 Found 45 hits of Enzyme Inhibition Constant Data
Found 45 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325632
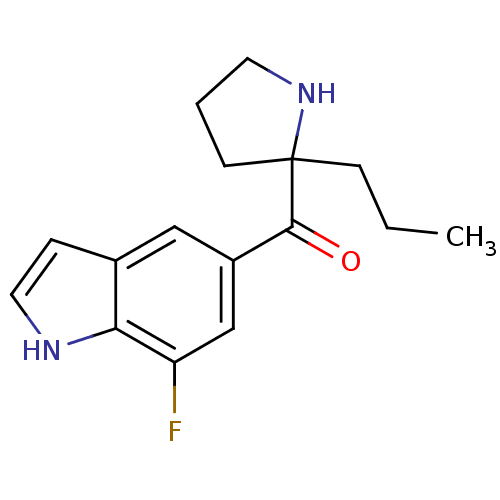
((7-fluoro-1H-indol-5-yl)(2-propylpyrrolidin-2-yl)m...)Show InChI InChI=1S/C16H19FN2O/c1-2-5-16(6-3-7-19-16)15(20)12-9-11-4-8-18-14(11)13(17)10-12/h4,8-10,18-19H,2-3,5-7H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325619
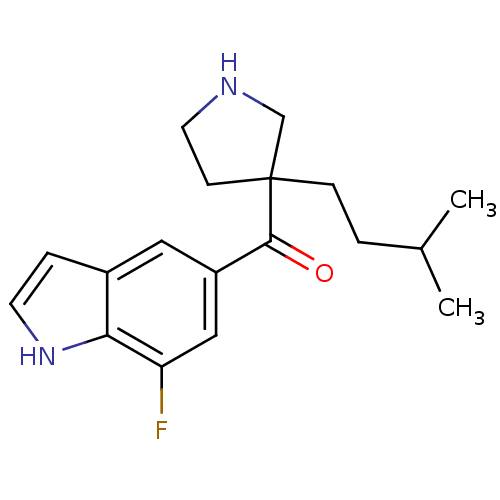
((7-fluoro-1H-indol-5-yl)(3-isopentyl pyrrolidin-3-...)Show InChI InChI=1S/C18H23FN2O/c1-12(2)3-5-18(6-8-20-11-18)17(22)14-9-13-4-7-21-16(13)15(19)10-14/h4,7,9-10,12,20-21H,3,5-6,8,11H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325614
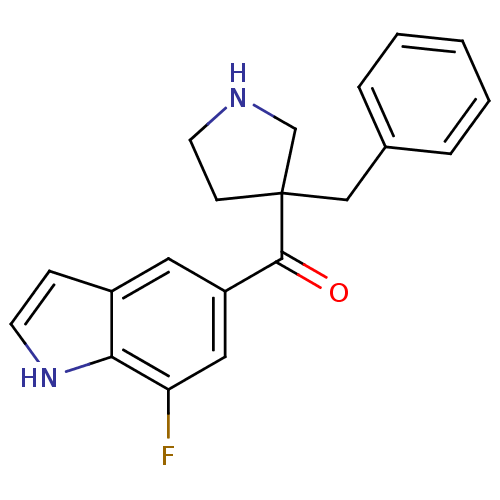
((3-benzyl pyrrolidin-3-yl)(7-fluoro-1H-indol-5-yl)...)Show InChI InChI=1S/C20H19FN2O/c21-17-11-16(10-15-6-8-23-18(15)17)19(24)20(7-9-22-13-20)12-14-4-2-1-3-5-14/h1-6,8,10-11,22-23H,7,9,12-13H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325614

((3-benzyl pyrrolidin-3-yl)(7-fluoro-1H-indol-5-yl)...)Show InChI InChI=1S/C20H19FN2O/c21-17-11-16(10-15-6-8-23-18(15)17)19(24)20(7-9-22-13-20)12-14-4-2-1-3-5-14/h1-6,8,10-11,22-23H,7,9,12-13H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325614

