 Found 16 hits of Enzyme Inhibition Constant Data
Found 16 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326399

((+/-)-trans-4-Butyl-1-[(9-deazaadenin-9-yl)methyl]...)Show SMILES CCCC[C@H]1CN(Cc2c[nH]c3c(N)ncnc23)C[C@@H]1O |r| Show InChI InChI=1S/C15H23N5O/c1-2-3-4-10-6-20(8-12(10)21)7-11-5-17-14-13(11)18-9-19-15(14)16/h5,9-10,12,17,21H,2-4,6-8H2,1H3,(H2,16,18,19)/t10-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326399

((+/-)-trans-4-Butyl-1-[(9-deazaadenin-9-yl)methyl]...)Show SMILES CCCC[C@H]1CN(Cc2c[nH]c3c(N)ncnc23)C[C@@H]1O |r| Show InChI InChI=1S/C15H23N5O/c1-2-3-4-10-6-20(8-12(10)21)7-11-5-17-14-13(11)18-9-19-15(14)16/h5,9-10,12,17,21H,2-4,6-8H2,1H3,(H2,16,18,19)/t10-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326400
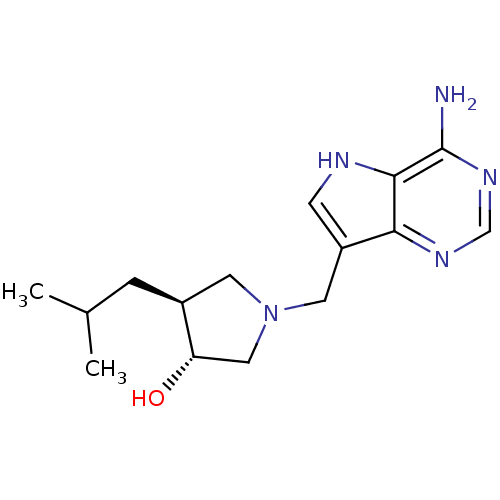
((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-3-hydro...)Show SMILES CC(C)C[C@H]1CN(Cc2c[nH]c3c(N)ncnc23)C[C@@H]1O |r| Show InChI InChI=1S/C15H23N5O/c1-9(2)3-10-5-20(7-12(10)21)6-11-4-17-14-13(11)18-8-19-15(14)16/h4,8-10,12,17,21H,3,5-7H2,1-2H3,(H2,16,18,19)/t10-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326402

