 Found 90 hits of Enzyme Inhibition Constant Data
Found 90 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50335574
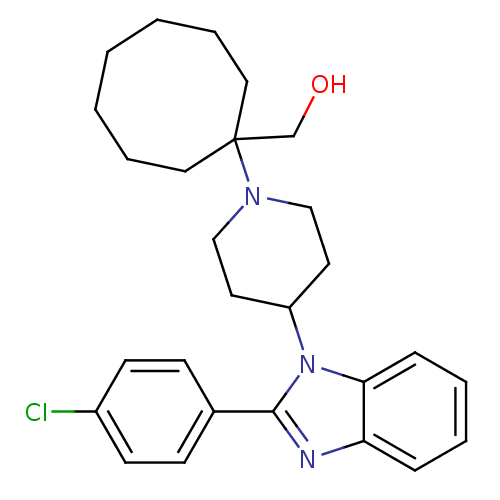
((1-{4-[2-(4-Chlorophenyl)-1H-benzimidazol-1-yl]pip...)Show SMILES OCC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C27H34ClN3O/c28-22-12-10-21(11-13-22)26-29-24-8-4-5-9-25(24)31(26)23-14-18-30(19-15-23)27(20-32)16-6-2-1-3-7-17-27/h4-5,8-13,23,32H,1-3,6-7,14-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004178
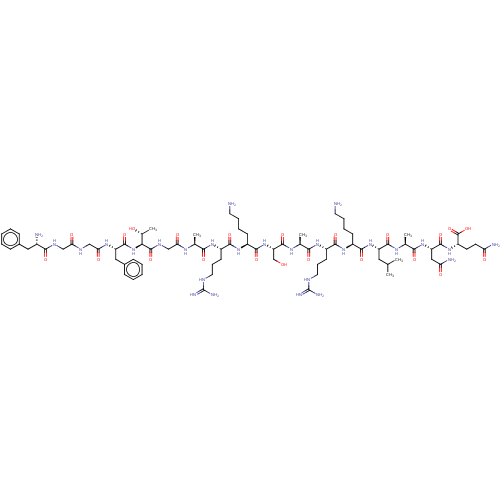
(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50335575
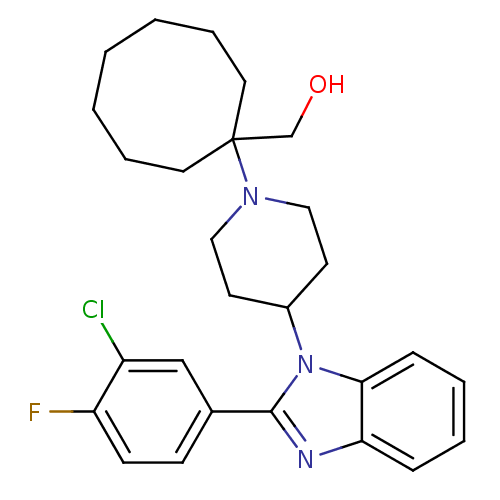
((1-{4-[2-(3-Chloro-4-fluorophenyl)-1H-benzimidazol...)Show SMILES OCC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C27H33ClFN3O/c28-22-18-20(10-11-23(22)29)26-30-24-8-4-5-9-25(24)32(26)21-12-16-31(17-13-21)27(19-33)14-6-2-1-3-7-15-27/h4-5,8-11,18,21,33H,1-3,6-7,12-17,19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50335568
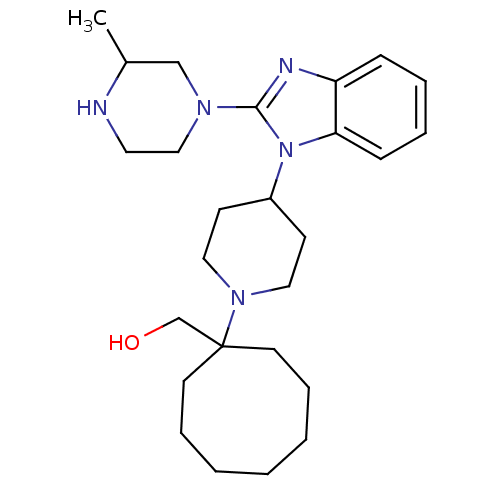
((1-{4-[2-(3-Methylpiperazin-1-yl)-1H-benzimidazol-...)Show SMILES CC1CN(CCN1)c1nc2ccccc2n1C1CCN(CC1)C1(CO)CCCCCCC1 Show InChI InChI=1S/C26H41N5O/c1-21-19-29(18-15-27-21)25-28-23-9-5-6-10-24(23)31(25)22-11-16-30(17-12-22)26(20-32)13-7-3-2-4-8-14-26/h5-6,9-10,21-22,27,32H,2-4,7-8,11-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50335570
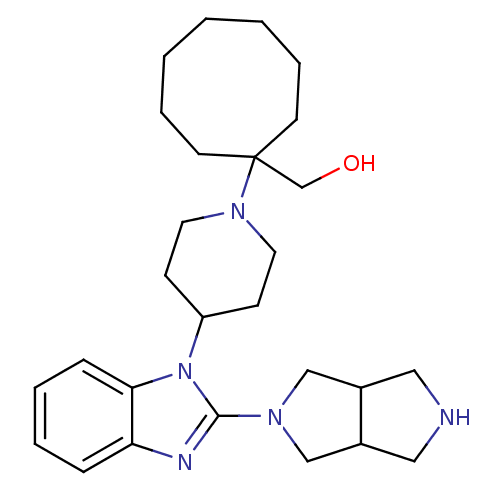
(CHEMBL1650844 | {1-[4-(2-{Hexahydropyrrolo[3,4-c]p...)Show SMILES OCC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)N1CC2CNCC2C1 Show InChI InChI=1S/C27H41N5O/c33-20-27(12-6-2-1-3-7-13-27)31-14-10-23(11-15-31)32-25-9-5-4-8-24(25)29-26(32)30-18-21-16-28-17-22(21)19-30/h4-5,8-9,21-23,28,33H,1-3,6-7,10-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50335567
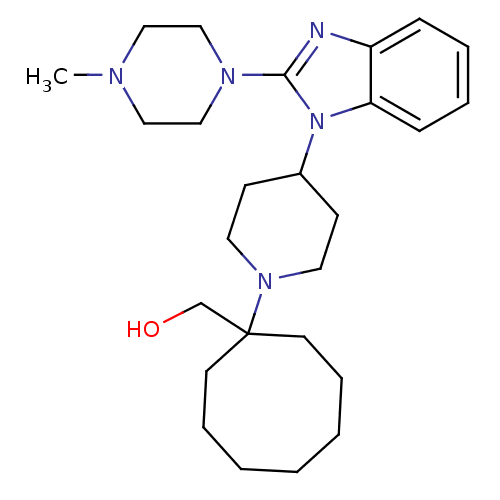
((1-{4-[2-(4-Methylpiperazin-1-yl)-1H-benzimidazol-...)Show SMILES CN1CCN(CC1)c1nc2ccccc2n1C1CCN(CC1)C1(CO)CCCCCCC1 Show InChI InChI=1S/C26H41N5O/c1-28-17-19-29(20-18-28)25-27-23-9-5-6-10-24(23)31(25)22-11-15-30(16-12-22)26(21-32)13-7-3-2-4-8-14-26/h5-6,9-10,22,32H,2-4,7-8,11-21H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50335569
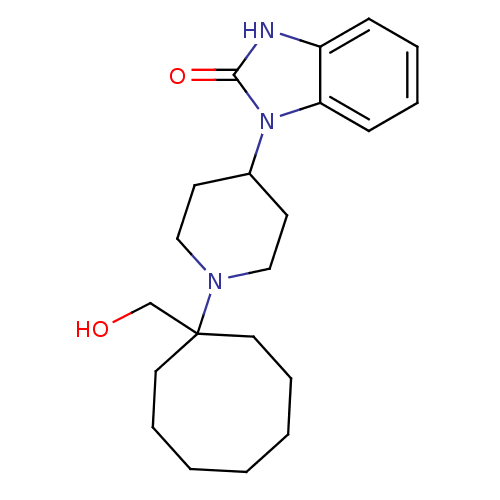
(1-{1-[1-(Hydroxymethyl)cyclooctyl]piperidin-4-yl}-...)Show InChI InChI=1S/C21H31N3O2/c25-16-21(12-6-2-1-3-7-13-21)23-14-10-17(11-15-23)24-19-9-5-4-8-18(19)22-20(24)26/h4-5,8-9,17,25H,1-3,6-7,10-16H2,(H,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50335576
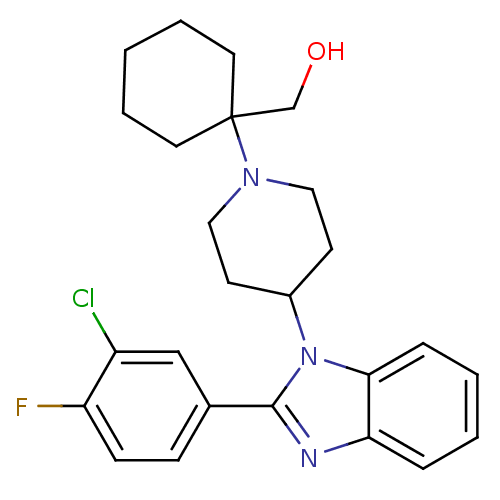
((1-{4-[2-(3-Chloro-4-fluorophenyl)-1H-benzimidazol...)Show SMILES OCC1(CCCCC1)N1CCC(CC1)n1c(nc2ccccc12)-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C25H29ClFN3O/c26-20-16-18(8-9-21(20)27)24-28-22-6-2-3-7-23(22)30(24)19-10-14-29(15-11-19)25(17-31)12-4-1-5-13-25/h2-3,6-9,16,19,31H,1,4-5,10-15,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50335573
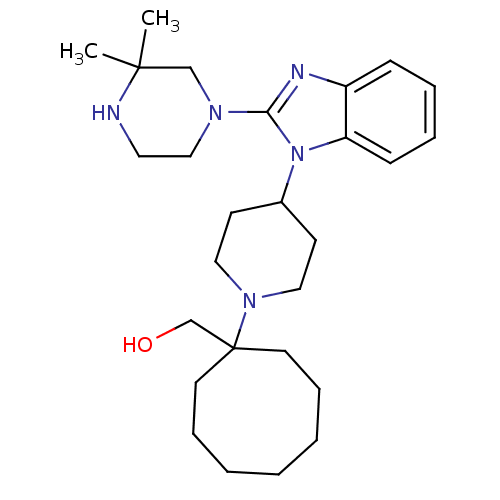
((1-{4-[2-(3,3-Dimethylpiperazin-1-yl)-1H-benzimida...)Show SMILES CC1(C)CN(CCN1)c1nc2ccccc2n1C1CCN(CC1)C1(CO)CCCCCCC1 Show InChI InChI=1S/C27H43N5O/c1-26(2)20-30(19-16-28-26)25-29-23-10-6-7-11-24(23)32(25)22-12-17-31(18-13-22)27(21-33)14-8-4-3-5-9-15-27/h6-7,10-11,22,28,33H,3-5,8-9,12-21H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50335571
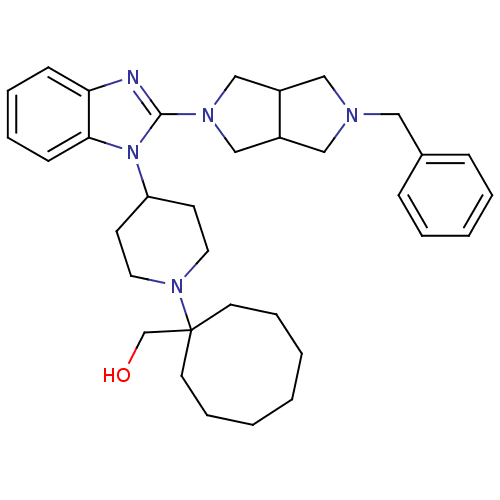
(CHEMBL1650845 | {1-[4-(2-{5-Benzylhexahydropyrrolo...)Show SMILES OCC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)N1CC2CN(Cc3ccccc3)CC2C1 Show InChI InChI=1S/C34H47N5O/c40-26-34(17-9-2-1-3-10-18-34)38-19-15-30(16-20-38)39-32-14-8-7-13-31(32)35-33(39)37-24-28-22-36(23-29(28)25-37)21-27-11-5-4-6-12-27/h4-8,11-14,28-30,40H,1-3,9-10,15-26H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50335569