((3-benzyl pyrrolidin-3-yl)(7-fluoro-1H-indol-5-yl)...)Show InChI InChI=1S/C20H19FN2O/c21-17-11-16(10-15-6-8-23-18(15)17)19(24)20(7-9-22-13-20)12-14-4-2-1-3-5-14/h1-6,8,10-11,22-23H,7,9,12-13H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325631
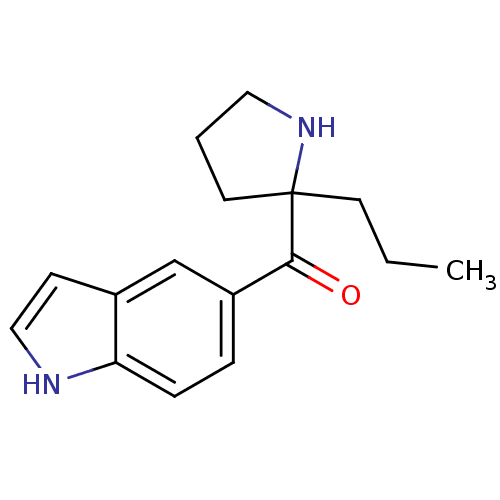
((1H-indol-5-yl)(2-propylpyrrolidin-2-yl)methanone ...)Show InChI InChI=1S/C16H20N2O/c1-2-7-16(8-3-9-18-16)15(19)13-4-5-14-12(11-13)6-10-17-14/h4-6,10-11,17-18H,2-3,7-9H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325639
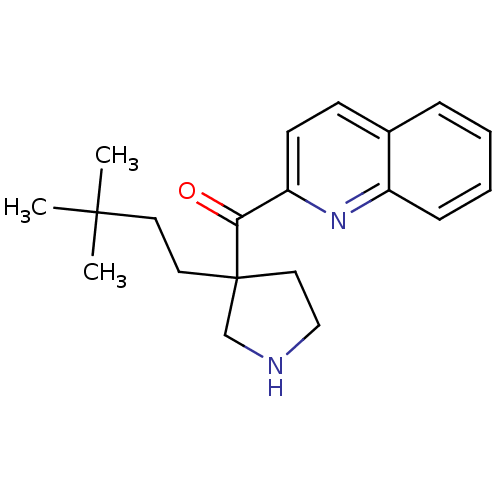
((3-(3,3-dimethylbutyl)pyrrolidin-3-yl)(quinolin-2-...)Show InChI InChI=1S/C20H26N2O/c1-19(2,3)10-11-20(12-13-21-14-20)18(23)17-9-8-15-6-4-5-7-16(15)22-17/h4-9,21H,10-14H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325618
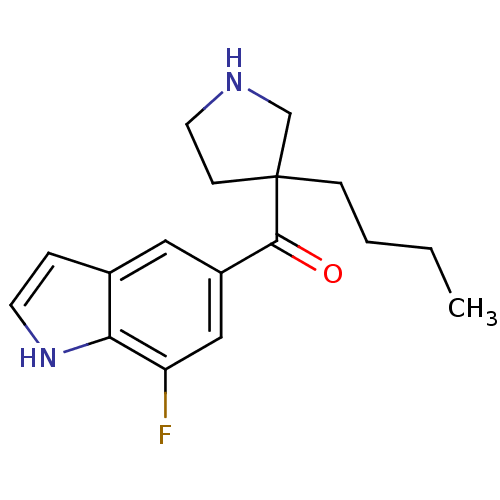
((3-butyl pyrrolidin-3-yl)(7-fluoro-1H-indol-5-yl)m...)Show InChI InChI=1S/C17H21FN2O/c1-2-3-5-17(6-8-19-11-17)16(21)13-9-12-4-7-20-15(12)14(18)10-13/h4,7,9-10,19-20H,2-3,5-6,8,11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325618

((3-butyl pyrrolidin-3-yl)(7-fluoro-1H-indol-5-yl)m...)Show InChI InChI=1S/C17H21FN2O/c1-2-3-5-17(6-8-19-11-17)16(21)13-9-12-4-7-20-15(12)14(18)10-13/h4,7,9-10,19-20H,2-3,5-6,8,11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325616
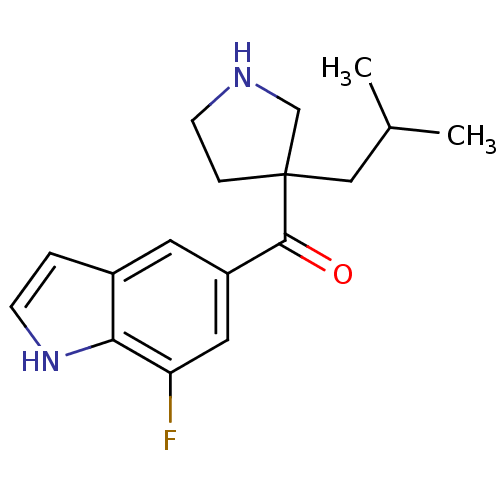
((7-fluoro-1H-indol-5-yl)(3-isobutyl pyrrolidin-3-y...)Show InChI InChI=1S/C17H21FN2O/c1-11(2)9-17(4-6-19-10-17)16(21)13-7-12-3-5-20-15(12)14(18)8-13/h3,5,7-8,11,19-20H,4,6,9-10H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325616

