

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325848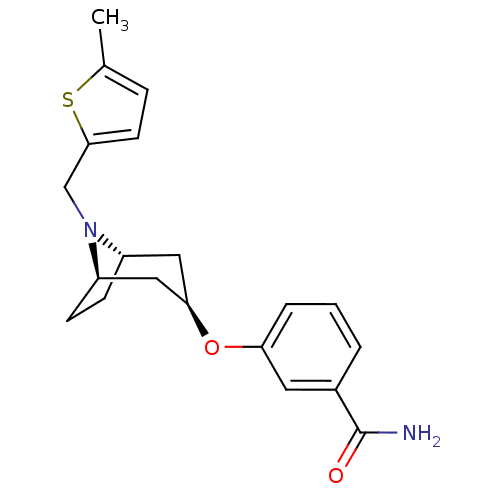 (8-azabicyclo[3.2.1]octan-3-yloxy-benzamide | CHEMB...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in HEK cells assessed as inhibition of SNC-80-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325853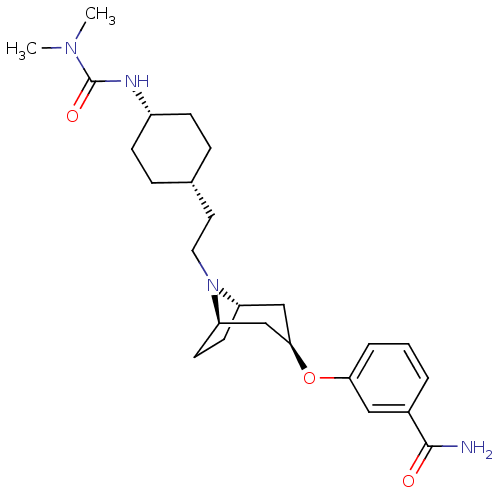 (CHEMBL1223872 | cis-3-((1R,3R,5S)-8-(2-((4S)-4-(3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325849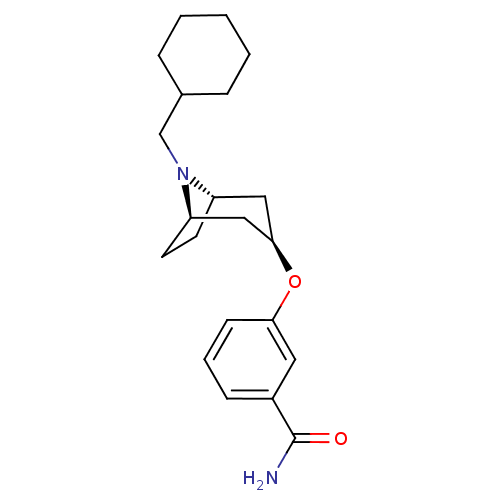 (3-((1R,3r,5S)-8-(cyclohexylmethyl)-8-azabicyclo[3....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 204 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 212 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in HEK cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325866 (CHEMBL1224139 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 222 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325857 (CHEMBL1223953 | exo-3-(((1R,5S)-8-benzyl-8-azabicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325848 (8-azabicyclo[3.2.1]octan-3-yloxy-benzamide | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325854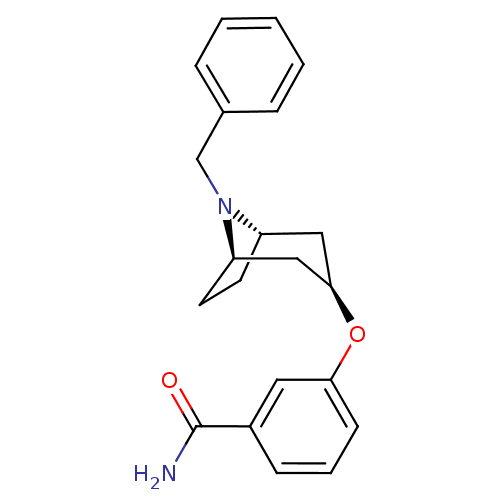 (CHEMBL1223873 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 286 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325858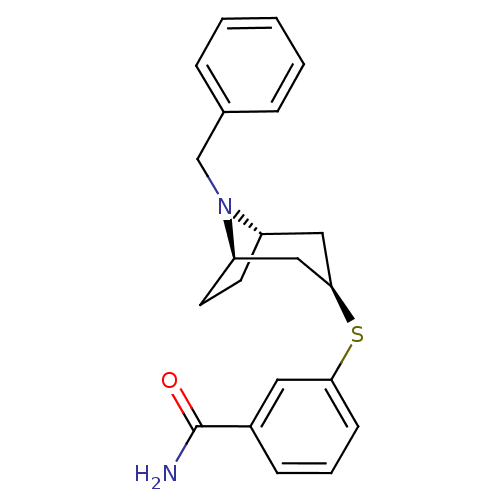 (CHEMBL1223954 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 338 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325878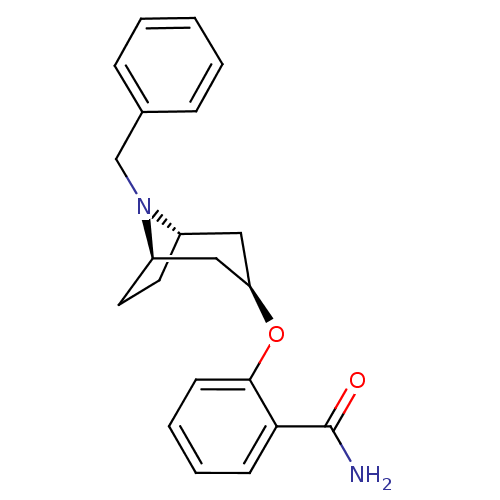 (CHEMBL1224213 | endo-2-((1R,5S)-8-benzyl-8-azabicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325877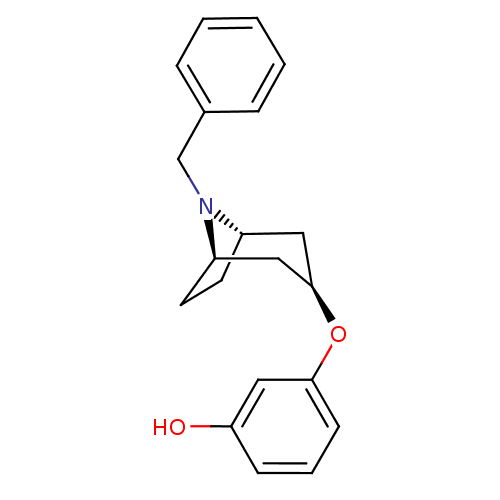 (CHEMBL1224288 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 347 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50325871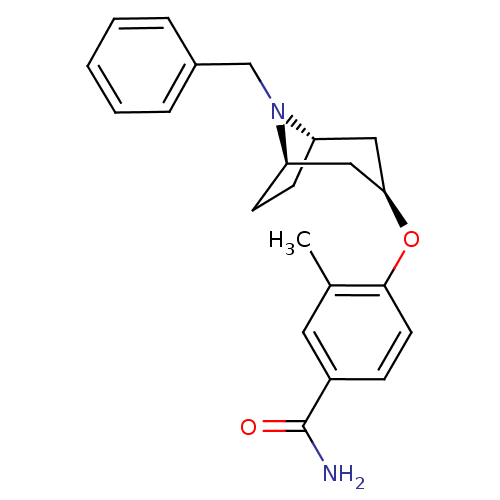 (CHEMBL1224215 | endo-4-((1R,5S)-8-benzyl-8-azabicy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 419 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in HEK cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325851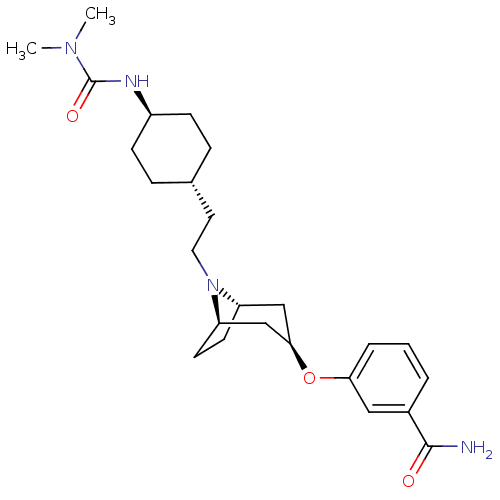 (CHEMBL1223870 | trans-3-((1R,3R,5S)-8-(2-((4R)-4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 451 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325871 (CHEMBL1224215 | endo-4-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 462 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325856 (CHEMBL1223952 | endo-3-(((1R,5S)-8-benzyl-8-azabic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 596 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325854 (CHEMBL1223873 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325867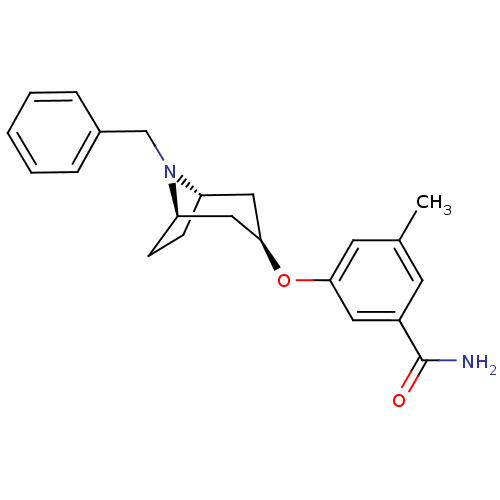 (CHEMBL1224140 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325870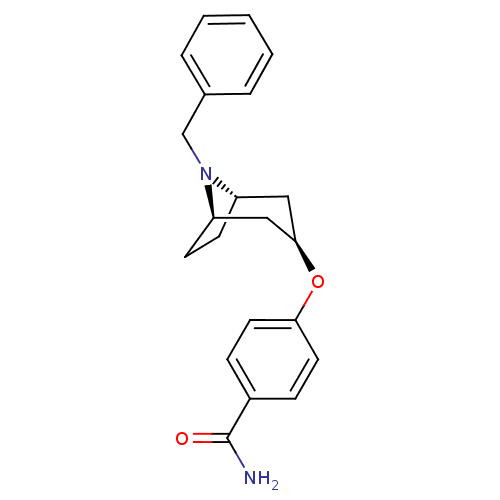 (CHEMBL1224214 | endo-4-((1R,5S)-8-benzyl-8-azabicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325866 (CHEMBL1224139 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50325848 (8-azabicyclo[3.2.1]octan-3-yloxy-benzamide | CHEMB...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 721 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in HEK cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325861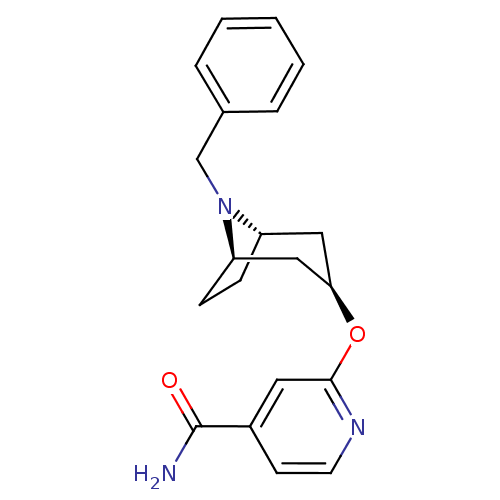 (CHEMBL1224066 | endo-2-((1R,5S)-8-benzyl-8-azabicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325864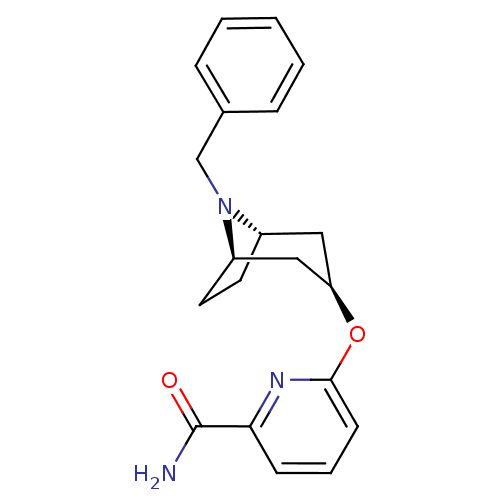 (CHEMBL1224069 | endo-6-((1R,5S)-8-benzyl-8-azabicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325871 (CHEMBL1224215 | endo-4-((1R,5S)-8-benzyl-8-azabicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325858 (CHEMBL1223954 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325869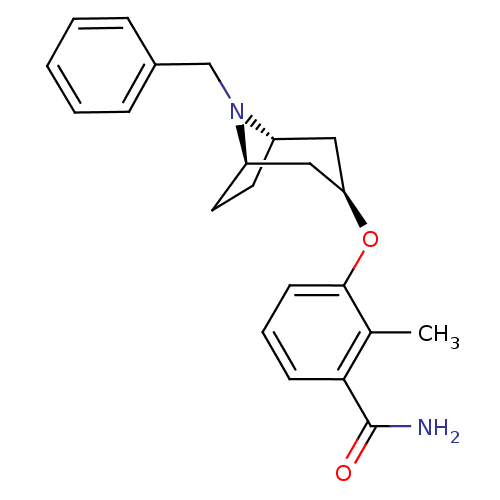 (CHEMBL1224142 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325860 (CHEMBL1224065 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325873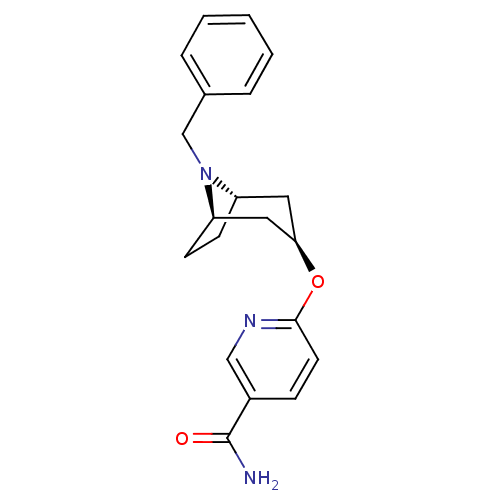 (CHEMBL1224217 | endo-6-((1R,5S)-8-benzyl-8-azabicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325850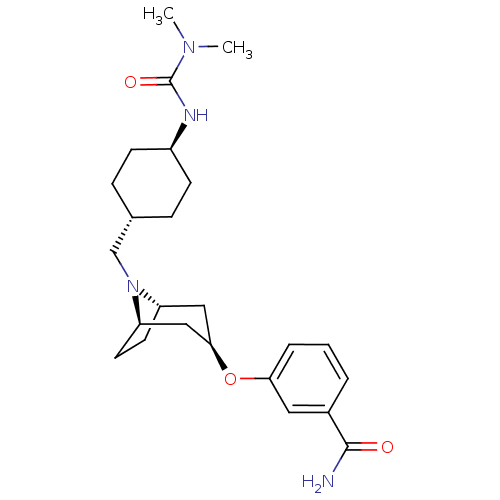 (CHEMBL1223817 | trans-3-((1R,3R,5S)-8-(((4R)-4-(3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325872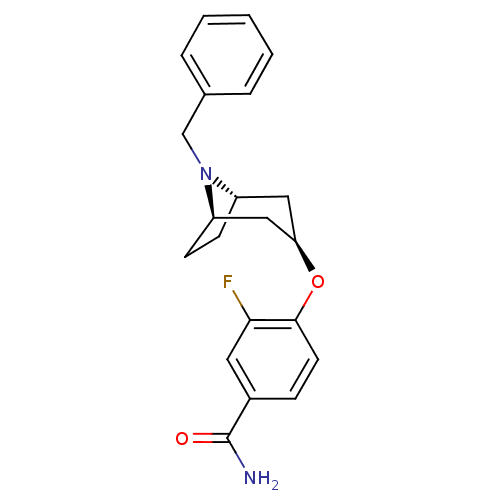 (CHEMBL1224216 | endo-4-((1R,5S)-8-benzyl-8-azabicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325860 (CHEMBL1224065 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325856 (CHEMBL1223952 | endo-3-(((1R,5S)-8-benzyl-8-azabic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325863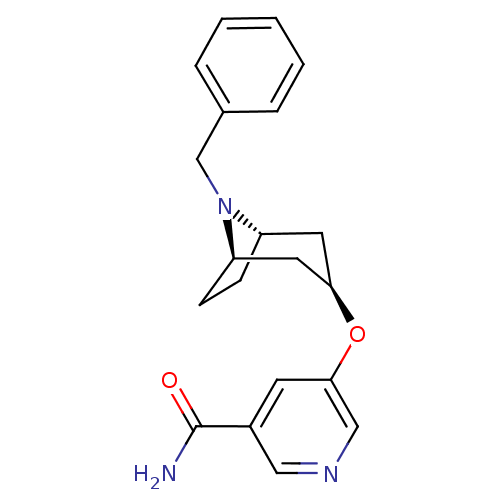 (CHEMBL1224068 | endo-5-((1R,5S)-8-benzyl-8-azabicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325877 (CHEMBL1224288 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325862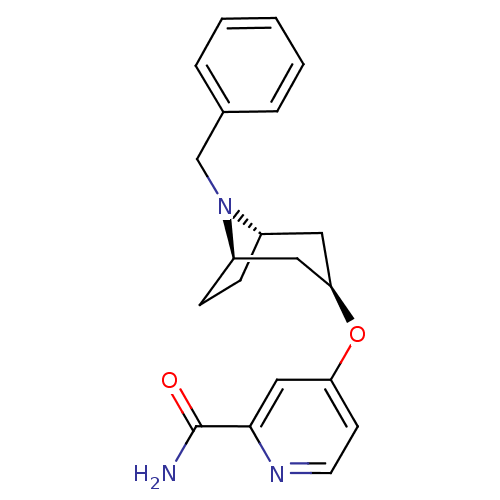 (CHEMBL1224067 | endo-4-((1R,5S)-8-benzyl-8-azabicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325867 (CHEMBL1224140 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50325849 (3-((1R,3r,5S)-8-(cyclohexylmethyl)-8-azabicyclo[3....) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in HEK cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325849 (3-((1R,3r,5S)-8-(cyclohexylmethyl)-8-azabicyclo[3....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325857 (CHEMBL1223953 | exo-3-(((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325872 (CHEMBL1224216 | endo-4-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325855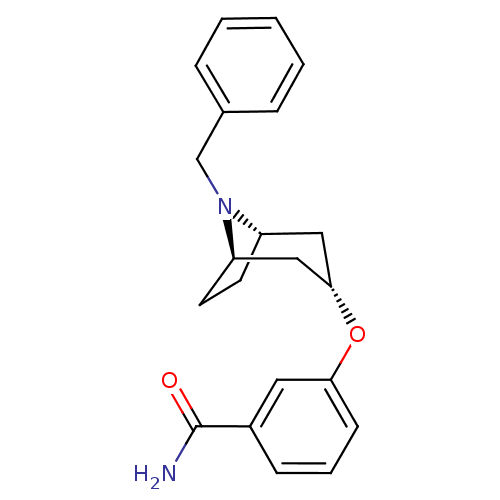 (CHEMBL1223951 | exo-3-((1R,3s,5S)-8-benzyl-8-azabi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50325858 (CHEMBL1223954 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in HEK cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325868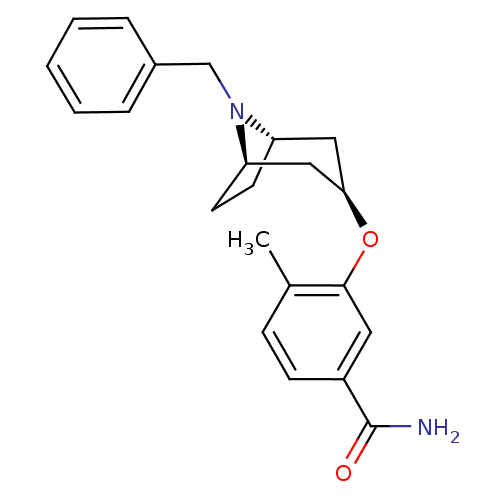 (CHEMBL1224141 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50325856 (CHEMBL1223952 | endo-3-(((1R,5S)-8-benzyl-8-azabic...