(1-{1-[1-(Hydroxymethyl)cyclooctyl]piperidin-4-yl}-...)Show InChI InChI=1S/C21H31N3O2/c25-16-21(12-6-2-1-3-7-13-21)23-14-10-17(11-15-23)24-19-9-5-4-8-18(19)22-20(24)26/h4-5,8-9,17,25H,1-3,6-7,10-16H2,(H,22,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOP receptor expressed in CHO-K1 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50335571

(CHEMBL1650845 | {1-[4-(2-{5-Benzylhexahydropyrrolo...)Show SMILES OCC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)N1CC2CN(Cc3ccccc3)CC2C1 Show InChI InChI=1S/C34H47N5O/c40-26-34(17-9-2-1-3-10-18-34)38-19-15-30(16-20-38)39-32-14-8-7-13-31(32)35-33(39)37-24-28-22-36(23-29(28)25-37)21-27-11-5-4-6-12-27/h4-8,11-14,28-30,40H,1-3,9-10,15-26H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]enadoline from human KOP receptor expressed in HEK-293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50335572
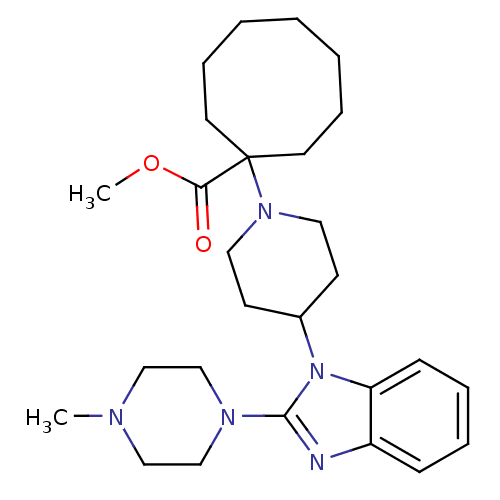
(CHEMBL1650846 | Methyl 1-{4-[2-(4-methylpiperazin-...)Show SMILES COC(=O)C1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)N1CCN(C)CC1 Show InChI InChI=1S/C27H41N5O2/c1-29-18-20-30(21-19-29)26-28-23-10-6-7-11-24(23)32(26)22-12-16-31(17-13-22)27(25(33)34-2)14-8-4-3-5-9-15-27/h6-7,10-11,22H,3-5,8-9,12-21H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50335571

(CHEMBL1650845 | {1-[4-(2-{5-Benzylhexahydropyrrolo...)Show SMILES OCC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)N1CC2CN(Cc3ccccc3)CC2C1 Show InChI InChI=1S/C34H47N5O/c40-26-34(17-9-2-1-3-10-18-34)38-19-15-30(16-20-38)39-32-14-8-7-13-31(32)35-33(39)37-24-28-22-36(23-29(28)25-37)21-27-11-5-4-6-12-27/h4-8,11-14,28-30,40H,1-3,9-10,15-26H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOP receptor expressed in CHO-K1 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50335574

((1-{4-[2-(4-Chlorophenyl)-1H-benzimidazol-1-yl]pip...)Show SMILES OCC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C27H34ClN3O/c28-22-12-10-21(11-13-22)26-29-24-8-4-5-9-25(24)31(26)23-14-18-30(19-15-23)27(20-32)16-6-2-1-3-7-17-27/h4-5,8-13,23,32H,1-3,6-7,14-20H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOP receptor expressed in CHO-K1 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50335570

(CHEMBL1650844 | {1-[4-(2-{Hexahydropyrrolo[3,4-c]p...)Show SMILES OCC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)N1CC2CNCC2C1 Show InChI InChI=1S/C27H41N5O/c33-20-27(12-6-2-1-3-7-13-27)31-14-10-23(11-15-31)32-25-9-5-4-8-24(25)29-26(32)30-18-21-16-28-17-22(21)19-30/h4-5,8-9,21-23,28,33H,1-3,6-7,10-20H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 201 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOP receptor expressed in CHO-K1 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50335574

((1-{4-[2-(4-Chlorophenyl)-1H-benzimidazol-1-yl]pip...)Show SMILES OCC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C27H34ClN3O/c28-22-12-10-21(11-13-22)26-29-24-8-4-5-9-25(24)31(26)23-14-18-30(19-15-23)27(20-32)16-6-2-1-3-7-17-27/h4-5,8-13,23,32H,1-3,6-7,14-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human DOP receptor expressed in CHO-K1 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50335567