((7-fluoro-1H-indol-5-yl)(3-isobutyl pyrrolidin-3-y...)Show InChI InChI=1S/C17H21FN2O/c1-11(2)9-17(4-6-19-10-17)16(21)13-7-12-3-5-20-15(12)14(18)8-13/h3,5,7-8,11,19-20H,4,6,9-10H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325620
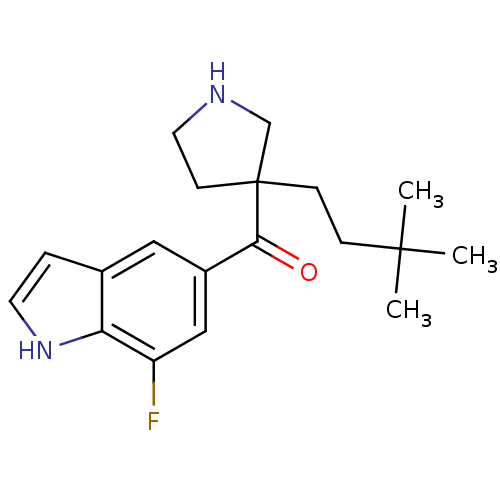
((3-(3,3-dimethylbutyl)pyrrolidin-3-yl)(7-fluoro-1H...)Show SMILES CC(C)(C)CCC1(CCNC1)C(=O)c1cc(F)c2[nH]ccc2c1 Show InChI InChI=1S/C19H25FN2O/c1-18(2,3)5-6-19(7-9-21-12-19)17(23)14-10-13-4-8-22-16(13)15(20)11-14/h4,8,10-11,21-22H,5-7,9,12H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325620

((3-(3,3-dimethylbutyl)pyrrolidin-3-yl)(7-fluoro-1H...)Show SMILES CC(C)(C)CCC1(CCNC1)C(=O)c1cc(F)c2[nH]ccc2c1 Show InChI InChI=1S/C19H25FN2O/c1-18(2,3)5-6-19(7-9-21-12-19)17(23)14-10-13-4-8-22-16(13)15(20)11-14/h4,8,10-11,21-22H,5-7,9,12H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325615
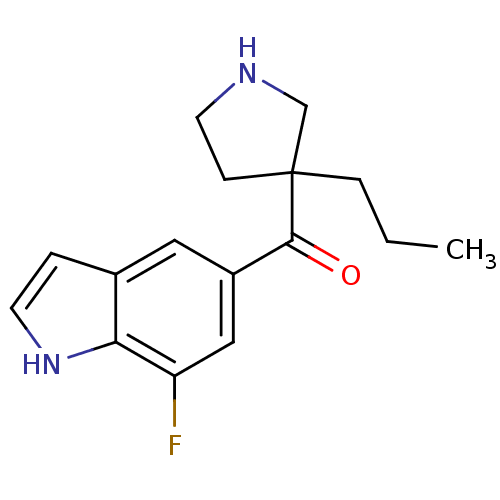
((7-fluoro-1H-indol-5-yl)(3-propyl pyrrolidin-3-yl)...)Show InChI InChI=1S/C16H19FN2O/c1-2-4-16(5-7-18-10-16)15(20)12-8-11-3-6-19-14(11)13(17)9-12/h3,6,8-9,18-19H,2,4-5,7,10H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325615

((7-fluoro-1H-indol-5-yl)(3-propyl pyrrolidin-3-yl)...)Show InChI InChI=1S/C16H19FN2O/c1-2-4-16(5-7-18-10-16)15(20)12-8-11-3-6-19-14(11)13(17)9-12/h3,6,8-9,18-19H,2,4-5,7,10H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325625
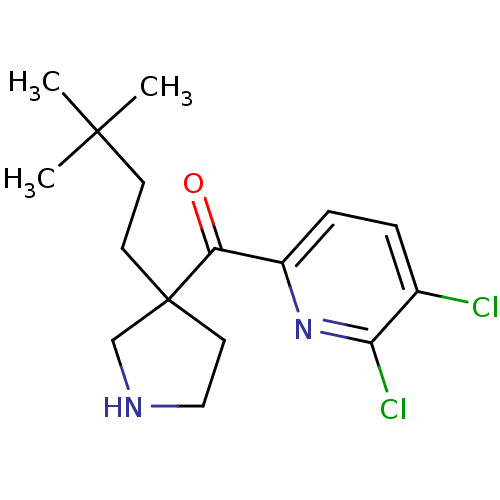
((5,6-dichloropyridin-2-yl)(3-(3,3-dimethylbutyl)py...)Show InChI InChI=1S/C16H22Cl2N2O/c1-15(2,3)6-7-16(8-9-19-10-16)13(21)12-5-4-11(17)14(18)20-12/h4-5,19H,6-10H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50325633
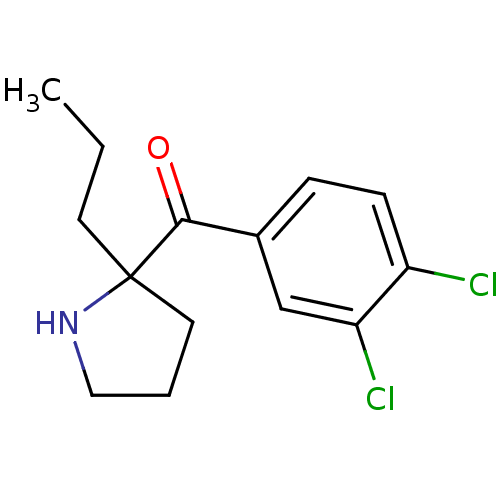
((3,4-dichloro phenyl)(2-propylpyrrolidin-2-yl)meth...)Show InChI InChI=1S/C14H17Cl2NO/c1-2-6-14(7-3-8-17-14)13(18)10-4-5-11(15)12(16)9-10/h4-5,9,17H,2-3,6-8H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325633