((+/-)-trans-4-Cyclopropyl-1-[(9-deazaadenin-9-yl)m...)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@H](C3)C3CC3)c[nH]c12 |r| Show InChI InChI=1S/C14H19N5O/c15-14-13-12(17-7-18-14)9(3-16-13)4-19-5-10(8-1-2-8)11(20)6-19/h3,7-8,10-11,16,20H,1-2,4-6H2,(H2,15,17,18)/t10-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326403
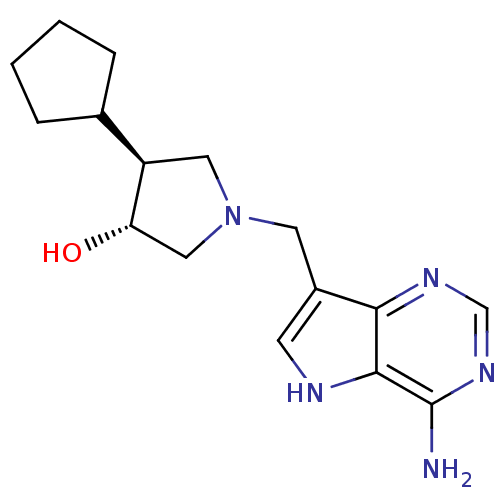
((+/-)-trans-4-Cyclopentyl-1-[(9-deazaadenin-9-yl)m...)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@H](C3)C3CCCC3)c[nH]c12 |r| Show InChI InChI=1S/C16H23N5O/c17-16-15-14(19-9-20-16)11(5-18-15)6-21-7-12(13(22)8-21)10-3-1-2-4-10/h5,9-10,12-13,18,22H,1-4,6-8H2,(H2,17,19,20)/t12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326407
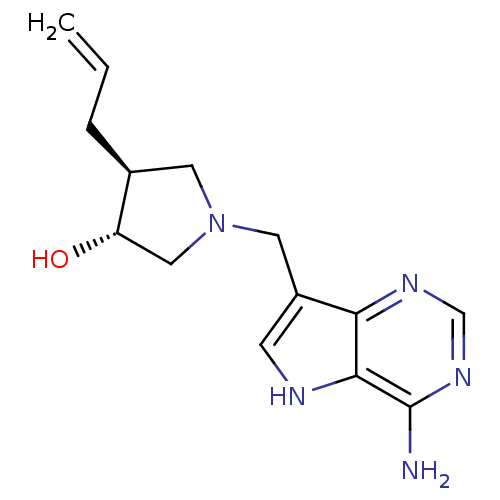
((+/-)-trans-4-Allyl-1-[(9-deazaadenin-9-yl)methyl]...)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@@H](CC=C)C3)c[nH]c12 |r| Show InChI InChI=1S/C14H19N5O/c1-2-3-9-5-19(7-11(9)20)6-10-4-16-13-12(10)17-8-18-14(13)15/h2,4,8-9,11,16,20H,1,3,5-7H2,(H2,15,17,18)/t9-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326406
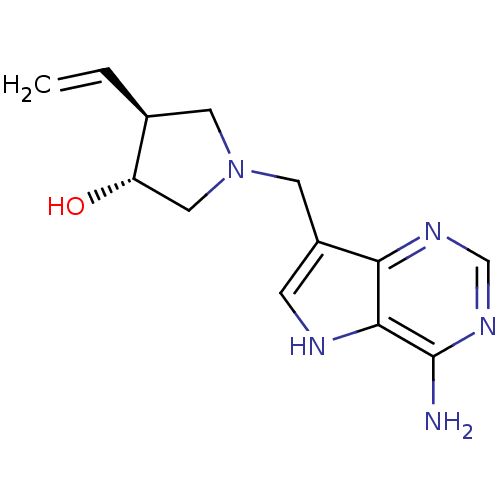
((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-3-hydro...)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@H](C3)C=C)c[nH]c12 |r| Show InChI InChI=1S/C13H17N5O/c1-2-8-4-18(6-10(8)19)5-9-3-15-12-11(9)16-7-17-13(12)14/h2-3,7-8,10,15,19H,1,4-6H2,(H2,14,16,17)/t8-,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326398

((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-4-ethyl...)Show SMILES CC[C@H]1CN(Cc2c[nH]c3c(N)ncnc23)C[C@@H]1O |r| Show InChI InChI=1S/C13H19N5O/c1-2-8-4-18(6-10(8)19)5-9-3-15-12-11(9)16-7-17-13(12)14/h3,7-8,10,15,19H,2,4-6H2,1H3,(H2,14,16,17)/t8-,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326398

((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-4-ethyl...)Show SMILES CC[C@H]1CN(Cc2c[nH]c3c(N)ncnc23)C[C@@H]1O |r| Show InChI InChI=1S/C13H19N5O/c1-2-8-4-18(6-10(8)19)5-9-3-15-12-11(9)16-7-17-13(12)14/h3,7-8,10,15,19H,2,4-6H2,1H3,(H2,14,16,17)/t8-,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326405
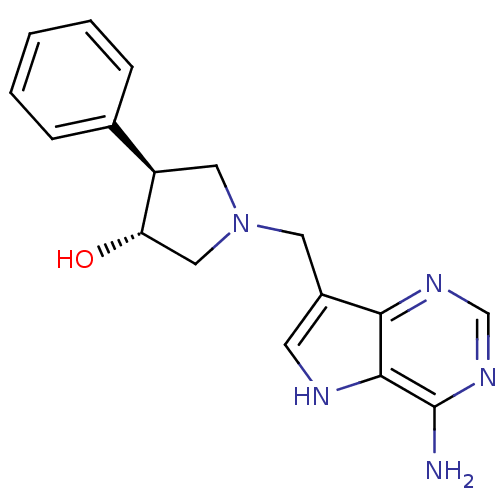
((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-3-hydro...)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@H](C3)c3ccccc3)c[nH]c12 |r| Show InChI InChI=1S/C17H19N5O/c18-17-16-15(20-10-21-17)12(6-19-16)7-22-8-13(14(23)9-22)11-4-2-1-3-5-11/h1-6,10,13-14,19,23H,7-9H2,(H2,18,20,21)/t13-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326404
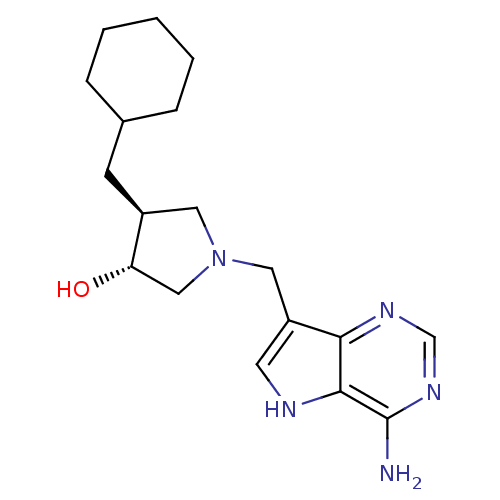
((+/-)-trans-4-(Cyclohexylmethyl)-1-[(9-deaza-adeni...)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@@H](CC4CCCCC4)C3)c[nH]c12 |r| Show InChI InChI=1S/C18H27N5O/c19-18-17-16(21-11-22-18)14(7-20-17)9-23-8-13(15(24)10-23)6-12-4-2-1-3-5-12/h7,11-13,15,20,24H,1-6,8-10H2,(H2,19,21,22)/t13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326401