((1-{4-[2-(4-Methylpiperazin-1-yl)-1H-benzimidazol-...)Show SMILES CN1CCN(CC1)c1nc2ccccc2n1C1CCN(CC1)C1(CO)CCCCCCC1 Show InChI InChI=1S/C26H41N5O/c1-28-17-19-29(20-18-28)25-27-23-9-5-6-10-24(23)31(25)22-11-15-30(16-12-22)26(21-32)13-7-3-2-4-8-14-26/h5-6,9-10,22,32H,2-4,7-8,11-21H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 229 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOP receptor expressed in CHO-K1 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50335569

(1-{1-[1-(Hydroxymethyl)cyclooctyl]piperidin-4-yl}-...)Show InChI InChI=1S/C21H31N3O2/c25-16-21(12-6-2-1-3-7-13-21)23-14-10-17(11-15-23)24-19-9-5-4-8-18(19)22-20(24)26/h4-5,8-9,17,25H,1-3,6-7,10-16H2,(H,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]enadoline from human KOP receptor expressed in HEK-293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50335577
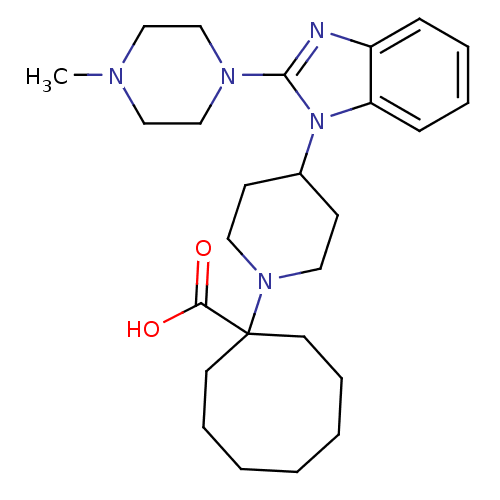
(1-{4-[2-(4-Methylpiperazin-1-yl)-1H-benzimidazol-1...)Show SMILES CN1CCN(CC1)c1nc2ccccc2n1C1CCN(CC1)C1(CCCCCCC1)C(O)=O Show InChI InChI=1S/C26H39N5O2/c1-28-17-19-29(20-18-28)25-27-22-9-5-6-10-23(22)31(25)21-11-15-30(16-12-21)26(24(32)33)13-7-3-2-4-8-14-26/h5-6,9-10,21H,2-4,7-8,11-20H2,1H3,(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50335571

(CHEMBL1650845 | {1-[4-(2-{5-Benzylhexahydropyrrolo...)Show SMILES OCC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)N1CC2CN(Cc3ccccc3)CC2C1 Show InChI InChI=1S/C34H47N5O/c40-26-34(17-9-2-1-3-10-18-34)38-19-15-30(16-20-38)39-32-14-8-7-13-31(32)35-33(39)37-24-28-22-36(23-29(28)25-37)21-27-11-5-4-6-12-27/h4-8,11-14,28-30,40H,1-3,9-10,15-26H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human DOP receptor expressed in CHO-K1 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50335568

((1-{4-[2-(3-Methylpiperazin-1-yl)-1H-benzimidazol-...)Show SMILES CC1CN(CCN1)c1nc2ccccc2n1C1CCN(CC1)C1(CO)CCCCCCC1 Show InChI InChI=1S/C26H41N5O/c1-21-19-29(18-15-27-21)25-28-23-9-5-6-10-24(23)31(25)22-11-16-30(17-12-22)26(20-32)13-7-3-2-4-8-14-26/h5-6,9-10,21-22,27,32H,2-4,7-8,11-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]enadoline from human KOP receptor expressed in HEK-293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50335575

((1-{4-[2-(3-Chloro-4-fluorophenyl)-1H-benzimidazol...)Show SMILES OCC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C27H33ClFN3O/c28-22-18-20(10-11-23(22)29)26-30-24-8-4-5-9-25(24)32(26)21-12-16-31(17-13-21)27(19-33)14-6-2-1-3-7-15-27/h4-5,8-11,18,21,33H,1-3,6-7,12-17,19H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOP receptor expressed in CHO-K1 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50335572

(CHEMBL1650846 | Methyl 1-{4-[2-(4-methylpiperazin-...)Show SMILES COC(=O)C1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)N1CCN(C)CC1 Show InChI InChI=1S/C27H41N5O2/c1-29-18-20-30(21-19-29)26-28-23-10-6-7-11-24(23)32(26)22-12-16-31(17-13-22)27(25(33)34-2)14-8-4-3-5-9-15-27/h6-7,10-11,22H,3-5,8-9,12-21H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 312 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human DOP receptor expressed in CHO-K1 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50335567

((1-{4-[2-(4-Methylpiperazin-1-yl)-1H-benzimidazol-...)Show SMILES CN1CCN(CC1)c1nc2ccccc2n1C1CCN(CC1)C1(CO)CCCCCCC1 Show InChI InChI=1S/C26H41N5O/c1-28-17-19-29(20-18-28)25-27-23-9-5-6-10-24(23)31(25)22-11-15-30(16-12-22)26(21-32)13-7-3-2-4-8-14-26/h5-6,9-10,22,32H,2-4,7-8,11-21H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 333 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]enadoline from human KOP receptor expressed in HEK-293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50335573

((1-{4-[2-(3,3-Dimethylpiperazin-1-yl)-1H-benzimida...)Show SMILES CC1(C)CN(CCN1)c1nc2ccccc2n1C1CCN(CC1)C1(CO)CCCCCCC1 Show InChI InChI=1S/C27H43N5O/c1-26(2)20-30(19-16-28-26)25-29-23-10-6-7-11-24(23)32(25)22-12-17-31(18-13-22)27(21-33)14-8-4-3-5-9-15-27/h6-7,10-11,22,28,33H,3-5,8-9,12-21H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 387 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]enadoline from human KOP receptor expressed in HEK-293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50335577

(1-{4-[2-(4-Methylpiperazin-1-yl)-1H-benzimidazol-1...)Show SMILES CN1CCN(CC1)c1nc2ccccc2n1C1CCN(CC1)C1(CCCCCCC1)C(O)=O Show InChI InChI=1S/C26H39N5O2/c1-28-17-19-29(20-18-28)25-27-22-9-5-6-10-23(22)31(25)21-11-15-30(16-12-21)26(24(32)33)13-7-3-2-4-8-14-26/h5-6,9-10,21H,2-4,7-8,11-20H2,1H3,(H,32,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOP receptor expressed in CHO-K1 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50335572

(CHEMBL1650846 | Methyl 1-{4-[2-(4-methylpiperazin-...)Show SMILES COC(=O)C1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)N1CCN(C)CC1 Show InChI InChI=1S/C27H41N5O2/c1-29-18-20-30(21-19-29)26-28-23-10-6-7-11-24(23)32(26)22-12-16-31(17-13-22)27(25(33)34-2)14-8-4-3-5-9-15-27/h6-7,10-11,22H,3-5,8-9,12-21H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOP receptor expressed in CHO-K1 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50335576

((1-{4-[2-(3-Chloro-4-fluorophenyl)-1H-benzimidazol...)Show SMILES OCC1(CCCCC1)N1CCC(CC1)n1c(nc2ccccc12)-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C25H29ClFN3O/c26-20-16-18(8-9-21(20)27)24-28-22-6-2-3-7-23(22)30(24)19-10-14-29(15-11-19)25(17-31)12-4-1-5-13-25/h2-3,6-9,16,19,31H,1,4-5,10-15,17H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOP receptor expressed in CHO-K1 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50335573

((1-{4-[2-(3,3-Dimethylpiperazin-1-yl)-1H-benzimida...)Show SMILES CC1(C)CN(CCN1)c1nc2ccccc2n1C1CCN(CC1)C1(CO)CCCCCCC1 Show InChI InChI=1S/C27H43N5O/c1-26(2)20-30(19-16-28-26)25-29-23-10-6-7-11-24(23)32(25)22-12-17-31(18-13-22)27(21-33)14-8-4-3-5-9-15-27/h6-7,10-11,22,28,33H,3-5,8-9,12-21H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOP receptor expressed in CHO-K1 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50335568