((3,4-dichloro phenyl)(2-propylpyrrolidin-2-yl)meth...)Show InChI InChI=1S/C14H17Cl2NO/c1-2-6-14(7-3-8-17-14)13(18)10-4-5-11(15)12(16)9-10/h4-5,9,17H,2-3,6-8H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325633

((3,4-dichloro phenyl)(2-propylpyrrolidin-2-yl)meth...)Show InChI InChI=1S/C14H17Cl2NO/c1-2-6-14(7-3-8-17-14)13(18)10-4-5-11(15)12(16)9-10/h4-5,9,17H,2-3,6-8H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325626
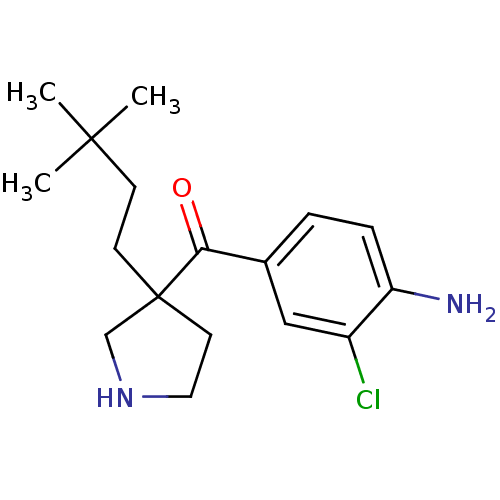
((4-amino-3-chlorophenyl)(3-(3,3-dimethylbutyl)pyrr...)Show InChI InChI=1S/C17H25ClN2O/c1-16(2,3)6-7-17(8-9-20-11-17)15(21)12-4-5-14(19)13(18)10-12/h4-5,10,20H,6-9,11,19H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325617
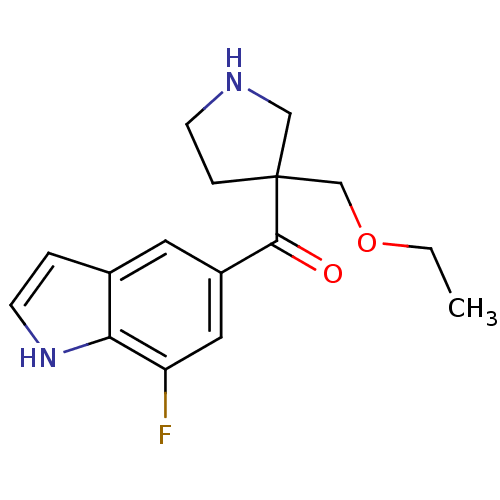
((3-(ethoxymethyl)pyrrolidin-3-yl)(7-fluoro-1H-indo...)Show InChI InChI=1S/C16H19FN2O2/c1-2-21-10-16(4-6-18-9-16)15(20)12-7-11-3-5-19-14(11)13(17)8-12/h3,5,7-8,18-19H,2,4,6,9-10H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50325632