((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-3-hydro...)Show SMILES CCC(CC)C[C@H]1CN(Cc2c[nH]c3c(N)ncnc23)C[C@@H]1O |r| Show InChI InChI=1S/C17H27N5O/c1-3-11(4-2)5-12-7-22(9-14(12)23)8-13-6-19-16-15(13)20-10-21-17(16)18/h6,10-12,14,19,23H,3-5,7-9H2,1-2H3,(H2,18,20,21)/t12-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326408
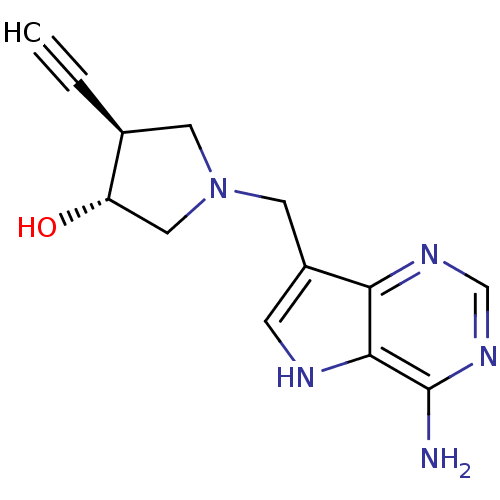
((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-4-ethyn...)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@H](C3)C#C)c[nH]c12 |r| Show InChI InChI=1S/C13H15N5O/c1-2-8-4-18(6-10(8)19)5-9-3-15-12-11(9)16-7-17-13(12)14/h1,3,7-8,10,15,19H,4-6H2,(H2,14,16,17)/t8-,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326411

((+/-)-Benzyl cis-3-(Benzoyloxy)-4-ethylpyrrolidine...)Show SMILES CC[C@H]1CN(Cc2c[nH]c3c(N)ncnc23)C[C@H]1O |r| Show InChI InChI=1S/C13H19N5O/c1-2-8-4-18(6-10(8)19)5-9-3-15-12-11(9)16-7-17-13(12)14/h3,7-8,10,15,19H,2,4-6H2,1H3,(H2,14,16,17)/t8-,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326409

((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-3-hydro...)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@H](C3)n3ccnn3)c[nH]c12 |r| Show InChI InChI=1S/C13H16N8O/c14-13-12-11(16-7-17-13)8(3-15-12)4-20-5-9(10(22)6-20)21-2-1-18-19-21/h1-3,7,9-10,15,22H,4-6H2,(H2,14,16,17)/t9-,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326410

((+/-)-trans-4-[3-(Benzylthio)propyl]-1-[(9-deazaad...)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@@H](CCCSCc4ccccc4)C3)c[nH]c12 |r| Show InChI InChI=1S/C21H27N5OS/c22-21-20-19(24-14-25-21)17(9-23-20)11-26-10-16(18(27)12-26)7-4-8-28-13-15-5-2-1-3-6-15/h1-3,5-6,9,14,16,18,23,27H,4,7-8,10-13H2,(H2,22,24,25)/t16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data