((1-{4-[2-(3-Methylpiperazin-1-yl)-1H-benzimidazol-...)Show SMILES CC1CN(CCN1)c1nc2ccccc2n1C1CCN(CC1)C1(CO)CCCCCCC1 Show InChI InChI=1S/C26H41N5O/c1-21-19-29(18-15-27-21)25-28-23-9-5-6-10-24(23)31(25)22-11-16-30(17-12-22)26(20-32)13-7-3-2-4-8-14-26/h5-6,9-10,21-22,27,32H,2-4,7-8,11-20H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOP receptor expressed in CHO-K1 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50335573

((1-{4-[2-(3,3-Dimethylpiperazin-1-yl)-1H-benzimida...)Show SMILES CC1(C)CN(CCN1)c1nc2ccccc2n1C1CCN(CC1)C1(CO)CCCCCCC1 Show InChI InChI=1S/C27H43N5O/c1-26(2)20-30(19-16-28-26)25-29-23-10-6-7-11-24(23)32(25)22-12-17-31(18-13-22)27(21-33)14-8-4-3-5-9-15-27/h6-7,10-11,22,28,33H,3-5,8-9,12-21H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human DOP receptor expressed in CHO-K1 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50335568

((1-{4-[2-(3-Methylpiperazin-1-yl)-1H-benzimidazol-...)Show SMILES CC1CN(CCN1)c1nc2ccccc2n1C1CCN(CC1)C1(CO)CCCCCCC1 Show InChI InChI=1S/C26H41N5O/c1-21-19-29(18-15-27-21)25-28-23-9-5-6-10-24(23)31(25)22-11-16-30(17-12-22)26(20-32)13-7-3-2-4-8-14-26/h5-6,9-10,21-22,27,32H,2-4,7-8,11-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human DOP receptor expressed in CHO-K1 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50335572

(CHEMBL1650846 | Methyl 1-{4-[2-(4-methylpiperazin-...)Show SMILES COC(=O)C1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)N1CCN(C)CC1 Show InChI InChI=1S/C27H41N5O2/c1-29-18-20-30(21-19-29)26-28-23-10-6-7-11-24(23)32(26)22-12-16-31(17-13-22)27(25(33)34-2)14-8-4-3-5-9-15-27/h6-7,10-11,22H,3-5,8-9,12-21H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]enadoline from human KOP receptor expressed in HEK-293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50335574

((1-{4-[2-(4-Chlorophenyl)-1H-benzimidazol-1-yl]pip...)Show SMILES OCC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C27H34ClN3O/c28-22-12-10-21(11-13-22)26-29-24-8-4-5-9-25(24)31(26)23-14-18-30(19-15-23)27(20-32)16-6-2-1-3-7-17-27/h4-5,8-13,23,32H,1-3,6-7,14-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]enadoline from human KOP receptor expressed in HEK-293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50335577

(1-{4-[2-(4-Methylpiperazin-1-yl)-1H-benzimidazol-1...)Show SMILES CN1CCN(CC1)c1nc2ccccc2n1C1CCN(CC1)C1(CCCCCCC1)C(O)=O Show InChI InChI=1S/C26H39N5O2/c1-28-17-19-29(20-18-28)25-27-22-9-5-6-10-23(22)31(25)21-11-15-30(16-12-21)26(24(32)33)13-7-3-2-4-8-14-26/h5-6,9-10,21H,2-4,7-8,11-20H2,1H3,(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]enadoline from human KOP receptor expressed in HEK-293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50335570

(CHEMBL1650844 | {1-[4-(2-{Hexahydropyrrolo[3,4-c]p...)Show SMILES OCC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)N1CC2CNCC2C1 Show InChI InChI=1S/C27H41N5O/c33-20-27(12-6-2-1-3-7-13-27)31-14-10-23(11-15-31)32-25-9-5-4-8-24(25)29-26(32)30-18-21-16-28-17-22(21)19-30/h4-5,8-9,21-23,28,33H,1-3,6-7,10-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >577 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]enadoline from human KOP receptor expressed in HEK-293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50335567

((1-{4-[2-(4-Methylpiperazin-1-yl)-1H-benzimidazol-...)Show SMILES CN1CCN(CC1)c1nc2ccccc2n1C1CCN(CC1)C1(CO)CCCCCCC1 Show InChI InChI=1S/C26H41N5O/c1-28-17-19-29(20-18-28)25-27-23-9-5-6-10-24(23)31(25)22-11-15-30(16-12-22)26(21-32)13-7-3-2-4-8-14-26/h5-6,9-10,22,32H,2-4,7-8,11-21H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 679 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human DOP receptor expressed in CHO-K1 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50004178

(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]enadoline from human KOP receptor expressed in HEK-293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50004178

(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human DOP receptor expressed in CHO-K1 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50004178

(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOP receptor expressed in CHO-K1 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50335570

(CHEMBL1650844 | {1-[4-(2-{Hexahydropyrrolo[3,4-c]p...)Show SMILES OCC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)N1CC2CNCC2C1 Show InChI InChI=1S/C27H41N5O/c33-20-27(12-6-2-1-3-7-13-27)31-14-10-23(11-15-31)32-25-9-5-4-8-24(25)29-26(32)30-18-21-16-28-17-22(21)19-30/h4-5,8-9,21-23,28,33H,1-3,6-7,10-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50335570

(CHEMBL1650844 | {1-[4-(2-{Hexahydropyrrolo[3,4-c]p...)Show SMILES OCC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)N1CC2CNCC2C1 Show InChI InChI=1S/C27H41N5O/c33-20-27(12-6-2-1-3-7-13-27)31-14-10-23(11-15-31)32-25-9-5-4-8-24(25)29-26(32)30-18-21-16-28-17-22(21)19-30/h4-5,8-9,21-23,28,33H,1-3,6-7,10-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human DOP receptor expressed in CHO-K1 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50335569

(1-{1-[1-(Hydroxymethyl)cyclooctyl]piperidin-4-yl}-...)Show InChI InChI=1S/C21H31N3O2/c25-16-21(12-6-2-1-3-7-13-21)23-14-10-17(11-15-23)24-19-9-5-4-8-18(19)22-20(24)26/h4-5,8-9,17,25H,1-3,6-7,10-16H2,(H,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human DOP receptor expressed in CHO-K1 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM15581

(CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...)Show InChI InChI=1S/C13H15Cl2NO/c1-3-7-16(2)8-4-9-17-13-6-5-11(14)10-12(13)15/h1,5-6,10H,4,7-9H2,2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.00490 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human monoamine oxidase A |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM15579

(CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...)Show InChI InChI=1S/C13H17N/c1-4-10-14(3)12(2)11-13-8-6-5-7-9-13/h1,5-9,12H,10-11H2,2-3H3/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human monoamine oxidase B |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Small conductance calcium-activated potassium channel protein 1
(RAT) | BDBM50260153
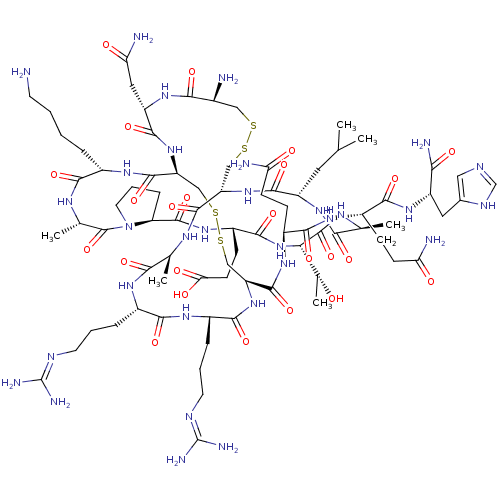
(Apamin | CHEMBL525408 | Octadecapeptide venom)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](C)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CSSC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N2)NC1=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(N)=O)[C@@H](C)O |r,wU:83.102,47.101,13.138,4.3,33.34,88.89,78.79,67.68,107.109,116.118,125.127,wD:26.30,52.106,56.57,17.17,135.140,8.8,38.39,93.94,(27.83,-36.7,;27.83,-35.16,;26.49,-34.38,;29.15,-34.38,;29.15,-32.85,;30.49,-32.08,;31.8,-32.85,;31.8,-34.38,;33.14,-32.08,;33.14,-30.53,;34.47,-32.85,;35.8,-32.08,;35.8,-30.53,;40.01,-33.02,;41.6,-32.04,;43.23,-32.89,;43.23,-34.42,;44.54,-32.11,;44.54,-30.57,;45.89,-29.8,;45.89,-28.26,;44.54,-27.49,;47.21,-27.49,;45.89,-32.89,;48.48,-32.23,;48.91,-30.87,;50.38,-34.89,;52.13,-34.44,;53.01,-35.96,;51.84,-36.95,;50.38,-36.43,;48.34,-38.91,;48.77,-40.45,;46.11,-38.58,;46.11,-37.03,;44.16,-39.41,;42.33,-38.62,;42.33,-37.09,;40.7,-39.47,;40.7,-40.99,;39.37,-41.76,;39.37,-43.3,;38.05,-44.06,;38.05,-45.59,;38.29,-38.35,;35.39,-39.38,;35.39,-40.91,;34.06,-38.61,;40.93,-30.71,;40.94,-27.32,;39.36,-26.12,;39.37,-23.14,;38.05,-22.37,;36.72,-23.15,;35.39,-22.38,;35.4,-20.84,;34.06,-23.15,;34.06,-24.68,;32.73,-25.46,;32.73,-26.98,;31.4,-27.31,;30.08,-26.97,;28.36,-27.74,;30.08,-25.44,;32.73,-22.39,;31.4,-23.16,;31.41,-24.7,;28.69,-22.34,;28.69,-20.8,;30.02,-20.04,;30.02,-18.5,;31.34,-17.73,;31.34,-16.2,;32.68,-15.42,;30.02,-15.42,;26.07,-23.55,;26.08,-25.1,;27.41,-25.88,;23.8,-26.94,;22.48,-26.19,;23.83,-29.77,;25.16,-30.53,;26.5,-29.77,;25.16,-32.08,;23.84,-32.85,;23.84,-34.38,;24.76,-36.3,;26.09,-37.07,;26.09,-38.61,;24.77,-39.38,;27.42,-39.38,;27.42,-40.91,;28.75,-38.61,;30.09,-39.38,;30.09,-40.91,;31.42,-41.68,;31.42,-43.23,;32.75,-40.91,;31.41,-38.61,;31.41,-37.07,;32.73,-39.38,;26.5,-32.85,;27.83,-32.08,;27.83,-30.53,;38.04,-20.83,;36.7,-20.07,;39.36,-20.06,;39.36,-18.52,;38.03,-17.74,;38.03,-16.21,;36.71,-15.43,;36.71,-13.9,;35.38,-16.21,;40.7,-17.74,;40.69,-16.21,;42.03,-18.52,;43.37,-17.74,;43.37,-16.2,;44.7,-15.42,;44.7,-13.89,;46.03,-13.1,;43.36,-13.12,;44.71,-18.51,;44.7,-20.05,;46.04,-17.73,;47.37,-18.52,;48.68,-17.74,;50.05,-18.53,;50.21,-20.07,;51.72,-20.4,;52.48,-19.07,;51.47,-17.91,;47.36,-20.05,;48.68,-20.83,;46.03,-20.83,;40.01,-34.57,;38.67,-35.33,;41.74,-35.33,)| Show InChI InChI=1S/C79H131N31O24S4/c1-35(2)26-49-70(127)107-51-31-136-135-30-41(81)63(120)105-50(28-57(84)114)71(128)108-53(73(130)99-42(12-7-8-22-80)64(121)96-38(5)77(134)110-25-11-15-54(110)75(132)102-47(18-21-58(115)116)69(126)109-59(39(6)111)76(133)95-37(4)62(119)104-49)33-138-137-32-52(106-66(123)44(14-10-24-92-79(88)89)98-65(122)43(13-9-23-91-78(86)87)97-61(118)36(3)94-72(51)129)74(131)101-45(16-19-55(82)112)67(124)100-46(17-20-56(83)113)68(125)103-48(60(85)117)27-40-29-90-34-93-40/h29,34-39,41-54,59,111H,7-28,30-33,80-81H2,1-6H3,(H2,82,112)(H2,83,113)(H2,84,114)(H2,85,117)(H,90,93)(H,94,129)(H,95,133)(H,96,121)(H,97,118)(H,98,122)(H,99,130)(H,100,124)(H,101,131)(H,102,132)(H,103,125)(H,104,119)(H,105,120)(H,106,123)(H,107,127)(H,108,128)(H,109,126)(H,115,116)(H4,86,87,91)(H4,88,89,92)/t36-,37-,38-,39+,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,59-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat SK Calcium channel |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50022775

((m-Hydroxyphenyl)trimethylammonium dimethylcarbama...)Show InChI InChI=1S/C12H19N2O2/c1-13(2)12(15)16-11-8-6-7-10(9-11)14(3,4)5/h6-9H,1-5H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholine esterase |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50009338
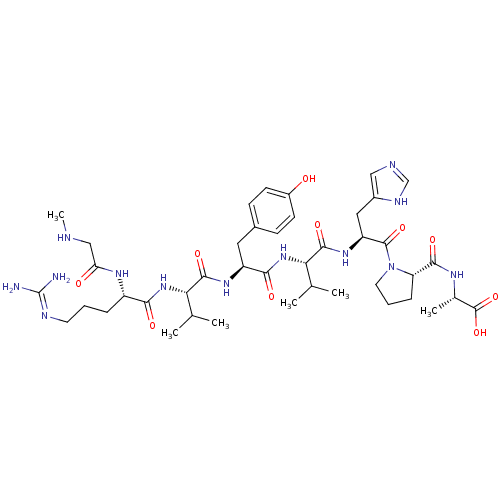
((S)-2-((S)-1-((S)-2-((S)-2-((S)-2-((S)-2-((S)-5-(d...)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O |r,wU:60.63,wD:24.23,36.36,43.43,56.59,17.16,6.5,(6.84,-31.4,;5.3,-31.4,;4.53,-30.07,;2.99,-30.07,;2.22,-31.4,;2.22,-28.73,;2.99,-27.4,;4.53,-27.4,;5.3,-26.07,;4.53,-24.73,;5.3,-23.4,;6.84,-23.4,;7.61,-24.73,;7.61,-22.07,;2.22,-26.07,;2.99,-24.73,;.68,-26.07,;-.09,-24.73,;.68,-23.4,;2.22,-23.4,;-.09,-22.07,;-1.63,-24.73,;-2.4,-23.4,;-2.4,-26.07,;-3.94,-26.07,;-4.71,-24.73,;-6.25,-24.73,;-7.02,-23.4,;-8.56,-23.4,;-9.33,-24.73,;-10.87,-24.73,;-8.56,-26.07,;-7.02,-26.07,;-4.71,-27.4,;-6.25,-27.4,;-3.94,-28.73,;-4.71,-30.07,;-6.25,-30.07,;-7.02,-28.73,;-7.02,-31.4,;-3.94,-31.4,;-2.4,-31.4,;-4.71,-32.73,;-3.94,-34.07,;-4.71,-35.4,;-6.25,-35.4,;-7.15,-34.16,;-8.62,-34.63,;-8.62,-36.17,;-7.15,-36.65,;-2.4,-34.07,;-1.63,-32.73,;-1.63,-35.4,;-2.26,-36.81,;-1.12,-37.84,;.22,-37.07,;-.1,-35.56,;.93,-34.42,;.45,-32.95,;2.42,-34.82,;3.5,-33.73,;3.11,-32.24,;5,-34.13,;6.09,-33.04,;5.39,-35.61,)| Show InChI InChI=1S/C42H65N13O10/c1-22(2)33(53-35(58)28(50-32(57)20-45-6)9-7-15-47-42(43)44)38(61)51-29(17-25-11-13-27(56)14-12-25)36(59)54-34(23(3)4)39(62)52-30(18-26-19-46-21-48-26)40(63)55-16-8-10-31(55)37(60)49-24(5)41(64)65/h11-14,19,21-24,28-31,33-34,45,56H,7-10,15-18,20H2,1-6H3,(H,46,48)(H,49,60)(H,50,57)(H,51,61)(H,52,62)(H,53,58)(H,54,59)(H,64,65)(H4,43,44,47)/t24-,28-,29-,30-,31-,33-,34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human angiotensin II AT2 receptor |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50009338