((7-fluoro-1H-indol-5-yl)(2-propylpyrrolidin-2-yl)m...)Show InChI InChI=1S/C16H19FN2O/c1-2-5-16(6-3-7-19-16)15(20)12-9-11-4-8-18-14(11)13(17)10-12/h4,8-10,18-19H,2-3,5-7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325637
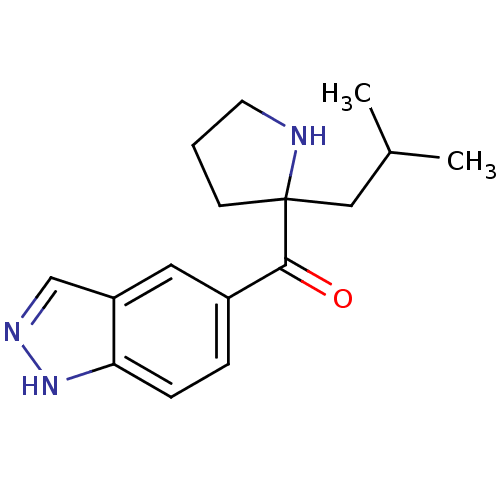
((1H-indazol-5-yl)(2-isobutylpyrrolidin-2-yl)methan...)Show InChI InChI=1S/C16H21N3O/c1-11(2)9-16(6-3-7-17-16)15(20)12-4-5-14-13(8-12)10-18-19-14/h4-5,8,10-11,17H,3,6-7,9H2,1-2H3,(H,18,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325634
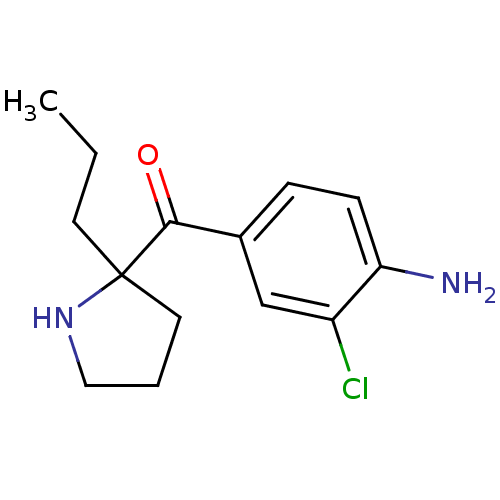
((4-amino-3-chlorophenyl)(2-propylpyrrolidin-2-yl)m...)Show InChI InChI=1S/C14H19ClN2O/c1-2-6-14(7-3-8-17-14)13(18)10-4-5-12(16)11(15)9-10/h4-5,9,17H,2-3,6-8,16H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325622
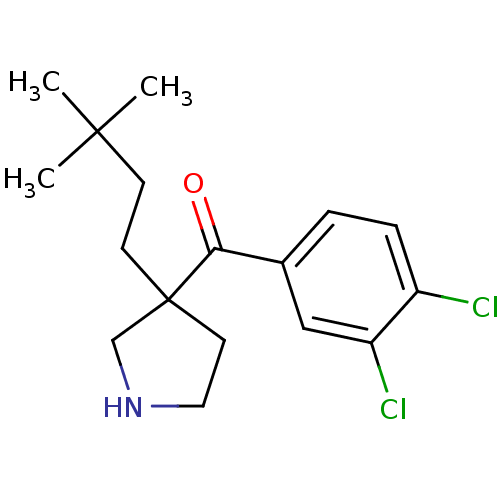
((3,4-dichloro phenyl)(3-(3,3-dimethylbutyl)pyrroli...)Show InChI InChI=1S/C17H23Cl2NO/c1-16(2,3)6-7-17(8-9-20-11-17)15(21)12-4-5-13(18)14(19)10-12/h4-5,10,20H,6-9,11H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325622

((3,4-dichloro phenyl)(3-(3,3-dimethylbutyl)pyrroli...)Show InChI InChI=1S/C17H23Cl2NO/c1-16(2,3)6-7-17(8-9-20-11-17)15(21)12-4-5-13(18)14(19)10-12/h4-5,10,20H,6-9,11H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325638
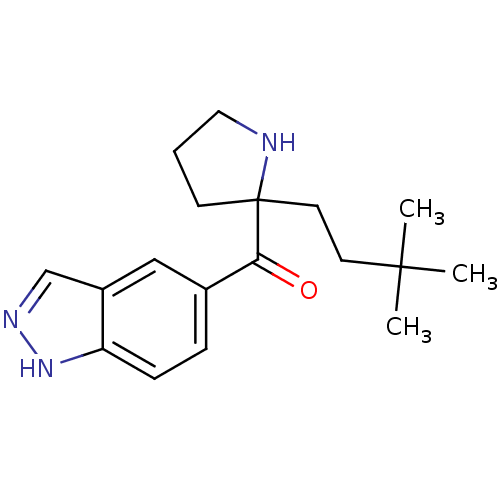
((2-(3,3-dimethylbutyl)pyrrolidin-2-yl)(1H-indazol-...)Show InChI InChI=1S/C18H25N3O/c1-17(2,3)8-9-18(7-4-10-19-18)16(22)13-5-6-15-14(11-13)12-20-21-15/h5-6,11-12,19H,4,7-10H2,1-3H3,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325640
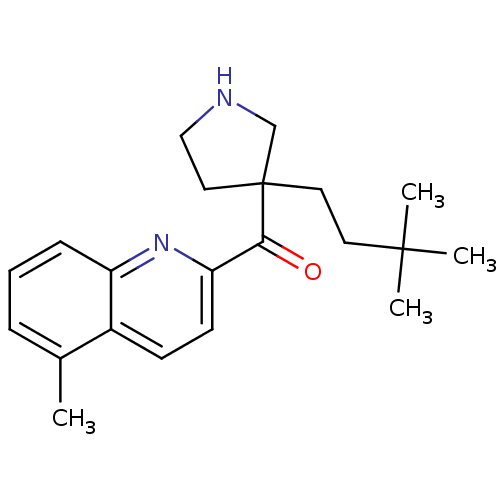
((3-(3,3-dimethylbutyl)pyrrolidin-3-yl)(5-methylqui...)Show InChI InChI=1S/C21H28N2O/c1-15-6-5-7-17-16(15)8-9-18(23-17)19(24)21(12-13-22-14-21)11-10-20(2,3)4/h5-9,22H,10-14H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50325637