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in HEK cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325869 (CHEMBL1224142 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325852 (CHEMBL1223871 | cis-3-((1R,3R,5S)-8-(((4S)-4-(3,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50325866 (CHEMBL1224139 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in HEK cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50325872 (CHEMBL1224216 | endo-4-((1R,5S)-8-benzyl-8-azabicy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in HEK cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325859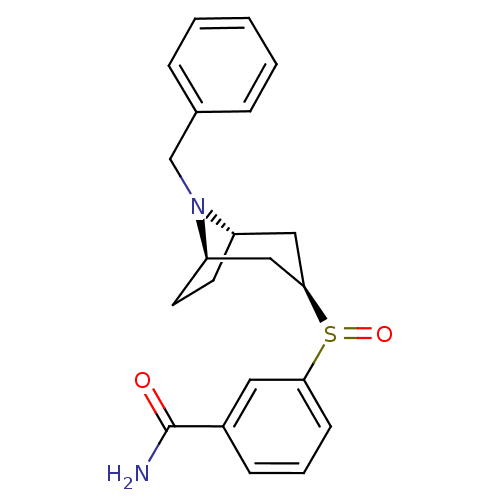 (CHEMBL1223955 | endo-3-((R)-((1R,5S)-8-benzyl-8-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50325848 (8-azabicyclo[3.2.1]octan-3-yloxy-benzamide | CHEMB...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in HEK cells assessed as inhibition of SNC-80-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325861 (CHEMBL1224066 | endo-2-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325855 (CHEMBL1223951 | exo-3-((1R,3s,5S)-8-benzyl-8-azabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50325854 (CHEMBL1223873 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in HEK cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325870 (CHEMBL1224214 | endo-4-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50325857 (CHEMBL1223953 | exo-3-(((1R,5S)-8-benzyl-8-azabicy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in HEK cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325862 (CHEMBL1224067 | endo-4-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50325860 (CHEMBL1224065 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in HEK cells assessed as inhibition of SNC-80-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325865 (CHEMBL1224138 | endo-6-((1R,5S)-8-benzyl-8-azabicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50325853 (CHEMBL1223872 | cis-3-((1R,3R,5S)-8-(2-((4S)-4-(3,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in HEK cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50325867 (CHEMBL1224140 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in HEK cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50325860 (CHEMBL1224065 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in HEK cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325863 (CHEMBL1224068 | endo-5-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50325852 (CHEMBL1223871 | cis-3-((1R,3R,5S)-8-(((4S)-4-(3,3-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in HEK cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50325851 (CHEMBL1223870 | trans-3-((1R,3R,5S)-8-(2-((4R)-4-(...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in HEK cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325873 (CHEMBL1224217 | endo-6-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325859 (CHEMBL1223955 | endo-3-((R)-((1R,5S)-8-benzyl-8-az...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50325877 (CHEMBL1224288 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in HEK cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50325868 (CHEMBL1224141 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in HEK cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50325857 (CHEMBL1223953 | exo-3-(((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in HEK cells assessed as inhibition of SNC-80-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50325869 (CHEMBL1224142 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in HEK cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50325854 (CHEMBL1223873 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in HEK cells assessed as inhibition of SNC-80-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50325858 (CHEMBL1223954 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in HEK cells assessed as inhibition of SNC-80-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50325856 (CHEMBL1223952 | endo-3-(((1R,5S)-8-benzyl-8-azabic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in HEK cells assessed as inhibition of SNC-80-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50325855 (CHEMBL1223951 | exo-3-((1R,3s,5S)-8-benzyl-8-azabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in HEK cells assessed as inhibition of SNC-80-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50325853 (CHEMBL1223872 | cis-3-((1R,3R,5S)-8-(2-((4S)-4-(3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in HEK cells assessed as inhibition of SNC-80-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50325852 (CHEMBL1223871 | cis-3-((1R,3R,5S)-8-(((4S)-4-(3,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in HEK cells assessed as inhibition of SNC-80-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325874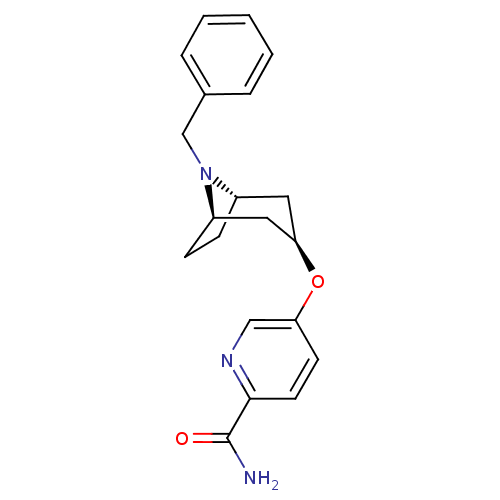 (CHEMBL1224285 | endo-5-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325876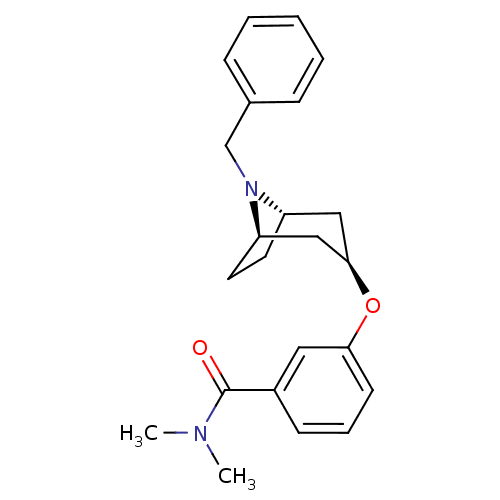 (CHEMBL1224287 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50325851 (CHEMBL1223870 | trans-3-((1R,3R,5S)-8-(2-((4R)-4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in HEK cells assessed as inhibition of SNC-80-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50325859 (CHEMBL1223955 | endo-3-((R)-((1R,5S)-8-benzyl-8-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in HEK cells assessed as inhibition of SNC-80-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50325849 (3-((1R,3r,5S)-8-(cyclohexylmethyl)-8-azabicyclo[3....