((S)-2-((S)-1-((S)-2-((S)-2-((S)-2-((S)-2-((S)-5-(d...)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O |r,wU:60.63,wD:24.23,36.36,43.43,56.59,17.16,6.5,(6.84,-31.4,;5.3,-31.4,;4.53,-30.07,;2.99,-30.07,;2.22,-31.4,;2.22,-28.73,;2.99,-27.4,;4.53,-27.4,;5.3,-26.07,;4.53,-24.73,;5.3,-23.4,;6.84,-23.4,;7.61,-24.73,;7.61,-22.07,;2.22,-26.07,;2.99,-24.73,;.68,-26.07,;-.09,-24.73,;.68,-23.4,;2.22,-23.4,;-.09,-22.07,;-1.63,-24.73,;-2.4,-23.4,;-2.4,-26.07,;-3.94,-26.07,;-4.71,-24.73,;-6.25,-24.73,;-7.02,-23.4,;-8.56,-23.4,;-9.33,-24.73,;-10.87,-24.73,;-8.56,-26.07,;-7.02,-26.07,;-4.71,-27.4,;-6.25,-27.4,;-3.94,-28.73,;-4.71,-30.07,;-6.25,-30.07,;-7.02,-28.73,;-7.02,-31.4,;-3.94,-31.4,;-2.4,-31.4,;-4.71,-32.73,;-3.94,-34.07,;-4.71,-35.4,;-6.25,-35.4,;-7.15,-34.16,;-8.62,-34.63,;-8.62,-36.17,;-7.15,-36.65,;-2.4,-34.07,;-1.63,-32.73,;-1.63,-35.4,;-2.26,-36.81,;-1.12,-37.84,;.22,-37.07,;-.1,-35.56,;.93,-34.42,;.45,-32.95,;2.42,-34.82,;3.5,-33.73,;3.11,-32.24,;5,-34.13,;6.09,-33.04,;5.39,-35.61,)| Show InChI InChI=1S/C42H65N13O10/c1-22(2)33(53-35(58)28(50-32(57)20-45-6)9-7-15-47-42(43)44)38(61)51-29(17-25-11-13-27(56)14-12-25)36(59)54-34(23(3)4)39(62)52-30(18-26-19-46-21-48-26)40(63)55-16-8-10-31(55)37(60)49-24(5)41(64)65/h11-14,19,21-24,28-31,33-34,45,56H,7-10,15-18,20H2,1-6H3,(H,46,48)(H,49,60)(H,50,57)(H,51,61)(H,52,62)(H,53,58)(H,54,59)(H,64,65)(H4,43,44,47)/t24-,28-,29-,30-,31-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human angiotensin II AT1 receptor |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D1 receptor |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Tyrosine 3-monooxygenase
(Rattus norvegicus) | BDBM37633
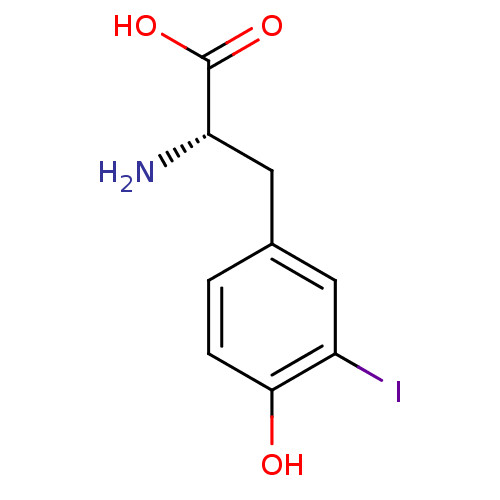
((2S)-2-amino-3-(4-hydroxy-3-iodo-phenyl)propionic ...)Show InChI InChI=1S/C9H10INO3/c10-6-3-5(1-2-8(6)12)4-7(11)9(13)14/h1-3,7,12H,4,11H2,(H,13,14)/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat tyrosine hydroxylase |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM22904

((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...)Show InChI InChI=1S/C6H11N3/c1-5(7)2-6-3-8-4-9-6/h3-5H,2,7H2,1H3,(H,8,9)/t5-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat histamine H3 receptor |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM22567

(3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...)Show InChI InChI=1S/C17H23N3O/c1-19(2)12-13-20(17-6-4-5-11-18-17)14-15-7-9-16(21-3)10-8-15/h4-11H,12-14H2,1-3H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig histamine H1 receptor |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176065
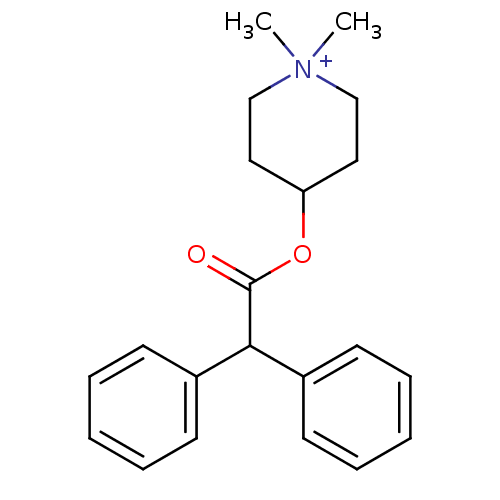
(4-DAMP | 4-Diphenylacetoxy-1,1-dimethyl-piperidini...)Show InChI InChI=1S/C21H26NO2/c1-22(2)15-13-19(14-16-22)24-21(23)20(17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human muscarinic M3 receptor |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50335566
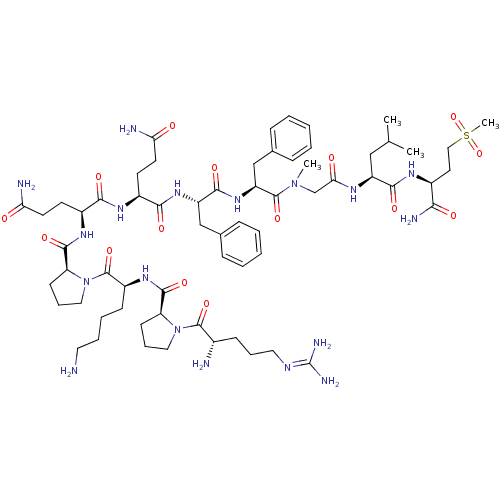
(CHEMBL1651026 | Substance P [Sar9,Met(O2)11])Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7](-[#6])-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]S([#6])(=O)=O)-[#6](-[#7])=O |r| Show InChI InChI=1S/C64H100N18O15S/c1-38(2)34-46(57(89)74-42(54(69)86)28-33-98(4,96)97)73-53(85)37-80(3)62(94)48(36-40-18-9-6-10-19-40)79-58(90)47(35-39-16-7-5-8-17-39)78-56(88)43(24-26-51(67)83)75-55(87)44(25-27-52(68)84)76-59(91)50-23-15-32-82(50)63(95)45(21-11-12-29-65)77-60(92)49-22-14-31-81(49)61(93)41(66)20-13-30-72-64(70)71/h5-10,16-19,38,41-50H,11-15,20-37,65-66H2,1-4H3,(H2,67,83)(H2,68,84)(H2,69,86)(H,73,85)(H,74,89)(H,75,87)(H,76,91)(H,77,92)(H,78,88)(H,79,90)(H4,70,71,72)/t41-,42-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human tachykinin NK1 receptor |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25768
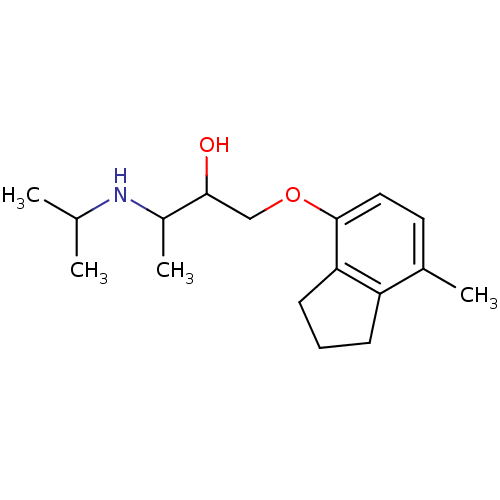
(1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]-3-(pro...)Show InChI InChI=1S/C17H27NO2/c1-11(2)18-13(4)16(19)10-20-17-9-8-12(3)14-6-5-7-15(14)17/h8-9,11,13,16,18-19H,5-7,10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human alpha2 adrenoceptor |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM21395