((1H-indazol-5-yl)(2-isobutylpyrrolidin-2-yl)methan...)Show InChI InChI=1S/C16H21N3O/c1-11(2)9-16(6-3-7-17-16)15(20)12-4-5-14-13(8-12)10-18-19-14/h4-5,8,10-11,17H,3,6-7,9H2,1-2H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50325638

((2-(3,3-dimethylbutyl)pyrrolidin-2-yl)(1H-indazol-...)Show InChI InChI=1S/C18H25N3O/c1-17(2,3)8-9-18(7-4-10-19-18)16(22)13-5-6-15-14(11-13)12-20-21-15/h5-6,11-12,19H,4,7-10H2,1-3H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50325636
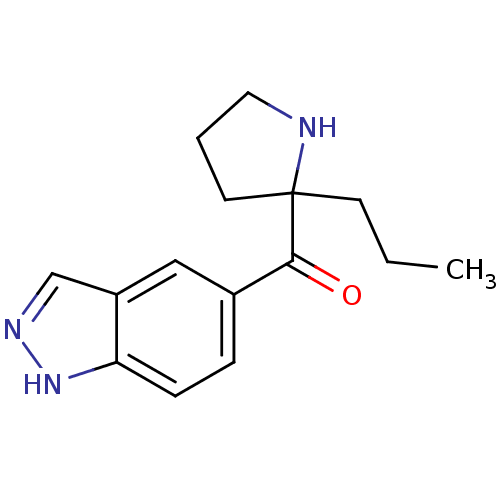
((1H-indazol-5-yl)(2-propylpyrrolidin-2-yl)methanon...)Show InChI InChI=1S/C15H19N3O/c1-2-6-15(7-3-8-16-15)14(19)11-4-5-13-12(9-11)10-17-18-13/h4-5,9-10,16H,2-3,6-8H2,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50325635
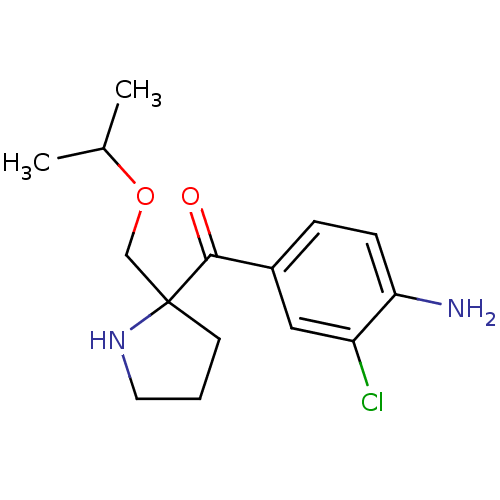
((4-amino-3-chlorophenyl)(2-(isopropoxymethyl)pyrro...)Show InChI InChI=1S/C15H21ClN2O2/c1-10(2)20-9-15(6-3-7-18-15)14(19)11-4-5-13(17)12(16)8-11/h4-5,8,10,18H,3,6-7,9,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50325631

((1H-indol-5-yl)(2-propylpyrrolidin-2-yl)methanone ...)Show InChI InChI=1S/C16H20N2O/c1-2-7-16(8-3-9-18-16)15(19)13-4-5-14-12(11-13)6-10-17-14/h4-6,10-11,17-18H,2-3,7-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50325634