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in HEK cells assessed as inhibition of SNC-80-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325878 (CHEMBL1224213 | endo-2-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325865 (CHEMBL1224138 | endo-6-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325875 (CHEMBL1224286 | endo-3-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325864 (CHEMBL1224069 | endo-6-((1R,5S)-8-benzyl-8-azabicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in HEK cells assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50325850 (CHEMBL1223817 | trans-3-((1R,3R,5S)-8-(((4R)-4-(3,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in HEK cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50325850 (CHEMBL1223817 | trans-3-((1R,3R,5S)-8-(((4R)-4-(3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in HEK cells assessed as inhibition of SNC-80-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50325861 (CHEMBL1224066 | endo-2-((1R,5S)-8-benzyl-8-azabicy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in HEK cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325852 (CHEMBL1223871 | cis-3-((1R,3R,5S)-8-(((4S)-4-(3,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325851 (CHEMBL1223870 | trans-3-((1R,3R,5S)-8-(2-((4R)-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325853 (CHEMBL1223872 | cis-3-((1R,3R,5S)-8-(2-((4S)-4-(3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50325850 (CHEMBL1223817 | trans-3-((1R,3R,5S)-8-(((4R)-4-(3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | Bioorg Med Chem Lett 20: 5405-10 (2010) Article DOI: 10.1016/j.bmcl.2010.07.112 BindingDB Entry DOI: 10.7270/Q2X92BHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||