(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 5-HT2A receptor |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50292408
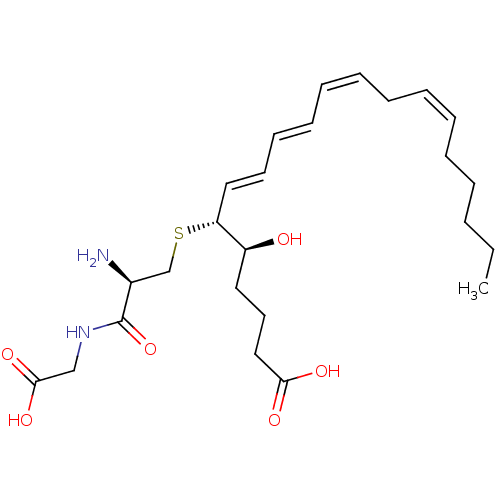
((R-(R*,S*-(E,E,Z,Z)))-N-(S-(1-(4-Carboxy-1-hydroxy...)Show SMILES CCCCC\C=C/C\C=C/C=C/C=C/[C@@H](SC[C@H](N)C(=O)NCC(O)=O)[C@@H](O)CCCC(O)=O |r| Show InChI InChI=1S/C25H40N2O6S/c1-2-3-4-5-6-7-8-9-10-11-12-13-16-22(21(28)15-14-17-23(29)30)34-19-20(26)25(33)27-18-24(31)32/h6-7,9-13,16,20-22,28H,2-5,8,14-15,17-19,26H2,1H3,(H,27,33)(H,29,30)(H,31,32)/b7-6-,10-9-,12-11+,16-13+/t20-,21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene D4 receptor |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM22165
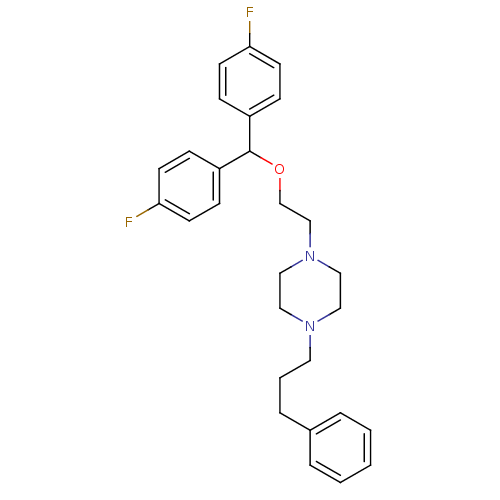
(1-{2-[bis(4-fluorophenyl)methoxy]ethyl}-4-(3-pheny...)Show SMILES Fc1ccc(cc1)C(OCCN1CCN(CCCc2ccccc2)CC1)c1ccc(F)cc1 Show InChI InChI=1S/C28H32F2N2O/c29-26-12-8-24(9-13-26)28(25-10-14-27(30)15-11-25)33-22-21-32-19-17-31(18-20-32)16-4-7-23-5-2-1-3-6-23/h1-3,5-6,8-15,28H,4,7,16-22H2 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine transporter |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50010859
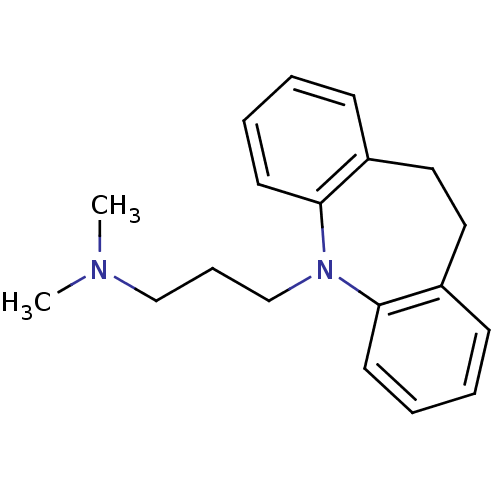
(CHEMBL11 | IMIPRAMINE HYDROCHLORIDE | IMIPRAMINE P...)Show InChI InChI=1S/C19H24N2/c1-20(2)14-7-15-21-18-10-5-3-8-16(18)12-13-17-9-4-6-11-19(17)21/h3-6,8-11H,7,12-15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 5-HT transporter |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50366495

((+)butaclamol | CHEMBL1255588)Show SMILES CC(C)(C)[C@@]1(O)CCN2C[C@@H]3c4ccccc4CCc4cccc([C@H]2C1)c34 |r| Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D3 receptor |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50366495

((+)butaclamol | CHEMBL1255588)Show SMILES CC(C)(C)[C@@]1(O)CCN2C[C@@H]3c4ccccc4CCc4cccc([C@H]2C1)c34 |r| Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D2 receptor |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cannabinoid CB1 receptor |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50176062
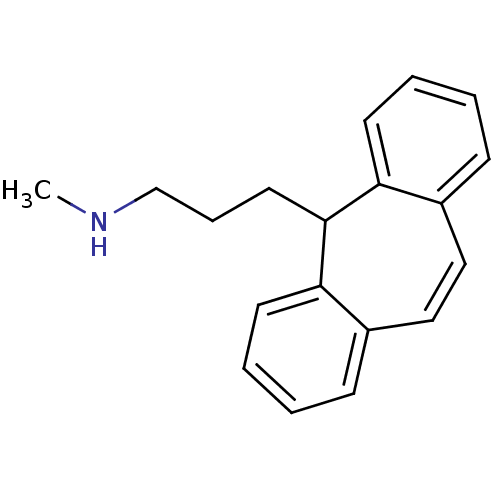
(3-(5H-dibenzo[a,d][7]annulen-5-yl)-N-methylpropan-...)Show InChI InChI=1S/C19H21N/c1-20-14-6-11-19-17-9-4-2-7-15(17)12-13-16-8-3-5-10-18(16)19/h2-5,7-10,12-13,19-20H,6,11,14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| DrugBank
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human aorepinephrine transporter |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM39341

(11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...)Show InChI InChI=1S/C19H21N5O2/c1-22-9-11-23(12-10-22)13-17(25)24-16-7-3-2-5-14(16)19(26)21-15-6-4-8-20-18(15)24/h2-8H,9-13H2,1H3,(H,21,26) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human muscarinic M1 receptor |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human adenosine A1 receptor |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50064176
(CHEMBL27673 | CHEMBL500996 | METHOCTRAMINE | N,N''...)Show SMILES COc1ccccc1CNCCCCCCNCCCCCCCCNCCCCCCNCc1ccccc1OC Show InChI InChI=1S/C36H62N4O2/c1-41-35-23-13-11-21-33(35)31-39-29-19-9-7-17-27-37-25-15-5-3-4-6-16-26-38-28-18-8-10-20-30-40-32-34-22-12-14-24-36(34)42-2/h11-14,21-24,37-40H,3-10,15-20,25-32H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human muscarinic M2 receptor |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human estrogen receptor beta |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human adenosine A2A receptor |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H2 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM22889
((Cimetidine) N-Methyl-N''''-[2-(5-methyl-1H-imidaz...)Show InChI InChI=1S/C10H16N6S/c1-8-9(16-7-15-8)5-17-4-3-13-10(12-2)14-6-11/h7H,3-5H2,1-2H3,(H,15,16)(H2,12,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig histamine H2 receptor |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM25753
(2-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl...)Show InChI InChI=1S/C14H22N2O3/c1-10(2)16-8-12(17)9-19-13-5-3-11(4-6-13)7-14(15)18/h3-6,10,12,16-17H,7-9H2,1-2H3,(H2,15,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human alpha1 adrenoceptor |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50335569

(1-{1-[1-(Hydroxymethyl)cyclooctyl]piperidin-4-yl}-...)Show InChI InChI=1S/C21H31N3O2/c25-16-21(12-6-2-1-3-7-13-21)23-14-10-17(11-15-23)24-19-9-5-4-8-18(19)22-20(24)26/h4-5,8-9,17,25H,1-3,6-7,10-16H2,(H,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 194 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human KOP receptor expressed in HEK-293 cells assessed as induction of [35S]GTPgammaS binding after 30 mins by liquid scintillati... |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50335575

((1-{4-[2-(3-Chloro-4-fluorophenyl)-1H-benzimidazol...)Show SMILES OCC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C27H33ClFN3O/c28-22-18-20(10-11-23(22)29)26-30-24-8-4-5-9-25(24)32(26)21-12-16-31(17-13-21)27(19-33)14-6-2-1-3-7-15-27/h4-5,8-11,18,21,33H,1-3,6-7,12-17,19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human NOP receptor expressed in HEK293 cells assessed as induction of [35S]GTPgammaS binding after 30 mins by liquid scintillatio... |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50335575