((4-amino-3-chlorophenyl)(2-propylpyrrolidin-2-yl)m...)Show InChI InChI=1S/C14H19ClN2O/c1-2-6-14(7-3-8-17-14)13(18)10-4-5-12(16)11(15)9-10/h4-5,9,17H,2-3,6-8,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325629
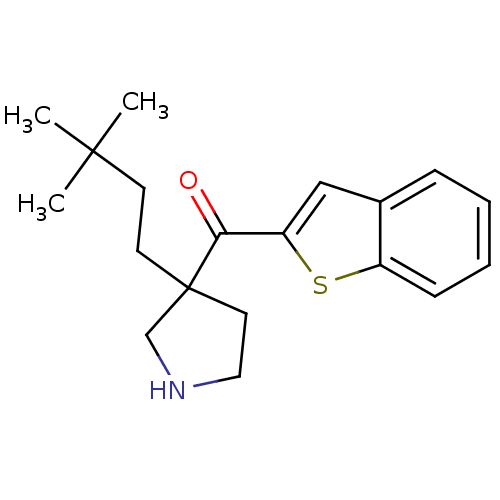
(CHEMBL1224237 | benzo[b]thiophen-2-yl(3-(3,3-dimet...)Show InChI InChI=1S/C19H25NOS/c1-18(2,3)8-9-19(10-11-20-13-19)17(21)16-12-14-6-4-5-7-15(14)22-16/h4-7,12,20H,8-11,13H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325641
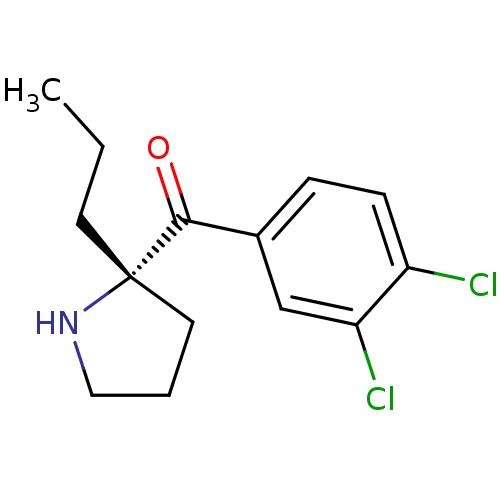
((S)-(3,4-dichlorophenyl)(2-propylpyrrolidin-2-yl)m...)Show InChI InChI=1S/C14H17Cl2NO/c1-2-6-14(7-3-8-17-14)13(18)10-4-5-11(15)12(16)9-10/h4-5,9,17H,2-3,6-8H2,1H3/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325642
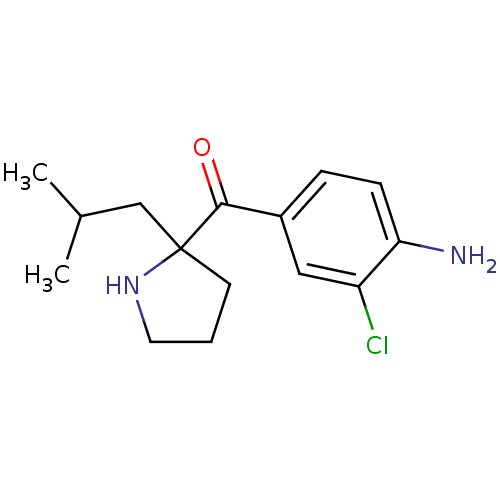
((4-amino-3-chlorophenyl)(2-isobutylpyrrolidin-2-yl...)Show InChI InChI=1S/C15H21ClN2O/c1-10(2)9-15(6-3-7-18-15)14(19)11-4-5-13(17)12(16)8-11/h4-5,8,10,18H,3,6-7,9,17H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325623
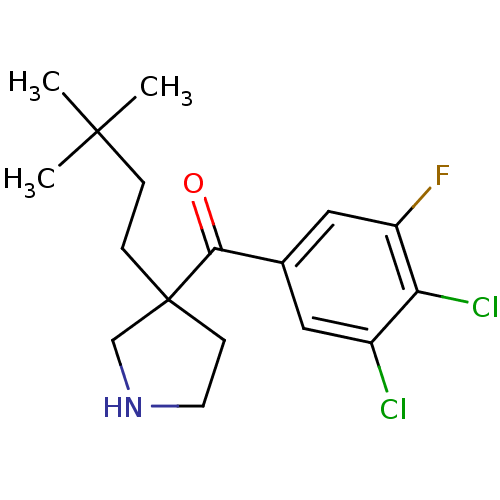
((3,4-dichloro-5-fluorophenyl)(3-(3,3-dimethylbutyl...)Show SMILES CC(C)(C)CCC1(CCNC1)C(=O)c1cc(F)c(Cl)c(Cl)c1 Show InChI InChI=1S/C17H22Cl2FNO/c1-16(2,3)4-5-17(6-7-21-10-17)15(22)11-8-12(18)14(19)13(20)9-11/h8-9,21H,4-7,10H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325621
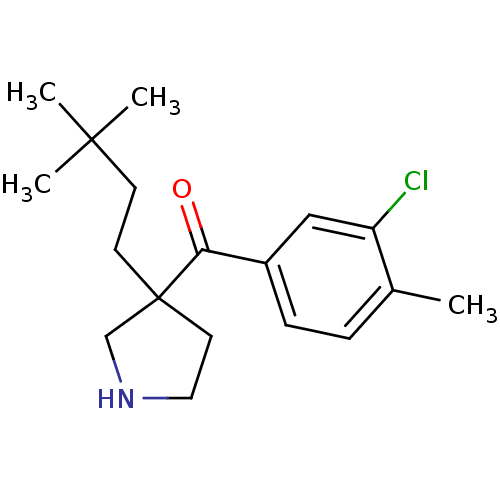
((3-chloro-4-methylphenyl)(3-(3,3-dimethylbutyl)pyr...)Show InChI InChI=1S/C18H26ClNO/c1-13-5-6-14(11-15(13)19)16(21)18(9-10-20-12-18)8-7-17(2,3)4/h5-6,11,20H,7-10,12H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325628
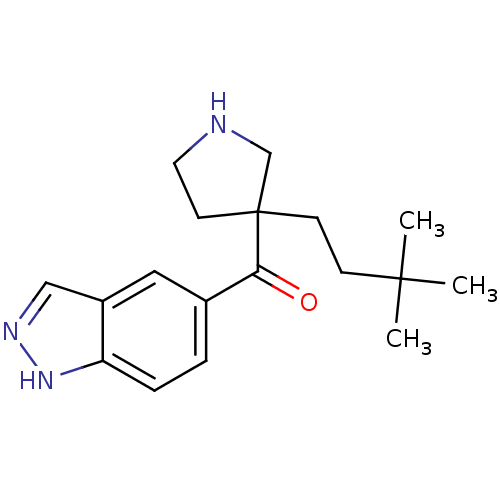
((3-(3,3-dimethylbutyl)pyrrolidin-3-yl)(1H-indazol-...)Show InChI InChI=1S/C18H25N3O/c1-17(2,3)6-7-18(8-9-19-12-18)16(22)13-4-5-15-14(10-13)11-20-21-15/h4-5,10-11,19H,6-9,12H2,1-3H3,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325635

