-3,8,10H2,1H3/t12-,17?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4-mediated testosterone oxidation |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50323359

((S)-2-{6-[(S)-1-(4-Chloro-phenyl)-ethyl]-5,5-dioxo...)Show SMILES C[C@H](N1Cc2cncn2C(C[C@H](O)c2ccccc2)S1(=O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H22ClN3O3S/c1-15(16-7-9-18(22)10-8-16)25-13-19-12-23-14-24(19)21(29(25,27)28)11-20(26)17-5-3-2-4-6-17/h2-10,12,14-15,20-21,26H,11,13H2,1H3/t15-,20-,21?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B1 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50323352

(6-[(R)-1-(4-Chloro-phenyl)-ethyl]-4-isobutyl-6,7-d...)Show SMILES CC(C)CC1n2cncc2CN([C@H](C)c2ccc(Cl)cc2)S1(=O)=O |r| Show InChI InChI=1S/C17H22ClN3O2S/c1-12(2)8-17-20-11-19-9-16(20)10-21(24(17,22)23)13(3)14-4-6-15(18)7-5-14/h4-7,9,11-13,17H,8,10H2,1-3H3/t13-,17?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 204 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B1 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50323356

(6-[(S)-1-(4-Chloro-phenyl)-ethyl]-4-isopropoxymeth...)Show SMILES CC(C)OCC1n2cncc2CN([C@@H](C)c2ccc(Cl)cc2)S1(=O)=O |r| Show InChI InChI=1S/C17H22ClN3O3S/c1-12(2)24-10-17-20-11-19-8-16(20)9-21(25(17,22)23)13(3)14-4-6-15(18)7-5-14/h4-8,11-13,17H,9-10H2,1-3H3/t13-,17?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 222 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B1 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50323358

((R)-2-{6-[(S)-1-(4-Chloro-phenyl)-ethyl]-5,5-dioxo...)Show SMILES C[C@H](N1Cc2cncn2C(C[C@@H](O)c2ccccc2)S1(=O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H22ClN3O3S/c1-15(16-7-9-18(22)10-8-16)25-13-19-12-23-14-24(19)21(29(25,27)28)11-20(26)17-5-3-2-4-6-17/h2-10,12,14-15,20-21,26H,11,13H2,1H3/t15-,20+,21?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 234 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B1 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50323357

(6-[(S)-1-(4-Chloro-phenyl)-ethyl]-4-cyclopropylmet...)Show SMILES C[C@H](N1Cc2cncn2C(CC2CC2)S1(=O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C17H20ClN3O2S/c1-12(14-4-6-15(18)7-5-14)21-10-16-9-19-11-20(16)17(24(21,22)23)8-13-2-3-13/h4-7,9,11-13,17H,2-3,8,10H2,1H3/t12-,17?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B1 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50323346

(CHEMBL1209556 | rac-4-Isobutyl-6-(4-trifluoromethy...)Show SMILES CC(C)CC1n2cncc2CN(Cc2ccc(cc2)C(F)(F)F)S1(=O)=O Show InChI InChI=1S/C17H20F3N3O2S/c1-12(2)7-16-23-11-21-8-15(23)10-22(26(16,24)25)9-13-3-5-14(6-4-13)17(18,19)20/h3-6,8,11-12,16H,7,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4-mediated testosterone oxidation |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50323354

(6-[(S)-1-(4-Chloro-phenyl)-ethyl]-4-(3-methyl-oxet...)Show SMILES C[C@H](N1Cc2cncn2C(CC2(C)COC2)S1(=O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C18H22ClN3O3S/c1-13(14-3-5-15(19)6-4-14)22-9-16-8-20-12-21(16)17(26(22,23)24)7-18(2)10-25-11-18/h3-6,8,12-13,17H,7,9-11H2,1-2H3/t13-,17?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B1 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50323353

(1-{6-[(S)-1-(4-Chloro-phenyl)-ethyl]-5,5-dioxo-4,5...)Show SMILES C[C@H](N1Cc2cncn2C(CC(C)(C)O)S1(=O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C17H22ClN3O3S/c1-12(13-4-6-14(18)7-5-13)21-10-15-9-19-11-20(15)16(25(21,23)24)8-17(2,3)22/h4-7,9,11-12,16,22H,8,10H2,1-3H3/t12-,16?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 471 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B1 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50323345

(4-Isobutyl-6-(4-trifluoromethoxy-benzyl)-6,7-dihyd...)Show SMILES CC(C)CC1n2cncc2CN(Cc2ccc(OC(F)(F)F)cc2)S1(=O)=O Show InChI InChI=1S/C17H20F3N3O3S/c1-12(2)7-16-23-11-21-8-14(23)10-22(27(16,24)25)9-13-3-5-15(6-4-13)26-17(18,19)20/h3-6,8,11-12,16H,7,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4-mediated testosterone oxidation |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50323355

(6-[(S)-1-(4-Chloro-phenyl)-ethyl]-4-(2-methoxy-2-m...)Show SMILES COC(C)(C)CC1n2cncc2CN([C@@H](C)c2ccc(Cl)cc2)S1(=O)=O |r| Show InChI InChI=1S/C18H24ClN3O3S/c1-13(14-5-7-15(19)8-6-14)22-11-16-10-20-12-21(16)17(26(22,23)24)9-18(2,3)25-4/h5-8,10,12-13,17H,9,11H2,1-4H3/t13-,17?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4-mediated testosterone oxidation |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50323345