((1-{4-[2-(3-Chloro-4-fluorophenyl)-1H-benzimidazol...)Show SMILES OCC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C27H33ClFN3O/c28-22-18-20(10-11-23(22)29)26-30-24-8-4-5-9-25(24)32(26)21-12-16-31(17-13-21)27(19-33)14-6-2-1-3-7-15-27/h4-5,8-11,18,21,33H,1-3,6-7,12-17,19H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human MOP receptor expressed in CHO-K1 cells assessed as induction of [35S]GTPgammaS binding after 30 mins by liquid scintillatio... |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50335574

((1-{4-[2-(4-Chlorophenyl)-1H-benzimidazol-1-yl]pip...)Show SMILES OCC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C27H34ClN3O/c28-22-12-10-21(11-13-22)26-29-24-8-4-5-9-25(24)31(26)23-14-18-30(19-15-23)27(20-32)16-6-2-1-3-7-17-27/h4-5,8-13,23,32H,1-3,6-7,14-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50335569

(1-{1-[1-(Hydroxymethyl)cyclooctyl]piperidin-4-yl}-...)Show InChI InChI=1S/C21H31N3O2/c25-16-21(12-6-2-1-3-7-13-21)23-14-10-17(11-15-23)24-19-9-5-4-8-18(19)22-20(24)26/h4-5,8-9,17,25H,1-3,6-7,10-16H2,(H,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human NOP receptor expressed in HEK293 cells assessed as induction of [35S]GTPgammaS binding after 30 mins by liquid scintillatio... |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50335567

((1-{4-[2-(4-Methylpiperazin-1-yl)-1H-benzimidazol-...)Show SMILES CN1CCN(CC1)c1nc2ccccc2n1C1CCN(CC1)C1(CO)CCCCCCC1 Show InChI InChI=1S/C26H41N5O/c1-28-17-19-29(20-18-28)25-27-23-9-5-6-10-24(23)31(25)22-11-15-30(16-12-22)26(21-32)13-7-3-2-4-8-14-26/h5-6,9-10,22,32H,2-4,7-8,11-21H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human NOP receptor expressed in HEK293 cells assessed as induction of [35S]GTPgammaS binding after 30 mins by liquid scintillatio... |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50335576

((1-{4-[2-(3-Chloro-4-fluorophenyl)-1H-benzimidazol...)Show SMILES OCC1(CCCCC1)N1CCC(CC1)n1c(nc2ccccc12)-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C25H29ClFN3O/c26-20-16-18(8-9-21(20)27)24-28-22-6-2-3-7-23(22)30(24)19-10-14-29(15-11-19)25(17-31)12-4-1-5-13-25/h2-3,6-9,16,19,31H,1,4-5,10-15,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 53 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human NOP receptor expressed in HEK293 cells assessed as induction of [35S]GTPgammaS binding after 30 mins by liquid scintillatio... |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50335567

((1-{4-[2-(4-Methylpiperazin-1-yl)-1H-benzimidazol-...)Show SMILES CN1CCN(CC1)c1nc2ccccc2n1C1CCN(CC1)C1(CO)CCCCCCC1 Show InChI InChI=1S/C26H41N5O/c1-28-17-19-29(20-18-28)25-27-23-9-5-6-10-24(23)31(25)22-11-15-30(16-12-22)26(21-32)13-7-3-2-4-8-14-26/h5-6,9-10,22,32H,2-4,7-8,11-21H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human KOP receptor expressed in HEK-293 cells assessed as induction of [35S]GTPgammaS binding after 30 mins by liquid scintillati... |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50335568

((1-{4-[2-(3-Methylpiperazin-1-yl)-1H-benzimidazol-...)Show SMILES CC1CN(CCN1)c1nc2ccccc2n1C1CCN(CC1)C1(CO)CCCCCCC1 Show InChI InChI=1S/C26H41N5O/c1-21-19-29(18-15-27-21)25-28-23-9-5-6-10-24(23)31(25)22-11-16-30(17-12-22)26(20-32)13-7-3-2-4-8-14-26/h5-6,9-10,21-22,27,32H,2-4,7-8,11-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 366 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human KOP receptor expressed in HEK-293 cells assessed as induction of [35S]GTPgammaS binding after 30 mins by liquid scintillati... |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50335574

((1-{4-[2-(4-Chlorophenyl)-1H-benzimidazol-1-yl]pip...)Show SMILES OCC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C27H34ClN3O/c28-22-12-10-21(11-13-22)26-29-24-8-4-5-9-25(24)31(26)23-14-18-30(19-15-23)27(20-32)16-6-2-1-3-7-17-27/h4-5,8-13,23,32H,1-3,6-7,14-20H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human MOP receptor expressed in CHO-K1 cells assessed as induction of [35S]GTPgammaS binding after 30 mins by liquid scintillatio... |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50335570

(CHEMBL1650844 | {1-[4-(2-{Hexahydropyrrolo[3,4-c]p...)Show SMILES OCC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)N1CC2CNCC2C1 Show InChI InChI=1S/C27H41N5O/c33-20-27(12-6-2-1-3-7-13-27)31-14-10-23(11-15-31)32-25-9-5-4-8-24(25)29-26(32)30-18-21-16-28-17-22(21)19-30/h4-5,8-9,21-23,28,33H,1-3,6-7,10-20H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human MOP receptor expressed in CHO-K1 cells assessed as induction of [35S]GTPgammaS binding after 30 mins by liquid scintillatio... |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50335569

(1-{1-[1-(Hydroxymethyl)cyclooctyl]piperidin-4-yl}-...)Show InChI InChI=1S/C21H31N3O2/c25-16-21(12-6-2-1-3-7-13-21)23-14-10-17(11-15-23)24-19-9-5-4-8-18(19)22-20(24)26/h4-5,8-9,17,25H,1-3,6-7,10-16H2,(H,22,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 144 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human MOP receptor expressed in CHO-K1 cells assessed as induction of [35S]GTPgammaS binding after 30 mins by liquid scintillatio... |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50335568

((1-{4-[2-(3-Methylpiperazin-1-yl)-1H-benzimidazol-...)Show SMILES CC1CN(CCN1)c1nc2ccccc2n1C1CCN(CC1)C1(CO)CCCCCCC1 Show InChI InChI=1S/C26H41N5O/c1-21-19-29(18-15-27-21)25-28-23-9-5-6-10-24(23)31(25)22-11-16-30(17-12-22)26(20-32)13-7-3-2-4-8-14-26/h5-6,9-10,21-22,27,32H,2-4,7-8,11-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 38 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human NOP receptor expressed in HEK293 cells assessed as induction of [35S]GTPgammaS binding after 30 mins by liquid scintillatio... |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50335568

((1-{4-[2-(3-Methylpiperazin-1-yl)-1H-benzimidazol-...)Show SMILES CC1CN(CCN1)c1nc2ccccc2n1C1CCN(CC1)C1(CO)CCCCCCC1 Show InChI InChI=1S/C26H41N5O/c1-21-19-29(18-15-27-21)25-28-23-9-5-6-10-24(23)31(25)22-11-16-30(17-12-22)26(20-32)13-7-3-2-4-8-14-26/h5-6,9-10,21-22,27,32H,2-4,7-8,11-20H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human MOP receptor expressed in CHO-K1 cells assessed as induction of [35S]GTPgammaS binding after 30 mins by liquid scintillatio... |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50335570

(CHEMBL1650844 | {1-[4-(2-{Hexahydropyrrolo[3,4-c]p...)Show SMILES OCC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)N1CC2CNCC2C1 Show InChI InChI=1S/C27H41N5O/c33-20-27(12-6-2-1-3-7-13-27)31-14-10-23(11-15-31)32-25-9-5-4-8-24(25)29-26(32)30-18-21-16-28-17-22(21)19-30/h4-5,8-9,21-23,28,33H,1-3,6-7,10-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50335573

((1-{4-[2-(3,3-Dimethylpiperazin-1-yl)-1H-benzimida...)Show SMILES CC1(C)CN(CCN1)c1nc2ccccc2n1C1CCN(CC1)C1(CO)CCCCCCC1 Show InChI InChI=1S/C27H43N5O/c1-26(2)20-30(19-16-28-26)25-29-23-10-6-7-11-24(23)32(25)22-12-17-31(18-13-22)27(21-33)14-8-4-3-5-9-15-27/h6-7,10-11,22,28,33H,3-5,8-9,12-21H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human NOP receptor expressed in HEK293 cells assessed as induction of [35S]GTPgammaS binding after 30 mins by liquid scintillatio... |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004178

(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human NOP receptor expressed in HEK293 cells assessed as induction of [35S]GTPgammaS binding after 30 mins by liquid scintillatio... |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data