((4-amino-3-chlorophenyl)(2-(isopropoxymethyl)pyrro...)Show InChI InChI=1S/C15H21ClN2O2/c1-10(2)20-9-15(6-3-7-18-15)14(19)11-4-5-13(17)12(16)8-11/h4-5,8,10,18H,3,6-7,9,17H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325636

((1H-indazol-5-yl)(2-propylpyrrolidin-2-yl)methanon...)Show InChI InChI=1S/C15H19N3O/c1-2-6-15(7-3-8-16-15)14(19)11-4-5-13-12(9-11)10-17-18-13/h4-5,9-10,16H,2-3,6-8H2,1H3,(H,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325624
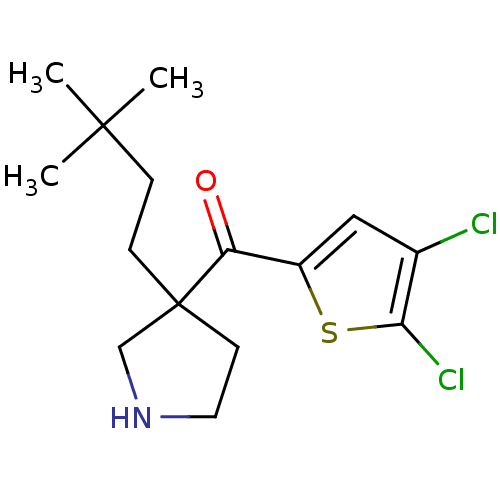
((4,5-dichlorothiophen-2-yl)(3-(3,3-dimethylbutyl)p...)Show InChI InChI=1S/C15H21Cl2NOS/c1-14(2,3)4-5-15(6-7-18-9-15)12(19)11-8-10(16)13(17)20-11/h8,18H,4-7,9H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325630
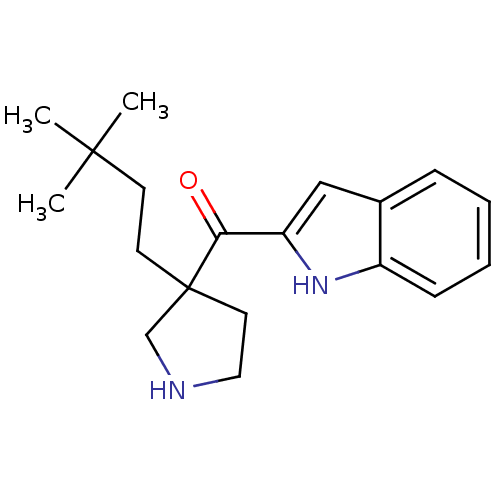
((3-(3,3-dimethylbutyl)pyrrolidin-3-yl)(1H-indol-2-...)Show InChI InChI=1S/C19H26N2O/c1-18(2,3)8-9-19(10-11-20-13-19)17(22)16-12-14-6-4-5-7-15(14)21-16/h4-7,12,20-21H,8-11,13H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50325627
((6-amino-5-chloropyridin-3-yl)(3-(3,3-dimethylbuty...)Show InChI InChI=1S/C16H24ClN3O/c1-15(2,3)4-5-16(6-7-19-10-16)13(21)11-8-12(17)14(18)20-9-11/h8-9,19H,4-7,10H2,1-3H3,(H2,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 5559-66 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.020
BindingDB Entry DOI: 10.7270/Q20865HF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data