(4-Isobutyl-6-(4-trifluoromethoxy-benzyl)-6,7-dihyd...)Show SMILES CC(C)CC1n2cncc2CN(Cc2ccc(OC(F)(F)F)cc2)S1(=O)=O Show InChI InChI=1S/C17H20F3N3O3S/c1-12(2)7-16-23-11-21-8-14(23)10-22(27(16,24)25)9-13-3-5-15(6-4-13)26-17(18,19)20/h3-6,8,11-12,16H,7,9-10H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 544 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B1 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50323346

(CHEMBL1209556 | rac-4-Isobutyl-6-(4-trifluoromethy...)Show SMILES CC(C)CC1n2cncc2CN(Cc2ccc(cc2)C(F)(F)F)S1(=O)=O Show InChI InChI=1S/C17H20F3N3O2S/c1-12(2)7-16-23-11-21-8-15(23)10-22(26(16,24)25)9-13-3-5-14(6-4-13)17(18,19)20/h3-6,8,11-12,16H,7,9-10H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 566 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B1 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50323350

(6-[1-(4-Chloro-phenyl)-cyclopropyl]-4-isobutyl-6,7...)Show SMILES CC(C)CC1n2cncc2CN(C2(CC2)c2ccc(Cl)cc2)S1(=O)=O Show InChI InChI=1S/C18H22ClN3O2S/c1-13(2)9-17-21-12-20-10-16(21)11-22(25(17,23)24)18(7-8-18)14-3-5-15(19)6-4-14/h3-6,10,12-13,17H,7-9,11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 628 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50323348

(6-Cyclopropylmethyl-4-isobutyl-6,7-dihydro-5-thia-...)Show InChI InChI=1S/C13H21N3O2S/c1-10(2)5-13-16-9-14-6-12(16)8-15(19(13,17)18)7-11-3-4-11/h6,9-11,13H,3-5,7-8H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 758 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50323358

((R)-2-{6-[(S)-1-(4-Chloro-phenyl)-ethyl]-5,5-dioxo...)Show SMILES C[C@H](N1Cc2cncn2C(C[C@@H](O)c2ccccc2)S1(=O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H22ClN3O3S/c1-15(16-7-9-18(22)10-8-16)25-13-19-12-23-14-24(19)21(29(25,27)28)11-20(26)17-5-3-2-4-6-17/h2-10,12,14-15,20-21,26H,11,13H2,1H3/t15-,20+,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4-mediated testosterone oxidation |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50323347
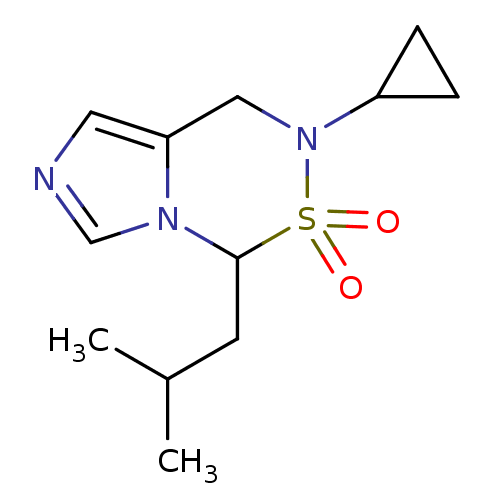
(6-Cyclopropyl-4-isobutyl-6,7-dihydro-5-thia-2,3a,6...)Show InChI InChI=1S/C12H19N3O2S/c1-9(2)5-12-14-8-13-6-11(14)7-15(10-3-4-10)18(12,16)17/h6,8-10,12H,3-5,7H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B1 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50323347

(6-Cyclopropyl-4-isobutyl-6,7-dihydro-5-thia-2,3a,6...)Show InChI InChI=1S/C12H19N3O2S/c1-9(2)5-12-14-8-13-6-11(14)7-15(10-3-4-10)18(12,16)17/h6,8-10,12H,3-5,7H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 by cell-based assay |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50323349

(4-Isobutyl-6-thiophen-2-ylmethyl-6,7-dihydro-5-thi...)Show InChI InChI=1S/C14H19N3O2S2/c1-11(2)6-14-17-10-15-7-12(17)8-16(21(14,18)19)9-13-4-3-5-20-13/h3-5,7,10-11,14H,6,8-9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4-mediated testosterone oxidation |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50323344

(6-Benzyl-4-isobutyl-6,7-dihydro-5-thia-2,3a,6-tria...)Show InChI InChI=1S/C16H21N3O2S/c1-13(2)8-16-19-12-17-9-15(19)11-18(22(16,20)21)10-14-6-4-3-5-7-14/h3-7,9,12-13,16H,8,10-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4-mediated testosterone oxidation |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50323359

((S)-2-{6-[(S)-1-(4-Chloro-phenyl)-ethyl]-5,5-dioxo...)Show SMILES C[C@H](N1Cc2cncn2C(C[C@H](O)c2ccccc2)S1(=O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H22ClN3O3S/c1-15(16-7-9-18(22)10-8-16)25-13-19-12-23-14-24(19)21(29(25,27)28)11-20(26)17-5-3-2-4-6-17/h2-10,12,14-15,20-21,26H,11,13H2,1H3/t15-,20-,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4-mediated testosterone oxidation |
Bioorg Med Chem Lett 20: 4324-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.086
BindingDB Entry DOI: 10.7270/Q2QJ7HGS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data